Professional Documents
Culture Documents
Group 1
Group 1
Uploaded by
MARY LEIANNE LEJATCopyright:
Available Formats
You might also like
- Unit 6 IAL ChemistryDocument11 pagesUnit 6 IAL ChemistryDonggyu Lee100% (1)
- Group I Cations: This Centrifuge Is Saved For Analysis of Group II-VDocument1 pageGroup I Cations: This Centrifuge Is Saved For Analysis of Group II-VpixiedustNo ratings yet
- Cations IV V and Anions I VDocument32 pagesCations IV V and Anions I Vwinnerq56No ratings yet
- Group Analysis For Qualitatively AnalysisDocument13 pagesGroup Analysis For Qualitatively AnalysisVishalNo ratings yet
- Experiment 2 CationsDocument16 pagesExperiment 2 Cationskirigayasmith1No ratings yet
- PROCEDURE 3. Precipitation of Group II CationsDocument2 pagesPROCEDURE 3. Precipitation of Group II CationsRicky SheldonNo ratings yet
- Analysis of Group I V AnionsDocument11 pagesAnalysis of Group I V AnionsRhea Jean Villalobos NobleNo ratings yet
- Chapter 62 Structured QuestionsDocument23 pagesChapter 62 Structured QuestionsytNo ratings yet
- Separation and Analysis of Binery and Te PDFDocument35 pagesSeparation and Analysis of Binery and Te PDFDiwakar PatelNo ratings yet
- Lab6 - Qualitative Analysis of Cations and Quantitative Analysis of Anion ChlorideDocument4 pagesLab6 - Qualitative Analysis of Cations and Quantitative Analysis of Anion ChlorideDounia MarbouhNo ratings yet
- Expt. 6 Isolation, Hydrolysis and Qualitative Analysis ofDocument16 pagesExpt. 6 Isolation, Hydrolysis and Qualitative Analysis ofLESLIE JANE BALUYOS JALANo ratings yet
- HandbookPharmaceutical Excipients-351Document1 pageHandbookPharmaceutical Excipients-351putri fatimahNo ratings yet
- Solutions PDFDocument129 pagesSolutions PDFRannyNo ratings yet
- Functional Group and Food StuffDocument6 pagesFunctional Group and Food StuffNandana MNo ratings yet
- Worksheet No. 10 Group IIIA CationsDocument2 pagesWorksheet No. 10 Group IIIA CationsAndrew CraigieNo ratings yet
- Organic Chemistry Practical Manual - Compound IdentificationDocument10 pagesOrganic Chemistry Practical Manual - Compound Identificationsp_douglas83% (35)
- Group 4 and 5 AnionsDocument2 pagesGroup 4 and 5 AnionsMarive Bonsai AlcomendrasNo ratings yet
- Qualitative Analysis: Identification of The AnionDocument40 pagesQualitative Analysis: Identification of The AniontwinkledreampoppiesNo ratings yet
- Chemistry Unit 1Document16 pagesChemistry Unit 1Bhairavi MNo ratings yet
- Analytical Chem - Post Lab NotesDocument11 pagesAnalytical Chem - Post Lab NotesMare5Der5No ratings yet
- Screenshot 2022-10-09 at 11.37.54 PMDocument6 pagesScreenshot 2022-10-09 at 11.37.54 PMpsyxs4tsv9No ratings yet
- IAL Chemistry SB2 Answers Topic20Document5 pagesIAL Chemistry SB2 Answers Topic20salmaNo ratings yet
- Procaine Hydrochloride 1605Document1 pageProcaine Hydrochloride 1605NTĐ ChannelNo ratings yet
- CHLORTALIDONEDocument2 pagesCHLORTALIDONEartemNo ratings yet
- Assignment On: Zinc SulfateDocument15 pagesAssignment On: Zinc SulfateFuad Hasan Pranto 1921147049100% (1)
- Experiment 5:: Blood BuffersDocument6 pagesExperiment 5:: Blood BuffersVianca Joy RedobleNo ratings yet
- Titrimetric AnalysisDocument3 pagesTitrimetric AnalysisKarthik BhatNo ratings yet
- RP 04 - Identification of Cations and AnionsDocument9 pagesRP 04 - Identification of Cations and AnionsAnything Anywhere AnytimeNo ratings yet
- Experiment 9 - Inorganic Qualitative AnalysisDocument8 pagesExperiment 9 - Inorganic Qualitative AnalysisCharles JimenezNo ratings yet
- Analisis Kation Dan AnionDocument11 pagesAnalisis Kation Dan AnionDhiyahNo ratings yet
- CPT ReadingsDocument24 pagesCPT Readingsom patelNo ratings yet
- Adobe Scan 26 Abr. 2023Document2 pagesAdobe Scan 26 Abr. 2023María Renee Quintanilla VidalNo ratings yet
