Professional Documents
Culture Documents
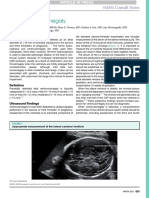
Cranial Ultrasound Scan - WoS
Cranial Ultrasound Scan - WoS
Uploaded by
drchiOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Cranial Ultrasound Scan - WoS
Cranial Ultrasound Scan - WoS
Uploaded by
drchiCopyright:
Available Formats
MCN for Neonatology
West of Scotland
Neonatal Guideline
Cranial Ultrasound:
A Guideline for the performance of routine cranial USS for preterm infants
This Guideline is applicable to medical staff and ANNPs caring for neonates in the West of
Scotland. All staff performing cranial ultrasound scans on neonates must first ensure that they
have received training in the correct use of the ultrasound scanner and the appropriate images
required, as outlined in this document. Except in an emergency, parents should be informed in
advance that head scans will be performed on their baby, and the reasons for this. This
information may be given verbally or in a printed form. The results of the scans should be
communicated in a timely manner by a senior member of staff able to interpret the images
and understand their prognostic value.
Introduction
This document will primarily cover standard cranial ultrasound techniques for recording peri-
ventricular haemorrhage (PVH) and peri-ventricular leucomalacia (PVL), including the
measurement of the resistive index and ventricular dilatation.
Cranial ultrasonography is the most widely used neuroimaging procedure in the neonatal
period. It plays a central role in the detection and management of some neonatal neurological
disease and can provide prognostic, as well as diagnostic, information to neonatologists. This
guideline provides a standard for clinicians in order to detect abnormalities that will alter
management and/or guide parental counselling.
Indications for Cranial Ultrasound scan
There are a diverse group of infants who will require scanning and ultimately a decision to
perform a scan on any unwell neonate can be taken by the consultant responsible for that
child. Common indications include:
Premature infants
Infants with neurological abnormalities e.g. seizures
Infants with antenatally detected abnormalities eg ventriculomegaly
Infants with hypoxic ischaemic encephalopathy
Infants with other congenital abnormalities
Infants with congenital or acquired CNS infection
Infants with a diagnosis of coagulopathy or thrombocytopenia
Page 1 of 12 WoS_CranialUltrasound_Neonates 07/05/2018
What pictures should be taken, stored & printed?
For auditing purposes it is necessary to store an adequate series of images for later review.
This is of particular importance for the benchmarking scans, for the diagnosis of the maximum
grade of PVH and the presence or absence of cystic PVL.
Coronal plane (6+ images)
• Anterior to the frontal horns of the lateral ventricles
• At the anterior horns of the lateral ventricles and
Sylvian fissures
• At the third ventricle and thalami
• At the posterior horns of the lateral ventricles (with
choroids)
• Posterior to the choroids (posterior brain substance)
• A measurement of the ventriculo-cranial ratio (VCR)
should be taken at the level of the foramina of
Munro, connecting the third and lateral ventricles,
when there is lateral ventricular dilatation
Sagittal plane (5+ images)
• Midline through 3rd ventricle, septum cavum
pellucidum, cerebellum with 4th ventricle and
foramen magnum
• Through each lateral ventricle showing the anterior
and posterior horns, with the caudothalamic notch
imaged if possible (germinal matrix area)
• Through each hemisphere lateral to the ventricle for
deep white matter
• When there is evidence of Hypoxic Ischaemic
Encephalopathy (HIE) or ventricular dilatation, the
resistance index should be measured using the mid-
sagittal plane. The anterior cerebral artery is
identified with colour Doppler at its vertical course,
immediately anterior to the genu of the corpus
callosum
See sample images below
Page 2 of 12 WoS_CranialUltrasound_Neonates 07/05/2018
Page 3 of 12 WoS_CranialUltrasound_Neonates 07/05/2018
Timing of routine scans for premature infants:
The following routine scans should be performed for all infants less than 32 weeks gestation:
Day 1
Day 3 For detection of periventricular haemorrhage
Day 7
Day 28 For detection of periventricular leucomalacia
Additional scans should be performed as clinically indicated.
