Professional Documents
Culture Documents
Cyclohexane Conformational Analysis
Cyclohexane Conformational Analysis
Uploaded by
cclatrumOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Cyclohexane Conformational Analysis
Cyclohexane Conformational Analysis
Uploaded by
cclatrumCopyright:
Available Formats
Cyclohexane Conformational Analysis
http://research.cm.utexas.edu/nbauld/teach/cyclohex.html
Cyclohexane Conformational Analysis Cyclohexane Conformations and Energies TABLE OF CONTENTS FOR THIS CHAPTER CYCLOHEXANE CONFORMATIONS CYCLOHEXANE CONFORMATIONAL ENERGY DIAGRAM AXIAL AND EQUATORIAL BONDS CHAIR/CHAIR INTERCONVERSION METHYLCYCLOHEXANE CONFORMATIONS DISUBSTITUTED CYCLOHEXANES CYCLOHEXANE CONFORMATIONS
1 of 17
20/09/2011 9:41 CH
Cyclohexane Conformational Analysis
http://research.cm.utexas.edu/nbauld/teach/cyclohex.html
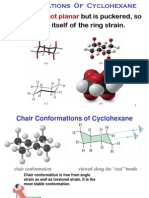
The simplest imaginable conformation for cyclohexane, the planar one, is not only not the ground state conformation, it is so high in energy that it plays no part in the conformational analysis of cyclohexane.This is mainly because of the large amount of torsional strain which is present in this form. Thus, all six C-C bonds in the planar form are eclipsed, so that we could crudely
2 of 17
20/09/2011 9:41 CH
Cyclohexane Conformational Analysis
http://research.cm.utexas.edu/nbauld/teach/cyclohex.html
estimate the torsional strain as being at least 6 times that in eclipsed ethane, which has one eclipsed C-C bond, or 18 kcal/mole. The ground state conformation of cyclohexane is a fully staggered conformation which is shaped somewhat like a "chair". In this conformation there is no torsional strain at all, and as we shall see later, no strain of any kind. Cyclohexane is unique in being the only cyclic hydrocarbon which is completely strain-free. So the six membered ring is the most stable of all.So we see that torsional effects, although individually small (per C-C bond), exert a tremendously important effect on the shapes of molecules, and shapes often effect reactivity. In the chair conformation, four carbon atoms are coplanar, and the other two are puckered out of this plane, one being puckered up above this plane(the carbon at the extreme right of the depicted chair form) and one being puckered down out of this plane(the one at the extreme left of the illustration). Another conformation which is important in any conformational analysis is the transition state, or maximum energy conformation on the rotational path.For cyclohexane this is the so-called "half-chair conformation", in which now 5 carbons are co-planar, and only one is puckered
3 of 17
20/09/2011 9:41 CH
Cyclohexane Conformational Analysis
http://research.cm.utexas.edu/nbauld/teach/cyclohex.html
out of the plane. In the illustration above, starting from the chair conformation, the half-chair form depicted arises from moving the left-hand-most carbon of the chair form , which is puckered down, upward into the plane of the four coplanar atoms. This creates considerable torsional strain, especially around the two carbon-carbon bonds involving the left-most carbon. The energy of this conformation is about 10 kcal/mol. The importance of this is that in any conformational changes in cyclohexane, 10 kcal is the energy barrier, and the pathway for such conformational changes must proceed through this relatively high energy conformation.Because this barrier is so much larger than that in ethane or butane, cyclohexane conformational changes can be "frozen out" at, e.g., -78 degrees. However, they are rapid at or near room temperature. For all practical purposes, only the chair form is populated. There is, as in the case of butane, another energy minimum structure which can be populated to a certain extent. This is the so- called "twist boat" conformation. However, since this conformation is 5.5 kcal/mol higher in energy than the chair conformation, it is not a highly populated state.Again, most of the strain energy results from bonds which are eclipsed or partially eclipsed, i.e.,
4 of 17
20/09/2011 9:41 CH
Cyclohexane Conformational Analysis
http://research.cm.utexas.edu/nbauld/teach/cyclohex.html
from torsional strain. CYCLOHEXANE CONFORMATIONAL ENERGY DIAGRAM
Axial and Equatorial Bonds in Cyclohexane Note that in chair cyclohexane, there are two different types of C-H bonds, and thus two chemically different types of hydrogens. The C-H bonds which point vertically upward or downward are called axial. There are 6 of these, 3 upward and 3 downward bonds, and they alternate up/down/up, etc., around the ring. The other six
5 of 17
20/09/2011 9:41 CH
Cyclohexane Conformational Analysis
http://research.cm.utexas.edu/nbauld/teach/cyclohex.html
bonds, which radiate away from the "equator" of the ring are called equatorial, and again there are six, 3 of which are "slant up" and 3 of whicdh are "slant down", again alternating around the ring. You should be able to quickly draw cyclohexane rings in which the axial and equatorial bonds are readily identifiable and distinguishable..