- Microscale Chemistry in A Plastic Petri Dish: Preparation and Chemical Properties of Chlorine GasDocument6 pagesMicroscale Chemistry in A Plastic Petri Dish: Preparation and Chemical Properties of Chlorine GasPaul SchumannNo ratings yet
- Ethanol PDFDocument4 pagesEthanol PDFThe First Song Was Titled MemoriesNo ratings yet
- Practica 4 Obtención de Butiraldehído"Document5 pagesPractica 4 Obtención de Butiraldehído"ethan pamatzNo ratings yet
- Expt. 3 Analysis of LipidsDocument18 pagesExpt. 3 Analysis of LipidsLESLIE JANE BALUYOS JALANo ratings yet
- Alvarezacosta Expt6Document9 pagesAlvarezacosta Expt6kiona100% (1)
- Salt Analysis-3 16/07/2021: Aim: To Analyze and Identify The Given SaltDocument2 pagesSalt Analysis-3 16/07/2021: Aim: To Analyze and Identify The Given SaltAryan PandeyNo ratings yet
- Compostela Valley State College Compostela Campus Compostela, Compostela Valley ProvinceDocument5 pagesCompostela Valley State College Compostela Campus Compostela, Compostela Valley ProvinceGizzelle LigutomNo ratings yet
- Hypo Normality: Normality5y s00.021 NDocument4 pagesHypo Normality: Normality5y s00.021 NAjaj AlamNo ratings yet
- Crystallization and PHDocument4 pagesCrystallization and PHsiddhantnayak025No ratings yet
- CHEM 102 Experiment 8 Aldehyde Ketone Version Covid19Document3 pagesCHEM 102 Experiment 8 Aldehyde Ketone Version Covid19Azizah MunitaNo ratings yet
- Chemistry Experiments - Though IncompleteDocument18 pagesChemistry Experiments - Though Incompletemurali kkNo ratings yet
- Exp.13.ALDEHYDE, Exp.14.KETONE, Exp.15. AMINEDocument4 pagesExp.13.ALDEHYDE, Exp.14.KETONE, Exp.15. AMINEArry DujNo ratings yet
- ChemDocument11 pagesChemextremegamer5908No ratings yet
- Chem27 Lab Results Post Lab E 1-3 ToolsDocument101 pagesChem27 Lab Results Post Lab E 1-3 ToolsAngelica Camille B. AbaoNo ratings yet
- Toaz - Info 59785464 Brain Lipid Writtendoc PRDocument18 pagesToaz - Info 59785464 Brain Lipid Writtendoc PRLOLONo ratings yet
- Anachem Guide Group 4 CationsDocument5 pagesAnachem Guide Group 4 CationsjudeNo ratings yet
- KEAM CREASH DPP Solutions - Alcohols, Phenols and EthersDocument6 pagesKEAM CREASH DPP Solutions - Alcohols, Phenols and EthersAlentNo ratings yet
- Chem 33 Postlabs Expt 10-13Document11 pagesChem 33 Postlabs Expt 10-13BelaNo ratings yet
- Chloride Test: MquantDocument1 pageChloride Test: MquantWijianto WijiantoNo ratings yet
- IodometryDocument16 pagesIodometryRichelle Lencioco100% (1)
- Chemical Tests For Detection of TanninDocument2 pagesChemical Tests For Detection of TanninPriyanka DasNo ratings yet
- CHEM 3L Group 4 Cation 1Document6 pagesCHEM 3L Group 4 Cation 1Beatrice AlejeNo ratings yet
- Adobe Scan 04 Dec 2023Document2 pagesAdobe Scan 04 Dec 2023lobrandi46No ratings yet
- Enrofloxacin For Veterinary UseDocument3 pagesEnrofloxacin For Veterinary Usenguyentuanson167No ratings yet
- MCQ Madness 10 PDFDocument11 pagesMCQ Madness 10 PDFnotabc gamerNo ratings yet
- Fluocinolone AcetonideDocument2 pagesFluocinolone AcetonideSidahmed SiDo BouchenakNo ratings yet
- Maunda BSMLS1GDocument3 pagesMaunda BSMLS1GSherwin OrdinariaNo ratings yet
- Compound Forming Extractants, Solvating Solvents and Inert Solvents: Iupac Chemical Data SeriesFrom EverandCompound Forming Extractants, Solvating Solvents and Inert Solvents: Iupac Chemical Data SeriesNo ratings yet
- Ref p03Document7 pagesRef p03nadhifah safitriNo ratings yet
- Physics 9 Icse Sample Paper 5Document5 pagesPhysics 9 Icse Sample Paper 5Study in an easy wayNo ratings yet
- Measuring The Speed Through Water by Wavex: by Dr. Scient. Rune GangeskarDocument10 pagesMeasuring The Speed Through Water by Wavex: by Dr. Scient. Rune GangeskarPrinceSadhotraNo ratings yet
