Professional Documents
Culture Documents
AQA Physics Topic 4 Atomic Structure Knowledge Organiser
AQA Physics Topic 4 Atomic Structure Knowledge Organiser
Uploaded by
Gabriel HoOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
AQA Physics Topic 4 Atomic Structure Knowledge Organiser
AQA Physics Topic 4 Atomic Structure Knowledge Organiser
Uploaded by
Gabriel HoCopyright:
Available Formats
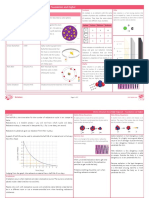
Atomic Structure Knowledge Organiser – Foundation and Higher Separate Science
Developing the Model of the Atom Isotopes Beta
An isotope is an element with the same Beta radiation is a fast moving electron that
Scientist Time Contribution
number of protons but a different number can be stopped by a piece of aluminium. Beta
John Dalton Start of 19th century Atoms were first described as solid spheres. of neutrons. They have the same atomic radiation is emitted by an atom when a neutron
splits into a proton and an electron.
number, but different mass numbers.
JJ Thomson 1897 Thomson suggested the plum 0
e
Isotope Protons Electrons Neutrons
pudding model – the atom is a 1
H 1 1 0
ball of charge with electrons 1 -1
scattered within it. 2
H 1 1 1
1
3
H 1 1 2
1
Ernest Rutherford 1909 Alpha Scattering experiment –
Some isotopes are unstable and, as a result,
Rutherford discovered that
decay and give out radiation. Ionising
the mass is concentrated at radiation is radiation that can knock
the centre and the nucleus electrons off atoms. Just how ionising this
is charged. Most of the mass radiation is, depends on how readily it can
is in the nucleus. Most atoms do that.
are empty space. Alpha Gamma
Niels Bohr Around 1911 Bohr theorised that the Alpha radiation is an alpha particle emitted A gamma wave is a wave of radiation and is
from the nucleus of a radioactive nuclei. It the most penetrating – stopped by thick lead
electrons were in shells
is made from two protons and two neutrons. and concrete.
orbiting the nucleus.
They can’t travel too far in the air and are the
least penetrating – stopped by skin and paper.
However, they are highly ionising because of
their size.
James Chadwick Around 1940 Chadwick discovered neutrons in the nucleus.
Page 1 of 3 visit twinkl.com
Atomic Structure Knowledge Organiser – Foundation and Higher Separate Science
Half-life Alpha Decay Equations Beta Decay Equations
The half-life is the time taken for the number of radioactive nuclei in an isotope to An alpha particle is made of two protons and two A neutron turns into a proton and releases a an
halve. neutrons. The atomic number goes down by two and its electron. The mass of the nucleus does not change
Radioactivity is a random process – you will not know which nuclei will decay. mass number decreases by four. but the number of protons increases.
Radioactive decay is measured in becquerels Bq. 1 Bq is one decay per second.
Radioactive substances give out radiation from their nucleus.
A graph of half-life can be used to calculate the half-life of a material and will always Gamma rays Alpha radiation is more dangerous inside
have this shape: There is no change to the nucleus when a radioactive the body. It is highly ionising and able to
source emits gamma radiation. It is the nucleus getting cause a lot of damage. Outside the body
rid of excess energy. it is less dangerous because it cannot
penetrate the skin.
Beta radiation is less dangerous inside
the body as some of the radiation is able
to escape. Outside the body it is more
Contamination dangerous as it can penetrate the skin.
When unwanted radioactive atoms get onto
an object, it is possible for the radioactive
Gamma radiation is the least dangerous
particles to get inside the body.
inside the body as most will pass out and
it is the least ionising. Gamma is more
Protective clothing should be worn when dangerous outside the body as it can
Judging from the graph, the radioactive material has a half-life of two days. handling radioactive material. penetrate the skin.
Irradiation
Irradiation occurs when materials are near a radioactive source. The source is sometimes
placed inside a lead-lined box to avoid this.
People who work with radioactive sources will sometimes stand behind a lead barrier,
be in a different room or use a remote-controlled arm when handling radioactive
substances.