For detection of Periventricular Haemorrhage (PVH)
• Routine Scans performed by neonatal staff - Day 1, Day 3, Day 7. The D1 scan should
be performed as soon as possible after birth to detect any lesions that may be
antenatal in origin. However this should be deferred if there is any concern that a
CrUSS at that point could destabilise the patient.
• Benchmarking Scan – To define maximum grade of PVH for Badger.
Where radiology input is available this should be booked for the 1st radiology visit
between Day 7 – 14. Otherwise the Day 7 routine scan should be utilised
• Clinical staff responsible for the baby should ensure that all of the above scans are
entered into Badger, using the categorisation in the badger database.
Monitoring of babies at risk of Post-haemorrhagic Ventricular dilatation (PHVD)
All babies who are diagnosed with PVH and any infant whose routine measurements of OFC
increase across centile lines should have further scans to monitor for evidence of PHVD
• Neonatal Staff – Additional USS scans are required in the 2nd-3rd week of life following
a diagnosis of PVH. The frequency of these scans will be determined based on the
severity of PVH and any indication of the development of PVHD.
A single scan should be considered in any infant whose OFC crosses centile lines on
routine monitoring
• Radiology – Where possible, a formal radiology scan should ideally be performed in any
infant in whom a ventricular drainage procedure is being considered. This may require
the transfer of the infant to a tertiary level unit.
Exceptions are necessary where there is acute symptomatology outside normal
radiology sessions.
For detection of Periventricular Leucomalacia (PVL)
• Routine scan performed by Neonatal staff – Day 28 or shortly thereafter.
• Benchmarking Scan – To define the presence or absence of cystic PVL for Badger
Where radiology input is available this should be ordered on day 28 to be performed
between 28 days and discharge. Otherwise the Day 28 scan should be utilised
Who should perform the scans?
All scans must be performed, or supervised, by a clinician with sufficient experience to ensure
the quality of the images produced and their interpretation. This would usually be a career
grade paediatrician with an interest in neonatology, or, a senior neonatal trainee. Less
experienced trainees must be supervised until this standard is achieved. Neonatal trainees
should be encouraged to attend a formal training course.
Level 3 units should consider seeking formal radiology input, for the benchmarking scans,
where this is not currently available.
Page 4 of 12 WoS_CranialUltrasound_Neonates 07/05/2018
Grading Periventricular Haemorrhage
PVH may be asymptomatic and without long term consequence. There is a strong association
between extensive PVH and early neonatal mortality, neurodevelopmental disability and post
haemorrhagic hydrocephalus. Most observers agree that the more severe grades of
haemorrhage are associated with a higher incidence of neuro-developmental handicap.
Appendix 1 provides estimates for the incidence of neurodevelopmental handicap with different
severities of PVH
A full description of the findings should be entered in the notes and, for the purposes of data
collection and benchmarking, PVH should be graded using the same system used by the
Vermont Oxford Network:
Grade 0: No subependymal or intraventricular haemorrhage
Grade 1: Subependymal germinal matrix haemorrhage only
Grade 2: Intraventricular blood, no ventricular dilation
Grade 3: Intraventricular blood, ventricular dilation
Grade 4: Intraparenchymal haemorrhage
NB – for Grade 3 PVH, the dilatation of the ventricle refers to dilatation due to the
volume of intraventricular blood. Dilatation of the ventricle due to CSF (Post
Haemorrhagic Ventricular Dilatation - PVHD) should be recorded as an additional
finding.
Grading Periventricular leucomalacia
Mild degrees of PVL may result in transient periventricular echodensities or mild enlargement
of the lateral ventricles. These changes are not associated with a significant increase in
neurodevelopmental handicap. It is therefore important that serial cranial ultrasound
scans are performed within the above timeframe to ensure that any echodensities
noted are assessed for either improvement or development of cystic PVL. With cystic
PVL, the risks of neurodevelopmental handicap are significantly increased as outlined in
Appendix 1. The risk of handicap also increases with more extensive and bilateral changes.
For the purposes of data collection and benchmarking PVL should be diagnosed only in the
presence of cystic PVL.
NB – It is common to find benign periventricular cysts on routine cranial scans. It is important
not to mistake these for cystic PVL or porencephaly. The diagram below indicates the typical
locations of of each type of periventricular cyst
If cysts are seen around the lateral ventricles, it is
important to determine their position in regard to
the upper part of the lateral ventricle .