- QuantumdotsDocument31 pagesQuantumdotsChocho DreamNo ratings yet
- Kaplan Wheel Turbine: 1. Scroll CasingDocument3 pagesKaplan Wheel Turbine: 1. Scroll CasingAnwaar SafdarNo ratings yet
- TP 2003 210788 MMOD ShieldingDocument115 pagesTP 2003 210788 MMOD ShieldingstefNo ratings yet
- Lab1 SampleDocument20 pagesLab1 SampleLee MingHweeNo ratings yet
- Concrete Technology MCQ PDF (Erexams - Com)Document69 pagesConcrete Technology MCQ PDF (Erexams - Com)krishna chaithanyaNo ratings yet
- MetrologyDocument639 pagesMetrologyranveerm100% (1)
- Downloaded From Manuals Search EngineDocument13 pagesDownloaded From Manuals Search EngineSandro CoelhoNo ratings yet
- Gate Aptitude (All Branches) - Updated Till 2017Document59 pagesGate Aptitude (All Branches) - Updated Till 2017NITISH KUMAR THAKURNo ratings yet
- Dow ELITE 5220G Enhanced Polyethylene ResinDocument2 pagesDow ELITE 5220G Enhanced Polyethylene Resinusman3549606No ratings yet
- Test 3 With SolutionsDocument33 pagesTest 3 With SolutionssvsvsvsvNo ratings yet
- Fujitsu Air Conditioning Pricelist 2010Document44 pagesFujitsu Air Conditioning Pricelist 2010adnannsaNo ratings yet
- Chapter 32 - Electromagnetic WavesDocument19 pagesChapter 32 - Electromagnetic WavesKarla PereraNo ratings yet
- CONVO User Manual of SY5000 Series AC DrivesDocument115 pagesCONVO User Manual of SY5000 Series AC DrivesRavshan SaidahmetovNo ratings yet
- ALT Series Service Parts List: 24 Volt AlternatorDocument4 pagesALT Series Service Parts List: 24 Volt AlternatorJacksonNo ratings yet
- cc12 Group 8 This Is An Example For The Assignement of General Chemistry in HcmutDocument17 pagescc12 Group 8 This Is An Example For The Assignement of General Chemistry in HcmutGIANG LẠI THUNo ratings yet
- Liebherr Slewing Bearings Product Catalogue en Metric WebDocument158 pagesLiebherr Slewing Bearings Product Catalogue en Metric WebИгорьNo ratings yet
- 02 Assignment CEDocument45 pages02 Assignment CEshafia100% (1)
- Physical Science Week 3 Day 1Document2 pagesPhysical Science Week 3 Day 1daniel loberizNo ratings yet
- SFP CalculationDocument6 pagesSFP CalculationMohammad IsmailNo ratings yet
- BHS-TEPC-SIEVERT-ACFM-2022-001-Rev.01 - ACFM Inspection (Hassyan Power Plant)Document11 pagesBHS-TEPC-SIEVERT-ACFM-2022-001-Rev.01 - ACFM Inspection (Hassyan Power Plant)Saddam HossainNo ratings yet
- 119A2032 Krishnaveni Doki ME Experiment 6Document7 pages119A2032 Krishnaveni Doki ME Experiment 6Ishika DokiNo ratings yet
- Fiber OpticDocument19 pagesFiber OpticJohn Carl Villavicencio100% (1)
- Bedroom Fan Coil Unit DS A4 - Layout 1Document3 pagesBedroom Fan Coil Unit DS A4 - Layout 1Dejan DosljakNo ratings yet
- Yu 2017Document10 pagesYu 2017Ehsan AbarghooeiNo ratings yet
- Lec.4 1Document43 pagesLec.4 1Mohammed ROSHDYNo ratings yet
- Jib Crane Specification 20190514 Jib Crane Specification The Jib CraneDocument10 pagesJib Crane Specification 20190514 Jib Crane Specification The Jib CraneMedardo Javier Olivares JuanicoNo ratings yet
- AES Sildes Spyros2018Document23 pagesAES Sildes Spyros2018坏豆腐No ratings yet
Group 1
Group 1
Uploaded by
MARY LEIANNE LEJATOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Group 1
Group 1
Uploaded by
MARY LEIANNE LEJATCopyright:
Available Formats
QUALITATIVE ANALYSIS OF
GROUP I CATIONS
INTRODUCTION
QUALITATIVE ANALYSIS
- A method used for identification of ions or compounds in a sample. It primarily involves the separation of ions or
compounds in a mixture. Qualitative analysis employs both ion precipitation reactions (solubility tests) and
chemical reactivity tests. The separation of ions is easily achieved by taking advantage of their solubility
properties.