CHAIR/CHAIR INTERCONVERSION OR RING FLIP Up to now we have not described in detail the rotational path of cyclohexane and what is the end result. We understand that the best path (the lowest energy path) available proceeds via the half chair and requires an energy input of 10 kcal/mol. This transition state proceeds to a twist boat energy minimum, but this is not highly popupulated and generally plays little or
6 of 17
20/09/2011 9:41 CH
Cyclohexane Conformational Analysis
http://research.cm.utexas.edu/nbauld/teach/cyclohex.html
no role in cyclohexane's structure or chemistry. However, the twist boat can interconvert with another equivalent twist boat (via the true boat conformation as a transition state) to give another chair structure, in which the sense of the ring puckering is reversed. This is significant in cyclohexane itself, because in this process the axial and equatorial hydrogens are interconverted.Since this interconversion or ring flip occrus rapidly at room temperature, all hydrogens spend 50% of their time as axial hydrogens and 50% of their time as equatorial hydrogens, so that on the time average all C-H bonds of cyclohexane are equivalent. However, at any given instant, there are always two types of hydrogen.
7 of 17
20/09/2011 9:41 CH
Cyclohexane Conformational Analysis
http://research.cm.utexas.edu/nbauld/teach/cyclohex.html
METHYLCYCLOHEXANE CONFORMATIONS
8 of 17
20/09/2011 9:41 CH
Cyclohexane Conformational Analysis
http://research.cm.utexas.edu/nbauld/teach/cyclohex.html
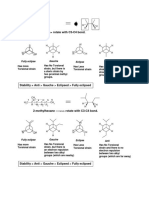
Since axial and equatorial bonds are non-equivalent, there are two non-equivalent positions in which to place any substituent. We use the simple methyl group as an example, but the same concept applies to any substituent. Equatorial methyl cyclohexane is the more stable conformation. When the ring flip occurs, however, it converts to axial methylcyclohexane. These two conformations are in rapid equilibrium at room temperature, but can be frozen out as distinct compounds at -78degrees. The equatorial conofrmation is favored in the equilibrium by a modest amount because the axial isomer has about 1.8 kcal/mol of steric strain. This strain arises from the interaction of one of the hydrogens of the axial methyl group with each of the two other axial hydrogens on the same side of the ring, as illustrated above.
9 of 17
20/09/2011 9:41 CH
Cyclohexane Conformational Analysis
http://research.cm.utexas.edu/nbauld/teach/cyclohex.html
Each of these steric interacgions is approximately equivalent to one gauche butane interaction of 0.9 kcal/mole, so the total is 1.8 kcal/mol. The point of the gauche butane comparison is that the H/H distance of the sterically hindered hydrogens is almost exactly the same in gauche butane as with axial methyl cyclohexane, except that there are two such H/H interactions in the latter case. The steric interactions in axial methylcyclohexane are referred to as "1,3-diaxial interactions", because the interactions involve two axial atoms or groups (one H and one CH3 and the carbons bearing these atoms or groups are 1,3 related. THE "EQUATORIALITY PRINCIPLE" PROVIDES THAT ANY SUBSTITUENT PREFERS TO OCCUPY THE LESS STERICALLY HINDERED EQUATORIAL POSITION, IF AT ALL POSSIBLE.IN THE CASE OF DI- OR POLY-SUBSTITUTED CYCLOHEXANES NOT ALL SUBSTITUENTS CAN OCCUPY ALL EQUATORIAL POSITIONS IN EVERY ISOMER, BUT THAT ISOMER WILL BE THE MOST STABLE IN WHICH ALL OF THE
10 of 17
20/09/2011 9:41 CH
Cyclohexane Conformational Analysis
http://research.cm.utexas.edu/nbauld/teach/cyclohex.html
SUBSTITUENTS OCCUPY EQUATORIAL POSITIONS(see examples below). Disubstituted Cyclohexanes 1,4-Dimethylcyclohexane
11 of 17