Page 2 of 3 visit twinkl.com
Atomic Structure Knowledge Organiser – Foundation and Higher Separate Science
Background Radiation Different Half-Lives of Radioactive Isotopes
This comes from natural sources like rocks, food and air. It also comes from man- All radioactive isotopes have different half-lives. Some are very short and others are
made sources such as nuclear weapons, nuclear waste or nuclear accidents. The dose of much longer. The uses of these will depend on the half-life. For example, you would use
radiation people receive varies dependent on how close they are to the source. Too much an isotope with a short half-life as a medical tracer so it is not in the body for too long.
exposure to radiation can cause radiation poisoning. Radiation dosage is measured in
Radiotherapy Fusion
sieverts (Sv).
High doses of radiation can be Nuclear fusion is the joining together of smaller
1000 millisieverts (mSv) = 1 sievert (Sv) used to treat cancer. Gamma radioactive nuclei to make a larger atom. Fusion
rays are focused directly onto occurs in the sun. This whole process releases a lot of
Uses of Nuclear Radiation
the cancer cell, killing the energy, much more than fission. However, a very high
Although radiation can be dangerous, it also has its uses. The risks are always considered
cancer cell but not killing temperature and pressure is needed for fusion to occur,
when using radiation. Gamma sources can be used as a medical tracer in the human
too many healthy cells. The so it is not used in the production of energy yet.
body; isotopes can be injected or swallowed. As the isotope goes around the body, it
damage to the healthy cells
can be monitored and medical issues can be spotted. Gamma radiation is emitted out
that may be close to the cancer
of the body and does not cause the cells to become ionised. The isotope used will have
can cause the patient to feel
a short half-life so it does not stay inside the body for too long. Tracers can be used to
ill. However, killing the cancer
diagnose potentially life-threatening conditions which otherwise would not be spotted.
cell makes it worth it.
The risk of using the radioactive tracer is much less than the risk of the condition they
may diagnose.
Fission
Nuclear fission is the splitting of large radioactive nuclei into smaller ones. A neutron is absorbed by a large unstable radioactive nucleus. Next, the nucleus
splits into smaller nuclei. As this happens, more neutrons and energy are released. The neutrons released go on to cause more reactions. This is
called a chain reaction.
Fission is carried out in a nuclear reactor in order to generate energy. It is controlled by control rods which, when they are lowered down,
slow down the reaction process. When they are raised, the reaction speeds up again. If this process is not controlled, then a nuclear weapon
has been produced.
Page 3 of 3 visit twinkl.com
You might also like
- AQA Physics Topic 4 Atomic Structure Knowledge OrganiserDocument2 pagesAQA Physics Topic 4 Atomic Structure Knowledge Organisergundavannessa27No ratings yet
- Atomic Structure Knowledge Organiser - Foundation and HigherDocument2 pagesAtomic Structure Knowledge Organiser - Foundation and HigheranqelineeNo ratings yet
- 1-2 Atoms (Part 1)Document3 pages1-2 Atoms (Part 1)api-3734333No ratings yet
- Chapter 4Document14 pagesChapter 4salma salmaNo ratings yet