1+2 = Germinolytic cysts and Pseudocysts are below
or at the level of the upper part of the lateral ventricle.
3 = Cystic periventricular leukomalacia is mostly above
this level
4 = Cysts as a result of a venous infarct
(porencephaly) are large and can be either above, at
or below this level
Page 5 of 12 WoS_CranialUltrasound_Neonates 07/05/2018
Assessment of Ventricular dilatation
In addition to routine Cranial USS imaging, as described above, all premature infants should
have measurements of their OFC performed weekly to identify cases of Post-haemorrhagic
Ventricular Dilatation (PVHD). All babies with PHVD need regular scans to monitor the
progression of the ventricular dilatation and to assess the possible requirement for ventricular
drainage. A number of measures have been described to monitor ventricular dilatation the
most widely used being the ventriculo-cranial ratio (VCR) and the ventricular index (VI). There
is no clear advantage to either measure but it is important to use the same measurement in an
individual patient to monitor change over time.
Ventriculo-cranial ratio (VCR)
Measurement of the ventricular system needs to be performed on a symmetrical, easily
reproducible view. The ventriculo-cranial ratio (VCR) is the ratio of distance between the
lateral sides of the ventricles and the biparietal diameter. This is usually expressed as a
percentage with a normal value of around 33-36% in a preterm infant. This value is of most
use in monitoring the degree of change between successive measurements. An increasing
VCR should trigger frequent reassessments with measurements of the cerebral resistive index
(see below).
Ventricular index (VI)
The Ventricular index, as described by Levine, is the absolute distance between the falx and
the lateral wall of the anterior horn in the coronal plane at the level of the third ventricle.
Values more than 4mm above the 97th centile for gestational age are indicative of significant
ventricular dilatation (see chart). A study by Brouwer et al compared early CSF drainage, at a
ventricular index of 4 mm above the 97th centile, to drainage beyond this threshold, and
showed that early treatment was associated with better development quotient at 2 years and a
decreased likelihood of shunt dependence; there was, however, no reduction in the rate of
cerebral palsy with early drainage.
Page 6 of 12 WoS_CranialUltrasound_Neonates 07/05/2018
Resistive Index (RI)
In 1976, Pourcelot introduced the concept of RI, which is calculated by the following formula:
RI = (S-D)/S where S and D stand for systolic and diastolic velocities measured in the cerebral
arteries. The infant’s blood pressure and carbon dioxide tension need to be taken into account
when measuring the RI as these can affect cerebral blood flow. Variations in the RI
demonstrate that cerebral blood flow is not well regulated and are often associated with
adverse outcomes. It is important to interpret the RI with caution as cerebral blood flow is
always changing due to haemodynamic alterations and there is also considerable inter and
intra observer variation.
Interpretation
High RI
A high RI (>0.85) corresponds to low blood flow velocity where vascular resistance is high (eg
hydrocephalus). Infants with values higher than this may require ventricular drainage to
reduce intracranial pressure.
NB. A high RI must be interpreted with caution in an infant with a PDA as these infants may
have a low diastolic velocity due to ductal steal. This will give a high RI value even in infants
with normal intracranial pressure.
Low RI
A low Resistive Index corresponds to high blood flow velocity where vascular resistance is low
(eg HIE). In normothermic infants, an RI of <0.55 has a positive predictive value for poor
neurological outcome of 84% (95% CI 73%, 91%). However in cooled infants the positive
predictive value is lower - 60% (95% CI 45%, 74%). (Elstad M et. al,)
Page 7 of 12 WoS_CranialUltrasound_Neonates 07/05/2018
Background
Abnormal findings in a preterm cranial ultrasound include:
1. Periventricular haemorrhage PVH (sometimes known as intraventricular or germinal
matrix haemorrhage)
2. Periventricular leucomalacia (PVL)
3. Ventricular dilatation
4. Hydrocephalus
Periventricular haemorrhage
This is most common in the premature population and usually occurs within the first week of
life. The incidence of PVH decreases with increasing gestational age, and is rare beyond 34
weeks gestation because of involution of the vascular germinal matrix. The incidence of PVH in
infants with a birthweight <1500 grams is decreasing and is quoted in the literature as
anywhere between 15% and 30%. Many infants are asymptomatic and these haemorrhages
are found on surveillance sonography.