GROUP 1 CATIONS
The systematic analysis of the common cations is based upon the successive precipitation of groups of ions, so that the
total number of ions can be broken down into a small number of groups, each containing a number of related cations.
Group I cations are composed of those common cations whose chlorides are relatively insoluble in dilute acids.
Please READ Chapter 12: Group 1 Cations (Qualitative Analysis by E.S Gilreath pp.185-189)
At the end of this activity, the student should be able to:
1. Identify the principles involved in the qualitative analysis of cations.
2. Describe the principles involved in the separation and identification of Group 1 cations.
3. Understand chemical reactions involved in the analysis of Group 1 cations.
4. Appreciate the intricate process of qualitative analysis.
The successful separation of a group of cations is determined by the relative solubility products of the compounds formed
by the cations with the precipitating anion. Enumerate all the insoluble chlorides of Group I cation with their solubility
product constants.
Insoluble Chloride Solubility Product Constant
From the data above, compare then describe their solubility products and whether they are completely or incompletely
precipitated.
CPMata,RPhMSPharm,RSLumang-ay,RPhMSPharm©2020JBadillaRPH©2020 36
Saint Louis University – Department of Pharmacy
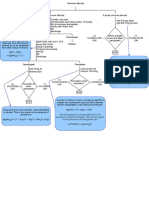
10 drops of Test
Solution
The original test solution may contain Chlorides of
Group I cation or oxychlorides of Antimony and Bismuth.
(+) 4 drops of 3F
Addition of Hydrochloric Acid causes the dissolution of the
HCl
latter; thereby, leaving the chlorides of Group I cation for
individual analysis.
Mix
Centrifuge
(+) 1 drop of 3F Slight excess of HCl not only causes a more complete
HCl precipitation of the chlorides of Group I cation, but also
prevents the formation of BiOCl and SbOCl.
Centrifuge
Separate
Centrifugate Precipitate
Wash
Lead Chloride may dissolve during the
Contains Group II-V 10 drops of cold
washing process. The addition of HCl
Cations water with 1 drop of
reduces its solubility by common-ion
*for further analysis 3F HCl
effect.
Centrifuge
Discard wash water White Precipitate
CPMata,RPhMSPharm,RSLumang-ay,RPhMSPharm©2020JBadillaRPH©2020 37
Saint Louis University – Department of Pharmacy
White precipitate (From Procedure 1) may be PbCl2, AgCl, and Hg2Cl2.
(+) 6-7 drops of
distilled water
Stir
Heat for 3 minutes
Centrifuge QUICKLY When separating keep the mixture hot in a water
bath, Why?
Separate
Centrifugate Precipitate
(+) 4 drops of 1F (+) 10 drops of
K2CrO4 NH3
Stir
Yellow precipitate Centrifuge
confirms the presence
of LEAD ION
Separate
Centrifugate Precipitate
Wash
Test for
(+) 3 F HNO3 (+) 10 drops of
acidity using Discard washing
until acidic water
litmus paper.
White precipitate (+) 2 drops
confirms the presence conc. HNO3
of SILVER ION
If the solution is not clear,
(+) 5 drops of
centrifuge then retain the
Distilled water
centrifugate.
(+) 1-2 drops of
SnCl2 solution
White/gray precipitate
confirms the presence
of MERCUROUS ION
CPMata,RPhMSPharm,RSLumang-ay,RPhMSPharm©2020JBadillaRPH©2020 38
Saint Louis University – Department of Pharmacy
On the initial Addition of 3 F Hydrochloric Acid
The test solution may contain a white precipitate which could either be the chlorides of Group I or the
oxychlorides of Bismuth (Bi) and Antimony (Sb). Both can be precipitated;
however, the addition of concentrated Hydrochloric acid (HCl) can
differentiate the two. The oxychlorides of Bi and Sb will dissolve, whereas NOTE:
the chlorides of Group I will not. The reaction is reversible and the
presence of oxychloride
precipitate depends on the
BiOCl + 2H3O + Bi + Cl + 3H2O
3+ -
Bismuth Hydronium Bismuth Chlorine Water concentration of Hydronium ions.
Oxychloride Ion
On the Addition of EXCESS Hydrochloric Acid
Apart from ensuring that the precipitation of Group I cation is
complete, by virtue of common-ion effect, the excess prevents the
formation of BiOCl and SbOCl.