- Atomic Structure Lecture 1234 PDFDocument58 pagesAtomic Structure Lecture 1234 PDFLokendra Shahi100% (1)
- Block 5: Atomic Physics: #Thenuclearatom #RadioactivityDocument70 pagesBlock 5: Atomic Physics: #Thenuclearatom #RadioactivityMac Justine JimenezNo ratings yet
- Atomic Structure - Development and ConclusionsDocument3 pagesAtomic Structure - Development and ConclusionsJack HuynhNo ratings yet
- Chemistry Reading Material Part 1Document21 pagesChemistry Reading Material Part 1RashpreetNo ratings yet
- Chapter 4Document11 pagesChapter 4anil.gelra5140100% (1)
- Psma PrelimsDocument42 pagesPsma PrelimsPrincess Rose GamboaNo ratings yet
- P AXgg 2 Gs FGte Z1 F MKOt 7Document11 pagesP AXgg 2 Gs FGte Z1 F MKOt 7Animesh SirNo ratings yet
- UntitledDocument11 pagesUntitledOjas SinghNo ratings yet
- Iesc 104Document11 pagesIesc 104Ty GravesNo ratings yet
- Chemistry-Ix: Chapter 4: Structure of The AtomDocument6 pagesChemistry-Ix: Chapter 4: Structure of The AtomShreyashkar JhaNo ratings yet
- Physics - The Physics of The AtomDocument8 pagesPhysics - The Physics of The AtomNaomi JohnsonNo ratings yet
- Nuclear Atom N RadioactivityDocument91 pagesNuclear Atom N RadioactivityLinaNo ratings yet
- Gen Chem Second PreDocument15 pagesGen Chem Second PreShayne Herrera IINo ratings yet
- Atomic Structure: Urning Back Time - The Theory of AtomsDocument14 pagesAtomic Structure: Urning Back Time - The Theory of AtomsUmer AzharNo ratings yet
- Iesc104 PDFDocument11 pagesIesc104 PDFPrasun ShrivastavNo ratings yet
- Chemistry Chapter OneDocument16 pagesChemistry Chapter Onedemro channelNo ratings yet
- Bohr S 1913 Cloud 1920 Chadwick1932 Dalton S 1800 Thomson S 1897 Rutherford S 1911Document1 pageBohr S 1913 Cloud 1920 Chadwick1932 Dalton S 1800 Thomson S 1897 Rutherford S 1911Mary RincónNo ratings yet
- Microsoft Word - 5. Atomic StructureDocument14 pagesMicrosoft Word - 5. Atomic Structureiamm.javed31No ratings yet
- GeneralChemistry Lecture 2Document28 pagesGeneralChemistry Lecture 2biyadgendeshewNo ratings yet
- 12 AtomsDocument14 pages12 AtomsRajathNo ratings yet
- Atomic StructureDocument80 pagesAtomic Structureshelley guptaNo ratings yet
- Physics Edexcel Unit 3 - Radioactivity and AstronomyDocument44 pagesPhysics Edexcel Unit 3 - Radioactivity and Astronomyshivensolanki31No ratings yet
- Chapter 4 (Atoms and Atomic Theory)Document47 pagesChapter 4 (Atoms and Atomic Theory)Raynan TabaldoNo ratings yet
- LESSON 2 Atoms, Ions and MoleculesDocument14 pagesLESSON 2 Atoms, Ions and MoleculesscientistgenerosoNo ratings yet
- 2 11 Atomic Structure and Mass SpectrometryDocument6 pages2 11 Atomic Structure and Mass SpectrometryAbdur RehmanNo ratings yet
- عائلة نيتروجينDocument9 pagesعائلة نيتروجينzahrakerim21No ratings yet
- ReviewerDocument2 pagesReviewerMarion PootenNo ratings yet
- Foundation &fundamentals of Chemistry Unit: 3 Atomic StructureDocument34 pagesFoundation &fundamentals of Chemistry Unit: 3 Atomic StructurePiyush KumarNo ratings yet
- Atomic ModelDocument46 pagesAtomic ModelShannelle Anne CaballeroNo ratings yet
- Atomic Struture Mass Spectrometry 2022Document49 pagesAtomic Struture Mass Spectrometry 2022Ciwan SahinNo ratings yet
- Physics RadioactivityDocument25 pagesPhysics Radioactivityazaanhasnat345No ratings yet
- Lesson - Atomic Structure: Scientist DiscoveryDocument4 pagesLesson - Atomic Structure: Scientist DiscoveryKokkilaa ParameswaranNo ratings yet