Aetiology / Risk factors for PVH
General pathogenetic factors may include antepartum, intrapartum and neonatal conditions:
Antepartum Intrapartum Neonatal
Prematurity Low umbilical artery pH Hyaline membrane disease
Lack of antenatal Delivery outside a tertiary unit Patent ductus arteriosus
corticosteroids Delivery mode (C-section Pneumothorax
Maternal pre-eclampsia protective)
Antepartum haemorrhage Low 1 minute Apgar
Chorioamnionitis Bruising at delivery
Pathogenesis
Crucial to the understanding of the pathogenesis of intraventricular haemorrhage is the
concept of impaired autoregulation of cerebral blood flow in the distressed fetus and newborn.
Most PVH is secondary to hypoxic ischaemic reperfusion injury of the germinal matrix. In
premature infants the germinal matrix is richly perfused with fragile vessels which are
particularly vulnerable to insult. Cerebrovascular autoregulation may be absent in sick preterm
infants and a patent ductus arteriosus may also ‘steal’ blood from the cerebral circulation.
Iatrogenic disturbances in intravascular volume and intrinsic disturbances in coagulation are
also associated with an increase in minor grades of PVH. A large periventricular haemorrhage
can result in a venous haemorrhagic infarct secondary to the obstruction of drainage.
Interventions
Only a limited number of interventions have been demonstrated to alter the risk of PVH:
Maternal transfer to a tertiary neonatal centre; Antenatal corticosteroids; and the use of
antimicrobial therapy in the expectant management of preterm rupture of membranes.
Page 8 of 12 WoS_CranialUltrasound_Neonates 07/05/2018
When should MRI / CT be considered?
Current guidance suggests that preterm infants with sequential cranial ultrasound that do not
show parenchymal haemorrhage, grade 3 or 4 intraventricular haemorrhage, cystic PVL or post
haemorrhagic ventricular dilatation are unlikely to suffer from cerebral palsy. Therefore it is
unlikely that conventional MRI will provide any significant additional diagnostic
or prognostic information.
MRI should be considered however, if there is evidence of overt parenchymal injury on
cranial ultrasound. An MRI may reveal abnormalities in the white matter, cerebellum and
posterior limb of internal capsule that were previously unrecognised on cranial ultrasound scan
and may be of prognostic significance.
MRI should be considered in babies with the following parenchymal abnormalities:-
• Cystic Periventricular Leucomalacia (PVL)
• Haemorrhagic parenchymal infarction (Grade 4 PVH)
• Moderate to severe post-haemorrhagic ventricular dilatation
• Echo densities persisting for more than 3-4weeks.
MRI may also be useful for preterm infants with unexplained abnormal neurological signs
because of its increased sensitivity for detecting acquired lesions and CNS malformations.
The optimal timing for MR imaging of the preterm infant is 38-42 weeks corrected gestation as
this allows for assessment of brain maturation and myelination in the posterior limb of the
internal capsule. In exceptional circumstances an earlier MRI may be beneficial if the
responsible consultant neonatologist considers it necessary to make an early diagnosis of
neurological disease and appropriate facilities for imaging the preterm infant are
available.
The final decision regarding any preterm infant undergoing an MRI scan should be made by the
patient’s consultant. A discussion with the patient’s parents is important prior to the scan to
explain the reasons for arranging an MRI and to also ensure that the parents understand that
further follow up post MRI is required to fully assess their child’s neurodevelopment.
A recent audit performed in a tertiary neonatal unit within NHS GGC has shown that this would
equate to approximately 9 extra MRI’s per year if the above criteria are used as guidance for
performing an MRI.
Therefore an MRI should be ideally booked on or shortly after the discharge date of the baby to
avoid the need for the neonatal transport service to become involved in the care of the patient.
In addition this is the optimum time for the baby to undergo the MRI by the ‘feed and sleep’
method rather than undergoing a general anaesthetic. MRI under general anaesthetic should
only be undertaken for diagnostic purposes or where interventions may be required, such as a
possible VP shunt insertion for post haemorrhagic ventricular dilatation.