A large concentration of HCl or
AgCl + 2Cl- AgCl3-
Chloride ion should be avoided, since
Silver Chloride Chloride ion Silver Trichloride
the chloride ion in high concentration
increases the solubility of the
precipitated chlorides. PbCl2 + 2Cl- PbCl4-
Lead Chloride Chloride ion Lead Tetrachloride
On the Addition of 3F Ammonia
If the ammoniacal solution is left in contact with the precipitate of Mercury (Hg) and Mercuric Amidochloride
(HgNH2Cl) for an appreciable length of time, the soluble silver complex ion may react with metallic Hg. Therefore, a
small concentration of Silver (Ag+) may not give the usual test for silver ion.
2Hg + 2Ag(NH3)2 + Hg2 2+ + 2Ag + 4NH3
Mercury Silver Diamine Mercurous ion Silver Ammonia
On the Addition of 3F Nitric acid
The solution must be acidified to convert the Ag(NH3)2 + to Silver Chloride (AgCl); otherwise, even though silver
ions are present, no precipitate will form.
On the remaining Precipitate after the Treatment of Ammonia
If mercurous ions are present, the residue left from the Ammonia (NH3) treatment must be black or greatly
discolored, because of the precipitation of colloidal mercury.
Hg2Cl2 + 2NH3 HgNH2Cl + Hg + NH4 + + Cl-
Mercury Ammonia Mercuric Mercury Ammonium Chloride
Chloride Amidochloride Ion Ion
CPMata,RPhMSPharm,RSLumang-ay,RPhMSPharm©2020JBadillaRPH©2020 39
Saint Louis University – Department of Pharmacy
CHEMICAL REACTIONS INVOLVED IN THE SEPARATION AND IDENTIFICATION OF CATIONS OF GROUP I
Group precipitation
Pb2+ + 2Cl- PbCl2
Lead, silver and mercurous ion give white precipitates
with the chloride ion in an acid solution. Ag+ + Cl- AgCl
Hg22+ + 2Cl- Hg2Cl2
Separation and Identification of Lead Ion
An incomplete separation of Lead Chloride (PbCl2) from
the group precipitate is affected with hot water. PbCl2 is Pb2+ + CrO4- PbCrO4
soluble to the extent of approximately 1 g/100 ml at room
temperature, and 3.34 g/ml at the temperature of boiling
water. The threefold increase insolubility is small, but sufficient NOTE:
to give a lead-ion concentration that can be detected with Lead Chromate is mush less soluble than Lead
chromate ions. Chloride
Treatment of Residue in Group I Analysis with Ammonia
When a mixture of Silver Chloride (AgCl) and Mercury
Chloride (HgCl2) is treated with Ammonia (NH3), the AgCl
AgCl + 2NH3 Ag(NH3)2 + Cl-
dissolves, leaving a black residue composed of mercury and
mercuric aminochloride.
Ammonia acts upon mercurous chloride to produce an internal redox reaction in which one mercurous ion is
produced and the other is oxidized to the mercuric state. Mercuric aminochloride is white and the finely divided mercury
is black. The blackening of the mixture is an identification test for the mercurous ion.
Hg2Cl2 + 2NH3 HgNH2Cl + Hg + NH4 + Cl-
Confirmation for the presence of Silver Ion
The centrifugate from the treatment of the Group I precipitate with ammonia contains Ag(NH 3)2+ and Cl- ions. If the
solution is made acidic with nitric acid (HNO3), the complex is destroyed and AgCl is reprecipitated.
Ag(NH3)2 + Cl- + 2H3O AgCl + 2NH4 + NH4 + 2H2O
Identification of Mercurous Ion
The residue from the treatment of mercurous chloride with ammonia is a mixture of Hg and HgNH 2Cl. Although the
production of black mixture is sufficient to indicate the presence of the mercurous ion, additional confirmation is
obtained by dissolving the mixture in nitric acid and testing with stannous chloride solution.
3Hg + 2NO3- + 8H3O+ 3Hg2+ + 2NO + 12H2O
2HgNH2Cl + 2NO3- + 4H3O- 2Hg2+ + N2 + 2NO + 2Cl- + 8H2O
CPMata,RPhMSPharm,RSLumang-ay,RPhMSPharm©2020JBadillaRPH©2020 40
Saint Louis University – Department of Pharmacy
In the presence of chloride ions, the mercuric ions tend to form the slightly dissociated mercuric chloride molecule,
HgCl2, or the complex ion, HgCl4-
Hg2+ + 2Cl- HgCl2 HgCl2 + 2Cl- HgCl4
- Low Concentration of Chloride ions - - High Concentration of Chloride ions -
Acid solutions containing HgCl2 and HgCl4- give precipitates with stannous ions. These precipitates may be white, gray,
or black, depending upon the relative concentrations of the reactants.