- Unit FDocument16 pagesUnit FVenkateswara Rao DoodalaNo ratings yet
- 4 CO1 Waonl MQK Xy Shye HVDocument20 pages4 CO1 Waonl MQK Xy Shye HVRoshni BhaliyaNo ratings yet
- ChemistryDocument78 pagesChemistryPromise Charles Sda KateteNo ratings yet
- General Chemistry 1: Module 3, Lesson 1: Atomic TheoryDocument4 pagesGeneral Chemistry 1: Module 3, Lesson 1: Atomic TheoryKeano GelmoNo ratings yet
- General Chemistry 2 1Document76 pagesGeneral Chemistry 2 1daudimgetamafweleNo ratings yet
- AQA Science: Physics Unit 4 Revision Notes - Radioactivity: AtomsDocument1 pageAQA Science: Physics Unit 4 Revision Notes - Radioactivity: AtomsAsa BizenjoNo ratings yet
- Chapter 3. Nuclear Chemistry Part ONEDocument17 pagesChapter 3. Nuclear Chemistry Part ONEChengNo ratings yet
- 2.2 The Atomic StructureDocument20 pages2.2 The Atomic StructureNurina SafiNo ratings yet
- Atomic StructureDocument34 pagesAtomic StructureArefin MahinNo ratings yet
- Structure of An Atom NotesDocument7 pagesStructure of An Atom NotesRajesh Kumar GuptaNo ratings yet
- Law of Conservation of Mass: Atomic TheoryDocument4 pagesLaw of Conservation of Mass: Atomic TheoryniloNo ratings yet
- Structure of An Atom (Form 4 Chemistry)Document7 pagesStructure of An Atom (Form 4 Chemistry)Adam NaqibNo ratings yet
- Self-Instructional Material: Atoms: Are You In?Document17 pagesSelf-Instructional Material: Atoms: Are You In?Richard ViseyNo ratings yet
- Atoms. Byju'sDocument75 pagesAtoms. Byju'sGaurav BhandariNo ratings yet
- Structure of An Atom and The Periodic TableDocument2 pagesStructure of An Atom and The Periodic TableErika Dela CruzNo ratings yet
- Atoms and Subatomic ParticlesDocument28 pagesAtoms and Subatomic ParticlesCooleenNo ratings yet
- Leaving Cert Chemistry NotesDocument116 pagesLeaving Cert Chemistry NotesSnivySerpentNo ratings yet
- "The Development of Atomic Theory": "Nothing Exists Except Atoms and Empty Space: Everything Else Is Opinion."Document2 pages"The Development of Atomic Theory": "Nothing Exists Except Atoms and Empty Space: Everything Else Is Opinion."Caryl SantosNo ratings yet
- Aakash Structure of AtomsDocument28 pagesAakash Structure of AtomsArsh TyagiNo ratings yet
- Sci9 Q2 Lesson 1 Atomic Model, Sub Atomic ParticlesDocument29 pagesSci9 Q2 Lesson 1 Atomic Model, Sub Atomic ParticlesFlorenze GonzalesNo ratings yet
- The Atom PPTDocument93 pagesThe Atom PPTCarlo AbellanaNo ratings yet
- Cell Biology Practice Exam QuestionsDocument20 pagesCell Biology Practice Exam QuestionsGabriel HoNo ratings yet
- Section Check in Mechanics Newton S Laws of MotionDocument7 pagesSection Check in Mechanics Newton S Laws of MotionGabriel HoNo ratings yet
- AQA Physics Topic 3 Particle Model of Matter Knowledge OrganiserDocument3 pagesAQA Physics Topic 3 Particle Model of Matter Knowledge OrganiserGabriel HoNo ratings yet
- AQA Physics Unit 3 Particle Model of Matter Student Progress SheetDocument3 pagesAQA Physics Unit 3 Particle Model of Matter Student Progress SheetGabriel HoNo ratings yet
- AQA Physics Unit 6 Waves Student Progress SheetDocument7 pagesAQA Physics Unit 6 Waves Student Progress SheetGabriel HoNo ratings yet
- AQA Physics Unit 1 Energy Student Progress SheetDocument3 pagesAQA Physics Unit 1 Energy Student Progress SheetGabriel HoNo ratings yet
- Islam Quotes For GcseDocument4 pagesIslam Quotes For GcseGabriel HoNo ratings yet
- L22 - Revision Lesson On HazardsDocument24 pagesL22 - Revision Lesson On HazardsGabriel HoNo ratings yet