Page 9 of 12 WoS_CranialUltrasound_Neonates 07/05/2018
Appendix - Prognosis for USS lesions
From - Phumza Nongena, Ash Ederies, Denis V Azzopardi, A David Edwards. Confidence in the
prediction of neurodevelopmental outcome by cranial ultrasound and MRI in preterm infants
Arch Dis Child Fetal Neonatal Ed 2010;95:F388-F390 doi:10.1136/adc.2009.168997
Table 1
Prediction of abnormal neuromotor function by cranial ultrasound
Cerebral palsy
Pre-test Likelihood ratios Post test probability
Ultrasound test result
probability (95% CI) (95% CI)
Normal scan 9% 0.5 (0.4 to 0.7) 5% (4% to 6%)
Grade 1 or 2 PVH 9% 1 (0.4 to 3) 9% (4% to 22%)
Grade 3 PVH 9% 4 (2 to 8) 26% (13% to 45%)
Grade 4 haemorrhage (any) 9% 11 (4 to 31) 53% (29% to 76%)
Cystic PVL 9% 29 (7 to 116) 74% (42% to 92%)
Ventricular dilatation 9% 3 (2 to 4) 22% (17% to 28%)
Hydrocephalus 9% 4 (1 to 13) 27% (10% to 56%)
Normal scan refers to absence of haemorrhage within the brain parenchyma or ventricles,
cysts or ventricular dilation. The grade of PVH (intraventricular haemorrhage) is given
according to the Papile classification. PVL indicates periventricular Leucomalacia. Ventricular
dilation indicates moderate to severe ventricular dilation not meeting the criterion for
hydrocephalus. Hydrocephalus indicates massive ventricular dilation >4 mm above the 97th
centile. Pre-test probability refers to the prevalence of cerebral palsy based on the Epipage
study.4 The likelihood ratio is the probability that a patient with cerebral palsy has a positive
test (abnormal ultrasound result). Post-test probability is the probability that a patient with a
specific abnormality on cranial ultrasound will have abnormal neuromotor function.
Page 10 of 12 WoS_CranialUltrasound_Neonates 07/05/2018
References
Westra S, Adler I, Batton D et al. Reader variability in the use of diagnostic terms to describe
white matter lesions seen on cranial scans of severely premature infants: the ELGAN study. J
Clin Ultrasound 2010;38(8):409-19
Davis PJ, Cox RM & Brooks J. Training in neonatal cranial ultrasound: a questionnaire survey.
Br J Radiol 2005;78(295):55-6
Rennie JM. Neonatal cerebral ultrasound. Cambridge University Press (1997)
Kuban KCK, Allred EN, O’Shea TM et al. Cranial ultrasound lesions in the NICU predict cerebral
palsy at age 2 years in children born at extremely low gestational age. J Child Neurol
2009;24(1):63-72
Berger R, Bender S, Sefkow S et al. Peri/intraventricular haemorrhage: a cranial ultrasound
study on 5286 neonates. Eur J Obstet Gynecol Reprod Biol. 1997;75(2):191-203
Horsch S, Skiold B, Hallberg B et al. Cranial ultrasound and MRI at term age in extremely
preterm infants. Arch Dis Child Fetal Neonatal Ed 2010;95(5):F310-4
Papile LA, Burstein J, Burstein R et al. Incidence and evolution of subependymal and
intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J
Pediatr 1978;92(4):529-34
Harding D, Kuschel C & Evans N. Should preterm infants born after 29 weeks’ gestation be
screened for intraventricular haemorrhage? J Paediatr Child Health 1998;34(1):57-9
Harris NJ, Palacio D, Ginzel A et al. Are routine cranial ultrasounds necessary in premature
infants greater than 30 weeks gestation? Am J Perinatol 2007;24(1):17-21
Boal DK, Watterberg KL, Miles S et al. Optimal cost-effective timing of cranial ultrasound
screening in low-birth-weight infants. Pediatr Radiol 1995;25:425-28
Veyrac C, Couture A, Saguintaah M et al. Brain ultrasonography in the premature infant.