2HgCl2 + SnCl4- Hg2Cl2 + SnCl6-
2HgCl4 + SnCl4- Hg2Cl2 + SnCl6- + 4Cl-
Further addition of stannous chloride reduces white Hg 2Cl2 to black, finely divided mercury. Generally, a gray mixture
of Hg2Cl2 and Hg is obtained.
Hg2Cl2 + SnCl4- 2Hg + SnCl6-
CPMata,RPhMSPharm,RSLumang-ay,RPhMSPharm©2020JBadillaRPH©2020 41
Saint Louis University – Department of Pharmacy
You might also like
- Unit 6 IAL ChemistryDocument11 pagesUnit 6 IAL ChemistryDonggyu Lee100% (1)
- Group I Cations: This Centrifuge Is Saved For Analysis of Group II-VDocument1 pageGroup I Cations: This Centrifuge Is Saved For Analysis of Group II-VpixiedustNo ratings yet
- Cations IV V and Anions I VDocument32 pagesCations IV V and Anions I Vwinnerq56No ratings yet
- Group Analysis For Qualitatively AnalysisDocument13 pagesGroup Analysis For Qualitatively AnalysisVishalNo ratings yet
- Experiment 2 CationsDocument16 pagesExperiment 2 Cationskirigayasmith1No ratings yet
- PROCEDURE 3. Precipitation of Group II CationsDocument2 pagesPROCEDURE 3. Precipitation of Group II CationsRicky SheldonNo ratings yet
- Analysis of Group I V AnionsDocument11 pagesAnalysis of Group I V AnionsRhea Jean Villalobos NobleNo ratings yet
- Chapter 62 Structured QuestionsDocument23 pagesChapter 62 Structured QuestionsytNo ratings yet
- Separation and Analysis of Binery and Te PDFDocument35 pagesSeparation and Analysis of Binery and Te PDFDiwakar PatelNo ratings yet
- Lab6 - Qualitative Analysis of Cations and Quantitative Analysis of Anion ChlorideDocument4 pagesLab6 - Qualitative Analysis of Cations and Quantitative Analysis of Anion ChlorideDounia MarbouhNo ratings yet
- Expt. 6 Isolation, Hydrolysis and Qualitative Analysis ofDocument16 pagesExpt. 6 Isolation, Hydrolysis and Qualitative Analysis ofLESLIE JANE BALUYOS JALANo ratings yet
- HandbookPharmaceutical Excipients-351Document1 pageHandbookPharmaceutical Excipients-351putri fatimahNo ratings yet
- Solutions PDFDocument129 pagesSolutions PDFRannyNo ratings yet
- Functional Group and Food StuffDocument6 pagesFunctional Group and Food StuffNandana MNo ratings yet
- Worksheet No. 10 Group IIIA CationsDocument2 pagesWorksheet No. 10 Group IIIA CationsAndrew CraigieNo ratings yet
- Organic Chemistry Practical Manual - Compound IdentificationDocument10 pagesOrganic Chemistry Practical Manual - Compound Identificationsp_douglas83% (35)
- Group 4 and 5 AnionsDocument2 pagesGroup 4 and 5 AnionsMarive Bonsai AlcomendrasNo ratings yet
- Qualitative Analysis: Identification of The AnionDocument40 pagesQualitative Analysis: Identification of The AniontwinkledreampoppiesNo ratings yet
- Chemistry Unit 1Document16 pagesChemistry Unit 1Bhairavi MNo ratings yet
- Analytical Chem - Post Lab NotesDocument11 pagesAnalytical Chem - Post Lab NotesMare5Der5No ratings yet
- Screenshot 2022-10-09 at 11.37.54 PMDocument6 pagesScreenshot 2022-10-09 at 11.37.54 PMpsyxs4tsv9No ratings yet
- IAL Chemistry SB2 Answers Topic20Document5 pagesIAL Chemistry SB2 Answers Topic20salmaNo ratings yet
- Procaine Hydrochloride 1605Document1 pageProcaine Hydrochloride 1605NTĐ ChannelNo ratings yet
- CHLORTALIDONEDocument2 pagesCHLORTALIDONEartemNo ratings yet
- Assignment On: Zinc SulfateDocument15 pagesAssignment On: Zinc SulfateFuad Hasan Pranto 1921147049100% (1)
- Experiment 5:: Blood BuffersDocument6 pagesExperiment 5:: Blood BuffersVianca Joy RedobleNo ratings yet
- Titrimetric AnalysisDocument3 pagesTitrimetric AnalysisKarthik BhatNo ratings yet
- RP 04 - Identification of Cations and AnionsDocument9 pagesRP 04 - Identification of Cations and AnionsAnything Anywhere AnytimeNo ratings yet