20/09/2011 9:41 CH
Cyclohexane Conformational Analysis
http://research.cm.utexas.edu/nbauld/teach/cyclohex.html
Note that with the 1,4-disubstitution pattern, the diequatorial (mosts stable) arrangement is what we call the "trans" isomer. in it, one substituent is "slant up" and one "slant down". Ring flipping gives a conformation in which the methyl substituents are anti (dihedral angle of
12 of 17
20/09/2011 9:41 CH
Cyclohexane Conformational Analysis
http://research.cm.utexas.edu/nbauld/teach/cyclohex.html
180) to each other, but this is still trans, and thisconformation is less stable because it has two axial substituents. There is another isomer of 1,4-dimethylcyclohexane, the cis isomer. Recall that cis and trans isomers are diastereoisomers, they are not different conformations of the same isomer and cannot be readily interconverted by a simple rotational process (a bond would have to be broken).In the cis isomer, here, one methyl group is equatorial and one axial, and ring flipping simply gives another equivalent conformation. Since one methyl is axial, this costs 1.8 kcal of steric strain. Consequently, the cis isomer is less thermodynamically stable than the trans, which has no steric strain in the more stable conformation. The energy difference is again, 1.8 kcal/mol. In the cis isomer, one substituent is vertically up (or down) and the other slant up (or down). So they both point, in a general sense, in the same direction, i.e., both up or both down. 1,3-Dimethylcyclohexane
13 of 17
20/09/2011 9:41 CH
Cyclohexane Conformational Analysis
http://research.cm.utexas.edu/nbauld/teach/cyclohex.html
In the 1,3-disubstitution pattern (whether it is dimethyl or any other 1,3-dibsubstitution), both groups can only be equatorial when they are both cis. So the cis isomer is the more stable isomer in this case. Ring flipping gives a conformation of the cis isomer which has both methyls axial. Worse than that, they are both on the same side of the ring, so it is not an axial methyl/axial hydrogen interaction any longer, it is an axial methy/axial methyl interaction, which is sterically much worse. Consequently
14 of 17
20/09/2011 9:41 CH
Cyclohexane Conformational Analysis
http://research.cm.utexas.edu/nbauld/teach/cyclohex.html
this ring flip is too energetically difficult, and this conformer can be neglected in he conformational analysis. The trans-1,3-dimethylcyclohexane isomer , on the other hand, has one methyl axial in both ring-flip conformers, so that it is less stable than the cis isomer by 1.8 kcal/mol. Both trans-1,4-dimethylcyclohexane and cis-1,3-dimethylcyclohexane have essentially the same energy, since neither one of them has any strain at all.
15 of 17
20/09/2011 9:41 CH
Cyclohexane Conformational Analysis
http://research.cm.utexas.edu/nbauld/teach/cyclohex.html
The 1,2-disubstitution pattern is very much like the 1,4 pattern, in that the two groups can only be equatorial if they are trans, so the trans isomer is more stable than the cis. The diaxial trans conformer has 3.6 kcal of steric strain, of course and is much less favored than the diequatorial conformer. The cis isomer is less stable than the trans because in it, one methyl must be axial. The cis isomer is therefore less stable than the trans by
16 of 17
20/09/2011 9:41 CH
Cyclohexane Conformational Analysis
http://research.cm.utexas.edu/nbauld/teach/cyclohex.html