Pediatr Radiol 2006;36:626-635
Technical Standard – Neonatal Cranial Ultrasound Scans by the British Society for Paediatric
Radiology: http://www.bspr.org.uk/docs/Headfinal2.pdf
Beek E & Groenendaal F. Neonatal Brain US. The Radiology Assistant by The Radiological
Society of the Netherlands: http://www.radiologyassistant.nl/en/440c93be7456f
Pourcelot L. Diagnostic ultrasound for vascular disease. In: Donald I, Levi S, editors. Present
and future in diagnostic ultrasound. Rotterdan: Kooker; 1976 p141.
Dani C, Poggi C, Bertini G et al. Method of delivery and intraventricular haemorrhage in
extremely preterm infants. J Matern Fetal Neonatal Med 2010;23(12):1419-23
Beaino G, Khoshnood B, Kaminski M et al. Predictors of cerebral palsy in very preterm infants:
the EPIPAGE prospective population-based cohort study. Dev Med Child Neurol
2010;52(6):e119-25
Laskin MD, Kingdom J, Toi A. Perinatal and neurodevelopmental outcome with isolated fetal
ventriculomegaly: a systematic review. J Matern Fetal Neonatal Med 2005;18(5):289-98
Bloom S, Bloom D, Dellanebbia C et al. The developmental outcome of children with antenatal
mild isolated ventriculomegaly. Obstet Gynecol 1997;90(1):93-7
Nomura ML, Barini R, De Andrade KC et al. Congenital hydrocephalus: gestational and
neonatal outcomes. Arch Gynecol Obstet 2010;282(6):607-11
Royal Prince Alfred Hospital Newborn Care Guidelines:
http://www.sswahs.nsw.gov.au/rpa/neonatal/
An audit of 2 year developmental outcomes for infants with PVL. Dr Stewart Guthrie
Roberton NRC. Textbook of Neonatology. Churchill Livingstone (1992)
Page 11 of 12 WoS_CranialUltrasound_Neonates 07/05/2018
Crowther CA, Hiller JE, Doyle LW, Haslam RR; Australasian Collaborative Trial of Magnesium
Sulphate (ACTOMg SO4) Collaborative Group. Effect of magnesium sulfate given for
neuroprotection before preterm birth: a randomized controlled trial. JAMA. 2003 Nov
26;290(20):2669-76.
Northern Neonatal Nursing Initiative Trial Group. Randomised trial of prophylactic early fresh
frozen plasma or gelatin or glucose in preterm babies. The Lancet 1996;348:229-232
Benson J, Drayton M, Hayward C et al. Multicentre trial of ethamsylate for prevention of
periventricular haemorrhage in very low birthweight infants. The Lancet 1986;ii:1297-1300
Sinha S, Davies J, Toner N et al. Vitamin E supplementation reduces the frequency of
periventricular haemorrhage in very preterm babies. The Lancet 1987;28:266-471
Fowlie PW. Prophylactic indomethacin: systemic review and meta-analysis. Cochrane Library
Elstad M, Whitelaw A, Thoresen M. Cerebral Resistance Index is less predictive in hypothermic
encephalopathic newborns. Acta Paediatr. 2011 Oct;100(10):1344-9. doi: 10.1111/j.1651-
2227.2011.02327.x. Epub 2011 May 18.
Brouwer A, Groenendaal F, van Haastert IL. . Neurodevelopmental outcome of preterm infants with
severe intraventricular hemorrhage and therapy for post-hemorrhagic ventricular dilatation. J
Pediatr 2008;152:648–54.