- Experiment 9 - Inorganic Qualitative AnalysisDocument8 pagesExperiment 9 - Inorganic Qualitative AnalysisCharles JimenezNo ratings yet
- Analisis Kation Dan AnionDocument11 pagesAnalisis Kation Dan AnionDhiyahNo ratings yet
- CPT ReadingsDocument24 pagesCPT Readingsom patelNo ratings yet
- Adobe Scan 26 Abr. 2023Document2 pagesAdobe Scan 26 Abr. 2023María Renee Quintanilla VidalNo ratings yet
- Microscale Chemistry in A Plastic Petri Dish: Preparation and Chemical Properties of Chlorine GasDocument6 pagesMicroscale Chemistry in A Plastic Petri Dish: Preparation and Chemical Properties of Chlorine GasPaul SchumannNo ratings yet
- Ethanol PDFDocument4 pagesEthanol PDFThe First Song Was Titled MemoriesNo ratings yet
- Practica 4 Obtención de Butiraldehído"Document5 pagesPractica 4 Obtención de Butiraldehído"ethan pamatzNo ratings yet
- Expt. 3 Analysis of LipidsDocument18 pagesExpt. 3 Analysis of LipidsLESLIE JANE BALUYOS JALANo ratings yet
- Alvarezacosta Expt6Document9 pagesAlvarezacosta Expt6kiona100% (1)
- Salt Analysis-3 16/07/2021: Aim: To Analyze and Identify The Given SaltDocument2 pagesSalt Analysis-3 16/07/2021: Aim: To Analyze and Identify The Given SaltAryan PandeyNo ratings yet
- Compostela Valley State College Compostela Campus Compostela, Compostela Valley ProvinceDocument5 pagesCompostela Valley State College Compostela Campus Compostela, Compostela Valley ProvinceGizzelle LigutomNo ratings yet
- Hypo Normality: Normality5y s00.021 NDocument4 pagesHypo Normality: Normality5y s00.021 NAjaj AlamNo ratings yet
- Crystallization and PHDocument4 pagesCrystallization and PHsiddhantnayak025No ratings yet
- CHEM 102 Experiment 8 Aldehyde Ketone Version Covid19Document3 pagesCHEM 102 Experiment 8 Aldehyde Ketone Version Covid19Azizah MunitaNo ratings yet
- Chemistry Experiments - Though IncompleteDocument18 pagesChemistry Experiments - Though Incompletemurali kkNo ratings yet
- Exp.13.ALDEHYDE, Exp.14.KETONE, Exp.15. AMINEDocument4 pagesExp.13.ALDEHYDE, Exp.14.KETONE, Exp.15. AMINEArry DujNo ratings yet
- ChemDocument11 pagesChemextremegamer5908No ratings yet
- Chem27 Lab Results Post Lab E 1-3 ToolsDocument101 pagesChem27 Lab Results Post Lab E 1-3 ToolsAngelica Camille B. AbaoNo ratings yet
- Toaz - Info 59785464 Brain Lipid Writtendoc PRDocument18 pagesToaz - Info 59785464 Brain Lipid Writtendoc PRLOLONo ratings yet
- Anachem Guide Group 4 CationsDocument5 pagesAnachem Guide Group 4 CationsjudeNo ratings yet
- KEAM CREASH DPP Solutions - Alcohols, Phenols and EthersDocument6 pagesKEAM CREASH DPP Solutions - Alcohols, Phenols and EthersAlentNo ratings yet
- Chem 33 Postlabs Expt 10-13Document11 pagesChem 33 Postlabs Expt 10-13BelaNo ratings yet
- Chloride Test: MquantDocument1 pageChloride Test: MquantWijianto WijiantoNo ratings yet
- IodometryDocument16 pagesIodometryRichelle Lencioco100% (1)
- Chemical Tests For Detection of TanninDocument2 pagesChemical Tests For Detection of TanninPriyanka DasNo ratings yet
- CHEM 3L Group 4 Cation 1Document6 pagesCHEM 3L Group 4 Cation 1Beatrice AlejeNo ratings yet
- Adobe Scan 04 Dec 2023Document2 pagesAdobe Scan 04 Dec 2023lobrandi46No ratings yet
- Enrofloxacin For Veterinary UseDocument3 pagesEnrofloxacin For Veterinary Usenguyentuanson167No ratings yet
- MCQ Madness 10 PDFDocument11 pagesMCQ Madness 10 PDFnotabc gamerNo ratings yet
- Fluocinolone AcetonideDocument2 pagesFluocinolone AcetonideSidahmed SiDo BouchenakNo ratings yet
- Maunda BSMLS1GDocument3 pagesMaunda BSMLS1GSherwin OrdinariaNo ratings yet