1.8 kcal. The key difference between the 1,2 and 1,4 patterns, is that in the diequatorial 1,2 conformation, the methyl groups are gauche as in gauche butane (remember that gauche essentially implies a 60 degree dihedral angle ). Consequently, this diequatorial conformation no longer is strain free, as was the 1,4-trans diequatorial conformation, where the methyls are very far apart. The 1,2-diequatorial isomer is 0.9 kcal/mol less stable than the 1,4-diequatorial isomer, because of this gauche butane-like interaction (recall that the gauche isomer in butane is destabilized by exactly this amount). BACK TO THE TOP OF THE PAGE ON TO THE NEXT PAGE BACK TO THE BAULD HOME PAGE
17 of 17
20/09/2011 9:41 CH
You might also like
- Barrier To Ring Inversion, Pyramidal Inversion, and 1,3-Diaxial Interaction EditedDocument12 pagesBarrier To Ring Inversion, Pyramidal Inversion, and 1,3-Diaxial Interaction EditedAnuja JoyNo ratings yet
- Functional Group Transformation NotebookDocument78 pagesFunctional Group Transformation NotebookchedhedNo ratings yet
- Isomerism DPP PDFDocument69 pagesIsomerism DPP PDFfootball 1063133% (3)
- PMR Spectroscopy: Solved Problems Volume : IIFrom EverandPMR Spectroscopy: Solved Problems Volume : IIRating: 5 out of 5 stars5/5 (3)
- Organic Chemistry - Morrison and BoydDocument390 pagesOrganic Chemistry - Morrison and Boydmadhavdhruv82% (22)
- Science Bowl Organic Chemistry NotesDocument44 pagesScience Bowl Organic Chemistry Notestaosat11No ratings yet
- Chapter 4 With Video LinksDocument37 pagesChapter 4 With Video LinksDoom RefugeNo ratings yet
- Conformational Analysis of CyclopentaneDocument11 pagesConformational Analysis of CyclopentaneNimra MalikNo ratings yet
- Ch221 Class 8 Chapter 4: Stereochemistry of Alkanes and Cycloalkanes, ContinuedDocument11 pagesCh221 Class 8 Chapter 4: Stereochemistry of Alkanes and Cycloalkanes, ContinuedSanjay Mani TripathiNo ratings yet
- GeneralChem LS 25 PDFDocument25 pagesGeneralChem LS 25 PDFSunil NahataNo ratings yet
- Notes Lecture 1 Conformational AnalysisDocument18 pagesNotes Lecture 1 Conformational AnalysisDianing Wismarani Putri100% (1)
- Conformational Analysis: Carey & Sundberg: Part A Chapter 3Document53 pagesConformational Analysis: Carey & Sundberg: Part A Chapter 3Dr-Dinesh Kumar100% (1)
- Butane - Rotate With C3-C4 Bond.: C H C H C H CH H H H HDocument11 pagesButane - Rotate With C3-C4 Bond.: C H C H C H CH H H H HMadison FullerNo ratings yet
- Symmetry NotesDocument8 pagesSymmetry NoteslillyammalNo ratings yet
- Vollhardt 6e Lecture PowerPoints - Chapter 11Document58 pagesVollhardt 6e Lecture PowerPoints - Chapter 11superfr3shmNo ratings yet
- Symmetry Ops and Elements and PT Gps 2Document35 pagesSymmetry Ops and Elements and PT Gps 2himel8189No ratings yet
- Module8 PDFDocument40 pagesModule8 PDFFaizan AhmadNo ratings yet
- Organic Chemistry 2021Document76 pagesOrganic Chemistry 2021Arah Mae BonillaNo ratings yet
- Isolobal AnalogyDocument4 pagesIsolobal Analogyindu priyaNo ratings yet
- Stereochemistry 22Document21 pagesStereochemistry 22Ahmed SideegNo ratings yet
- Loudon, Organic Chemistry ErrataDocument13 pagesLoudon, Organic Chemistry Errataig12345No ratings yet
- Organometal Chem PDFDocument55 pagesOrganometal Chem PDFSushmita Dey100% (1)
- Point Group PDFDocument46 pagesPoint Group PDFDharamsingh WaskaleNo ratings yet
- OrganometallicsDocument58 pagesOrganometallicsRohit ChaudharyNo ratings yet
- Organic Chemistry Chapter 8Document41 pagesOrganic Chemistry Chapter 8채종희No ratings yet
- 10 5Document15 pages10 5AZIZ ALBAR ROFI'UDDAROJADNo ratings yet
- Curriculum Tracker 2025 (Chemistry) - MathonGoDocument35 pagesCurriculum Tracker 2025 (Chemistry) - MathonGoSangam SadotraNo ratings yet
- Stereochemistry of CarbohydratesDocument11 pagesStereochemistry of CarbohydratesRabia Hussain100% (2)
- Group Theory (Theory) - Inorganic Chemistry Virtual Lab - Chemical SciencesDocument16 pagesGroup Theory (Theory) - Inorganic Chemistry Virtual Lab - Chemical SciencesNicholas ThompsonNo ratings yet