Phumza Nongena, Ash Ederies, Denis V Azzopardi, A David Edwards. Confidence in the
prediction of neurodevelopmental outcome by cranial ultrasound and MRI in preterm infants
Arch Dis Child Fetal Neonatal Ed 2010;95:F388-F390 doi:10.1136/adc.2009.168997
British Association of Perinatal Medicine; Fetal and Neonatal Brain Magnetic Resonance
Imaging: Clinical Indications, Acquisitions and Reporting; February 2016; Available URL:
https://www.bapm.org/publications/documents/guidelines/BAPM%20MRI%20standards%20for
%20fetal%20neonatal%20brain%20imaging_FINAL%20SUBMISSION%20080216.pdf
An audit reviewing diagnosis of brain injury on CrUSS – Dr Andrew Brunton
Author
Dr. Martina Rodie – SpR Paediatrics
Dr Andrew Powls – Neonatal Consultant PRM
Update (September 2017)
Dr. Andrew Brunton – ST7 Neonatal GRID Trainee
Other Professionals Consulted
Dr. Anne Marie Heuchan – Neonatal consultant RHSC
Dr. Andrew Watt – Radiology Consultant RHSC
Document Title
WoS_CranialUltrasound_Neonates
Implementation / Review Dates
Implementation 06/11/12 Latest Review 08/05/18 Review Date 01/05/21
Page 12 of 12 WoS_CranialUltrasound_Neonates 07/05/2018
You might also like
- Intracranial Hemorrhage: Peer Review Status: Internally Peer ReviewedDocument4 pagesIntracranial Hemorrhage: Peer Review Status: Internally Peer ReviewedSam Ang ChhengNo ratings yet
- OBS Scanning ProtocolDocument15 pagesOBS Scanning Protocoltafi66100% (1)
- Murphy2002 Posthaemorrhagic Ventricular DilatationDocument6 pagesMurphy2002 Posthaemorrhagic Ventricular DilatationModou NianeNo ratings yet
- FetalEcho Statement FINAL publishedinUOGAugust2008 PDFDocument4 pagesFetalEcho Statement FINAL publishedinUOGAugust2008 PDFLizbeth QuinteroNo ratings yet
- Aplicaciones Diagnosticas Del Ultrasonido en PediatriaDocument19 pagesAplicaciones Diagnosticas Del Ultrasonido en PediatriaFelipe Villarroel RomeroNo ratings yet
- Ivh and PHVDDocument12 pagesIvh and PHVDSebastian Mora LópezNo ratings yet
- AMNIOCENTESISDocument9 pagesAMNIOCENTESISSANCHAYEETA100% (1)
- Placenta Praevia and AccretaDocument7 pagesPlacenta Praevia and AccretaAnonymous xvRlqMf2zNo ratings yet
- C-Obs - 11 Management of Breech Presentation at Term Rewrite March 2013Document9 pagesC-Obs - 11 Management of Breech Presentation at Term Rewrite March 2013Ke XuNo ratings yet
- 2008 High Risk Ob Us GuidelinesDocument15 pages2008 High Risk Ob Us GuidelinesCristina DruzianNo ratings yet
- Obg Procedures FinalDocument60 pagesObg Procedures FinalVeena DalmeidaNo ratings yet
- Canadian Guidelines For Prenatal Diagnosis: Echniques of Renatal IagnosisDocument9 pagesCanadian Guidelines For Prenatal Diagnosis: Echniques of Renatal IagnosisRuxandra CretuNo ratings yet
- Case Study AccretaDocument35 pagesCase Study AccretaM Clarisse ParicoNo ratings yet
- VentrikulomegaliDocument2 pagesVentrikulomegaliAida NurwidyaNo ratings yet
- CNS Part 2 Targeted NeurosonographyDocument11 pagesCNS Part 2 Targeted NeurosonographyLeydi Laura QuirozNo ratings yet
- Cryo RopDocument7 pagesCryo RopJuan Pablo OlveraNo ratings yet
- Surgical Management of Hydrocephalus Secondary To Intraventricular Hemorrhage in The Preterm InfantDocument7 pagesSurgical Management of Hydrocephalus Secondary To Intraventricular Hemorrhage in The Preterm InfantcogajoNo ratings yet
- Fetal VenriculomegalyDocument4 pagesFetal VenriculomegalyServicio DigitalNo ratings yet
- Criteria For Transvaginal Sonographic Diagnosis of Ectopic PregnancyDocument6 pagesCriteria For Transvaginal Sonographic Diagnosis of Ectopic PregnancyDinorah MarcelaNo ratings yet
- CT1Document18 pagesCT1Merlin MaelissaNo ratings yet
- Prenatal DiagnosisDocument5 pagesPrenatal DiagnosisSadaf AfzalNo ratings yet