- Compound Forming Extractants, Solvating Solvents and Inert Solvents: Iupac Chemical Data SeriesFrom EverandCompound Forming Extractants, Solvating Solvents and Inert Solvents: Iupac Chemical Data SeriesNo ratings yet
- Ref p03Document7 pagesRef p03nadhifah safitriNo ratings yet
- Physics 9 Icse Sample Paper 5Document5 pagesPhysics 9 Icse Sample Paper 5Study in an easy wayNo ratings yet
- Measuring The Speed Through Water by Wavex: by Dr. Scient. Rune GangeskarDocument10 pagesMeasuring The Speed Through Water by Wavex: by Dr. Scient. Rune GangeskarPrinceSadhotraNo ratings yet
- QuantumdotsDocument31 pagesQuantumdotsChocho DreamNo ratings yet
- Kaplan Wheel Turbine: 1. Scroll CasingDocument3 pagesKaplan Wheel Turbine: 1. Scroll CasingAnwaar SafdarNo ratings yet
- TP 2003 210788 MMOD ShieldingDocument115 pagesTP 2003 210788 MMOD ShieldingstefNo ratings yet
- Lab1 SampleDocument20 pagesLab1 SampleLee MingHweeNo ratings yet
- Concrete Technology MCQ PDF (Erexams - Com)Document69 pagesConcrete Technology MCQ PDF (Erexams - Com)krishna chaithanyaNo ratings yet
- MetrologyDocument639 pagesMetrologyranveerm100% (1)
- Downloaded From Manuals Search EngineDocument13 pagesDownloaded From Manuals Search EngineSandro CoelhoNo ratings yet
- Gate Aptitude (All Branches) - Updated Till 2017Document59 pagesGate Aptitude (All Branches) - Updated Till 2017NITISH KUMAR THAKURNo ratings yet
- Dow ELITE 5220G Enhanced Polyethylene ResinDocument2 pagesDow ELITE 5220G Enhanced Polyethylene Resinusman3549606No ratings yet
- Test 3 With SolutionsDocument33 pagesTest 3 With SolutionssvsvsvsvNo ratings yet
- Fujitsu Air Conditioning Pricelist 2010Document44 pagesFujitsu Air Conditioning Pricelist 2010adnannsaNo ratings yet
- Chapter 32 - Electromagnetic WavesDocument19 pagesChapter 32 - Electromagnetic WavesKarla PereraNo ratings yet
- CONVO User Manual of SY5000 Series AC DrivesDocument115 pagesCONVO User Manual of SY5000 Series AC DrivesRavshan SaidahmetovNo ratings yet
- ALT Series Service Parts List: 24 Volt AlternatorDocument4 pagesALT Series Service Parts List: 24 Volt AlternatorJacksonNo ratings yet
- cc12 Group 8 This Is An Example For The Assignement of General Chemistry in HcmutDocument17 pagescc12 Group 8 This Is An Example For The Assignement of General Chemistry in HcmutGIANG LẠI THUNo ratings yet
- Liebherr Slewing Bearings Product Catalogue en Metric WebDocument158 pagesLiebherr Slewing Bearings Product Catalogue en Metric WebИгорьNo ratings yet
- 02 Assignment CEDocument45 pages02 Assignment CEshafia100% (1)
- Physical Science Week 3 Day 1Document2 pagesPhysical Science Week 3 Day 1daniel loberizNo ratings yet
- SFP CalculationDocument6 pagesSFP CalculationMohammad IsmailNo ratings yet
- BHS-TEPC-SIEVERT-ACFM-2022-001-Rev.01 - ACFM Inspection (Hassyan Power Plant)Document11 pagesBHS-TEPC-SIEVERT-ACFM-2022-001-Rev.01 - ACFM Inspection (Hassyan Power Plant)Saddam HossainNo ratings yet
- 119A2032 Krishnaveni Doki ME Experiment 6Document7 pages119A2032 Krishnaveni Doki ME Experiment 6Ishika DokiNo ratings yet
- Fiber OpticDocument19 pagesFiber OpticJohn Carl Villavicencio100% (1)
- Bedroom Fan Coil Unit DS A4 - Layout 1Document3 pagesBedroom Fan Coil Unit DS A4 - Layout 1Dejan DosljakNo ratings yet
- Yu 2017Document10 pagesYu 2017Ehsan AbarghooeiNo ratings yet
- Lec.4 1Document43 pagesLec.4 1Mohammed ROSHDYNo ratings yet
- Jib Crane Specification 20190514 Jib Crane Specification The Jib CraneDocument10 pagesJib Crane Specification 20190514 Jib Crane Specification The Jib CraneMedardo Javier Olivares JuanicoNo ratings yet
- AES Sildes Spyros2018Document23 pagesAES Sildes Spyros2018坏豆腐No ratings yet