- Chemical Bonding Class11th by PS Sir IIT JEEDocument44 pagesChemical Bonding Class11th by PS Sir IIT JEEMahendra PandaNo ratings yet
- StereochemistryDocument53 pagesStereochemistryDeshDeepak AbhayPratap SinghChauhan100% (1)
- 5 Stereoisomerism MMDocument32 pages5 Stereoisomerism MMShifa GhannamNo ratings yet
- Boger CourseDocument477 pagesBoger CourseharrypoutreurNo ratings yet
- Retrosynthetic Analysis PDFDocument6 pagesRetrosynthetic Analysis PDFNoleNo ratings yet
- Structure and Synthesis of Alcohols: Organic Chemistry, 7Document52 pagesStructure and Synthesis of Alcohols: Organic Chemistry, 7haha_le12100% (1)
- Chemistry Chapter 11 Alcohol, Phenol and EtherDocument32 pagesChemistry Chapter 11 Alcohol, Phenol and EtherVidyakulNo ratings yet
- Isomerism in Coordination Compounds PDFDocument7 pagesIsomerism in Coordination Compounds PDFmf720383270100% (1)
- Organometallics Notes PDFDocument130 pagesOrganometallics Notes PDFBiswa Bhusan NayakNo ratings yet
- Organic Chemistry Practice ProblemsDocument1 pageOrganic Chemistry Practice ProblemsSushant KumarNo ratings yet
- Spin-Spin Coupling in NMRDocument17 pagesSpin-Spin Coupling in NMRBenjamín Marc Ridgway de SassouNo ratings yet
- Iupac Nomenclature OrganicDocument14 pagesIupac Nomenclature Organicaj619624No ratings yet
- Introduction of Organic Chemistry by Eyes of Ajnish Kumar Gupta (AKG)Document24 pagesIntroduction of Organic Chemistry by Eyes of Ajnish Kumar Gupta (AKG)ajju_208180% (5)
- Rotational Spectroscopy Part 1Document17 pagesRotational Spectroscopy Part 1shrivastavashubhang02No ratings yet
- Colligative Properties of SolutionDocument15 pagesColligative Properties of SolutionJoon Bok NamleeNo ratings yet
- Sn2 and Sn1 Reactions: Sethuranga Skanthan.P.TDocument12 pagesSn2 and Sn1 Reactions: Sethuranga Skanthan.P.TSethuNo ratings yet
- Chapter 4 Chemical BondingDocument45 pagesChapter 4 Chemical BondingRavinder singhNo ratings yet
- Thermochemistry: - Petrucci, Herring Madura and BissonnetteDocument49 pagesThermochemistry: - Petrucci, Herring Madura and BissonnetteYousif Khalid100% (1)
- Stereochemistry PDFDocument3 pagesStereochemistry PDFbencleese100% (1)
- Hyperconjugation: - Devyani JoshiDocument21 pagesHyperconjugation: - Devyani JoshiEisha SaleemNo ratings yet
- HybridisationDocument10 pagesHybridisationSuresh KannanNo ratings yet
- ConformationsDocument8 pagesConformationsrameshiitNo ratings yet
- Stereochemistry MSCDocument42 pagesStereochemistry MSCBapu Thorat100% (1)
- N. Tewari, Vol-2, Organic ChemistryDocument920 pagesN. Tewari, Vol-2, Organic ChemistryVijay RaghavaNo ratings yet
- Ionic EquilibriumDocument46 pagesIonic Equilibriumabhinavsaurabh75% (4)
- Inductive EffectDocument38 pagesInductive EffectJoe JNo ratings yet
- Various Tools and Techniques Used For Structural ElucidationDocument28 pagesVarious Tools and Techniques Used For Structural ElucidationAvinashNo ratings yet
- Learn: Chapter-10 Haloalkanes & Haloarenes Class-XII Subject-ChemistryDocument32 pagesLearn: Chapter-10 Haloalkanes & Haloarenes Class-XII Subject-Chemistryprajaktac506100% (1)
- Organic Chemistry 32-235 Practice Questions For Exam #2: 2. Consider The SDocument9 pagesOrganic Chemistry 32-235 Practice Questions For Exam #2: 2. Consider The Ssweta KushwahaNo ratings yet
- Course Title: Organic Chemistry-I Course Code: Chm-553, Chm-507 Semester: MSC 1, Bs 5Document18 pagesCourse Title: Organic Chemistry-I Course Code: Chm-553, Chm-507 Semester: MSC 1, Bs 5Mian Naveed AhmedNo ratings yet
- 17 de Thi Hoc Ki 1 Mon Hoa Hoc Lop 10Document379 pages17 de Thi Hoc Ki 1 Mon Hoa Hoc Lop 10cclatrumNo ratings yet
- .Moleculary Symmetry, Projection Diagrams, and Point GroupsDocument23 pages.Moleculary Symmetry, Projection Diagrams, and Point GroupscclatrumNo ratings yet