- 458-Radhika Polypack - Amd - xlsx1.Document2 pages458-Radhika Polypack - Amd - xlsx1.MUNINo ratings yet
- History of UniverseDocument1 pageHistory of UniversemajdaNo ratings yet
- Jargeous - Product - Catalog Ver 1220 Compressed PDFDocument16 pagesJargeous - Product - Catalog Ver 1220 Compressed PDFFirdaus YahyaNo ratings yet
- Scribd ResumeDocument2 pagesScribd ResumeaaronschrankNo ratings yet
- IGBT MitsubishiDocument4 pagesIGBT Mitsubishimadhuvariar100% (3)
- DM No. 149 S. 2023 Addendum To Division Memorandum No. 412 S. 2022Document14 pagesDM No. 149 S. 2023 Addendum To Division Memorandum No. 412 S. 2022Jessa GeronNo ratings yet
- Business Case Part 1 - Phase 2 - Group 212053 - 17 FDocument14 pagesBusiness Case Part 1 - Phase 2 - Group 212053 - 17 Fkaren stefania hortua curzNo ratings yet
- Department of Labor: PassaicDocument2 pagesDepartment of Labor: PassaicUSA_DepartmentOfLaborNo ratings yet
- IMIL KYC + Sales TNCDocument5 pagesIMIL KYC + Sales TNCbanavaram1No ratings yet
- 20 - Feb - 2019 - 170439480IDKL5X0QAnnexPFRDocument44 pages20 - Feb - 2019 - 170439480IDKL5X0QAnnexPFRGavoutha BisnisNo ratings yet
- Evaluate Vendor LTSADocument2 pagesEvaluate Vendor LTSAApri Kartiwan100% (1)
- Advanced Digital Controls Improve PFC PerformanceDocument18 pagesAdvanced Digital Controls Improve PFC Performancediablo diablolordNo ratings yet
- Installation and Maintenance Manual For The GP Poro-Stone Gas FilterDocument4 pagesInstallation and Maintenance Manual For The GP Poro-Stone Gas FilterMahmoud El-abdNo ratings yet
- Organised By: Dr. Poonam S. TiwariDocument2 pagesOrganised By: Dr. Poonam S. Tiwarisayed kundumon100% (1)
- GE - Nine CellDocument12 pagesGE - Nine CellNikita SangalNo ratings yet
- CH 01 Wooldridge 5e PPTDocument23 pagesCH 01 Wooldridge 5e PPTKrithiga Soundrajan100% (1)
- Enforcement of IPRs at Border Book No.03Document32 pagesEnforcement of IPRs at Border Book No.03dhriti tutejaNo ratings yet
- PR 2001219Document10 pagesPR 2001219Amr Mohamed GalalNo ratings yet
- Pharmanex®: Food Supplement With Green Tea ExtractDocument2 pagesPharmanex®: Food Supplement With Green Tea ExtractMiriam Hui KungNo ratings yet
- Ielts Reading Test 1Document7 pagesIelts Reading Test 1Bách XuânNo ratings yet
- The Toyota Way 1Document32 pagesThe Toyota Way 1kumamech100% (4)
- RecursionDocument9 pagesRecursionMada BaskoroNo ratings yet
- SpeechDocument5 pagesSpeechNiza Pinky IchiyuNo ratings yet
- Green Light HPS Laser Operator ManualDocument81 pagesGreen Light HPS Laser Operator ManualPhillip V Mitchell0% (1)
- Kinematics Free Fall SolsDocument5 pagesKinematics Free Fall SolsDean JezerNo ratings yet
- William Stallings Computer Organization and Architecture 9 EditionDocument28 pagesWilliam Stallings Computer Organization and Architecture 9 EditionAnggi Riza Amirullah SidhartaNo ratings yet
- Saarc - 25 Years of Regional Integration in South Asia: Tomislav DelinićDocument15 pagesSaarc - 25 Years of Regional Integration in South Asia: Tomislav DelinićGagandeep KaurNo ratings yet
- Module 17 TAI - TC.MTO Basic Knowledge Examination Summary DataDocument27 pagesModule 17 TAI - TC.MTO Basic Knowledge Examination Summary DataChatchai PrasertsukNo ratings yet
- NCMEC Sextortion Fact SheetDocument3 pagesNCMEC Sextortion Fact SheetJacob Rodriguez100% (1)
- Pink Floyd - LyricsDocument97 pagesPink Floyd - LyricsИван ВащенкоNo ratings yet