- Elements Pics+Words 11x8.5Document2 pagesElements Pics+Words 11x8.5Juan Carlos Zuñiga100% (1)
- Transition Metal Chemistry - The Valence Shell in D-Block Chemistry - GerlochDocument219 pagesTransition Metal Chemistry - The Valence Shell in D-Block Chemistry - Gerlochd-fbuser-2994093833% (3)
- Iupac2011 BookDocument567 pagesIupac2011 BookcclatrumNo ratings yet
- Chemistry 3719 Old ExamsDocument300 pagesChemistry 3719 Old ExamsHY-11 Đỗ Quốc TiệpNo ratings yet
- BHU MSC EntranceDocument90 pagesBHU MSC EntranceAbhay VishwakarmaNo ratings yet
- General & Academic Branch-Iv J' Section: OrderDocument59 pagesGeneral & Academic Branch-Iv J' Section: OrderMohammed ZiyadNo ratings yet
- Organic Net PyqDocument537 pagesOrganic Net Pyqpranjal jangid100% (1)
- Chapter 9B General Organic Chemistry IIDocument55 pagesChapter 9B General Organic Chemistry IImrityunjaibharti2502No ratings yet
- Exercise 5 (Introduction To Stereochemistry)Document11 pagesExercise 5 (Introduction To Stereochemistry)Wendell Kim LlanetaNo ratings yet
- Full Ebook of Basic Concepts in Organic Stereochemistry 1St Edition Sunil Kumar Talapatra Online PDF All ChapterDocument69 pagesFull Ebook of Basic Concepts in Organic Stereochemistry 1St Edition Sunil Kumar Talapatra Online PDF All Chapterchristalmasamedda575100% (8)
- Elimination Reactions Mechanism Lecture NotesDocument17 pagesElimination Reactions Mechanism Lecture NotesveluselvamaniNo ratings yet
- CHM133 PRELIM EXAM Part2Document7 pagesCHM133 PRELIM EXAM Part2Rohaisa FaisalNo ratings yet
- H2S Scavenger TheoryDocument144 pagesH2S Scavenger TheoryMuhammad Basuki RakhmadNo ratings yet
- 2014 Exp 1 Data Sheet PDFDocument5 pages2014 Exp 1 Data Sheet PDFMichael QuinnNo ratings yet
- Organic - Reagents FinalDocument27 pagesOrganic - Reagents FinalSankar AdhikariNo ratings yet
- Alkanes, Cycloalkanes & Their Conformations Alkanes, Cycloalkanes & Their ConformationsDocument35 pagesAlkanes, Cycloalkanes & Their Conformations Alkanes, Cycloalkanes & Their Conformationsasdfasdfwedfr2345r34No ratings yet
- Cyclohexane ConformersDocument12 pagesCyclohexane ConformersShahzad KarimNo ratings yet
- Acyclic Conformational AnalysisDocument11 pagesAcyclic Conformational AnalysisRojo JohnNo ratings yet
- 429 SampleDocument40 pages429 Samplejerrica thomasNo ratings yet
- 01 Ch02 Alkanes 31 SlidesDocument31 pages01 Ch02 Alkanes 31 SlidesVo Trung Kien B2100780No ratings yet
- Resonance - : Resonance Structures Things To RememberDocument29 pagesResonance - : Resonance Structures Things To RememberDan Sebastian TilaoNo ratings yet
- TEST BANK For Organic Chemistry 6th Edition by Janice Smith, Verified Chapters 1 - 29, Complete Newest VersionDocument51 pagesTEST BANK For Organic Chemistry 6th Edition by Janice Smith, Verified Chapters 1 - 29, Complete Newest Versionkevinkariuki227No ratings yet
- Chapter 3Document27 pagesChapter 3Siddarth PalletiNo ratings yet
- Chapter Four, Cycloalkanes (Part One - Monocyclic Alkane)Document8 pagesChapter Four, Cycloalkanes (Part One - Monocyclic Alkane)Amin JamjahNo ratings yet
- Classification of CarbohydratesDocument13 pagesClassification of Carbohydratesprajesh_bilvaNo ratings yet
- Chair Conformation: Newman Projection of The Boat ConformationDocument11 pagesChair Conformation: Newman Projection of The Boat ConformationSunil ChoudharyNo ratings yet
- 11.konformasi Alkana Dan SikloalkanaDocument25 pages11.konformasi Alkana Dan SikloalkanasatriaramdhaniNo ratings yet
- Organic Chemistry: Electronic Structure and Bonding Acids and BasesDocument12 pagesOrganic Chemistry: Electronic Structure and Bonding Acids and Basesapi-3708473No ratings yet