- Cesarean Scar PregnancyDocument36 pagesCesarean Scar Pregnancyanu manojNo ratings yet
- Tto Crup NatureDocument7 pagesTto Crup NatureCarolina Mora RuedaNo ratings yet
- High Risk PregnancyDocument44 pagesHigh Risk PregnancyKavipriyaNo ratings yet
- O@g Maternal and Fetal Mesures ContentDocument22 pagesO@g Maternal and Fetal Mesures Contentjeya maniNo ratings yet
- Bass An 2006Document8 pagesBass An 2006Arjun .MNo ratings yet
- Hysterosalpingography A Re - Emerging Study With CDocument7 pagesHysterosalpingography A Re - Emerging Study With CTuyul Yelsi 1No ratings yet
- Screening of High-Risk Pregnancy, Newer Modalities of DiagnosisDocument12 pagesScreening of High-Risk Pregnancy, Newer Modalities of DiagnosisSanthosh.S.U100% (10)
- OBST ACOG Practice Bulletin No 101 Ultrasonography in PregnancyDocument11 pagesOBST ACOG Practice Bulletin No 101 Ultrasonography in PregnancyEduardo Agustín Vargas CamposNo ratings yet
- Acta Obstet Gynecol Scand - 2019 - Litwinska - Ventriculo Amniotic Shunting For Severe Fetal VentriculomegalyDocument6 pagesActa Obstet Gynecol Scand - 2019 - Litwinska - Ventriculo Amniotic Shunting For Severe Fetal VentriculomegalyApotik ApotekNo ratings yet
- Pregnancy Trauma NotesDocument8 pagesPregnancy Trauma NotesHisham FahadNo ratings yet
- Universal Cranial Ultrasound Screening in Preterm Infants With Gestational Age 33 36 WeeksDocument5 pagesUniversal Cranial Ultrasound Screening in Preterm Infants With Gestational Age 33 36 WeeksAnonymous Bx3ZJRIJFNo ratings yet
- Culdocentesis: G. Richard Braen and David Lee PierceDocument6 pagesCuldocentesis: G. Richard Braen and David Lee PierceebolynsurivNo ratings yet
- Imaging Techniques in Obstetrics: UltrasoundDocument8 pagesImaging Techniques in Obstetrics: UltrasoundAmmar AlnajjarNo ratings yet
- Hemorragia y EmbrazoDocument6 pagesHemorragia y Embrazoana catalinaNo ratings yet
- Umbilical Cord Prolapse: Green-Top Guideline No. 50Document13 pagesUmbilical Cord Prolapse: Green-Top Guideline No. 50Nurul Aimi Abdul RahmanNo ratings yet
- Kaplan1998 PDFDocument6 pagesKaplan1998 PDFAmsir LimbongNo ratings yet
- Hydrocephalus: Mira Octavionita Michael SalimDocument17 pagesHydrocephalus: Mira Octavionita Michael SalimMichelle SalimNo ratings yet
- Tugas 9Document9 pagesTugas 9Ainun Jariah FahayNo ratings yet
- UltrasoundDocument15 pagesUltrasoundNexgen TechnologyNo ratings yet
- Placenta Praevia, Placenta Accreta and Vasa Praevia Managment and Diagnosis Royal College of ObgDocument26 pagesPlacenta Praevia, Placenta Accreta and Vasa Praevia Managment and Diagnosis Royal College of ObgDiana PanaitNo ratings yet
- Literature Review Placenta PreviaDocument7 pagesLiterature Review Placenta Previaeygyabvkg100% (2)
- LEORAG OBGYN Formulation wk5Document3 pagesLEORAG OBGYN Formulation wk5FreakyRustlee LeoragNo ratings yet
- Detection of Placenta Acreta in The First Trimester by 2d Transvaginal Ultrasound and Colour DopplerDocument14 pagesDetection of Placenta Acreta in The First Trimester by 2d Transvaginal Ultrasound and Colour DopplerInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Current Approach To The Diagnosis and Emergency Department Management of Appendicitis in ChildrenDocument6 pagesCurrent Approach To The Diagnosis and Emergency Department Management of Appendicitis in ChildrenmaithamNo ratings yet
- Placenta Previa InfoDocument5 pagesPlacenta Previa Infoirene joyNo ratings yet
- High Risk Pregnancy Assessment and ManagementDocument48 pagesHigh Risk Pregnancy Assessment and ManagementWilfredo PesanteNo ratings yet
- Valoración PreanestesicaDocument5 pagesValoración PreanestesicaSebastian Hdez BolañosNo ratings yet
- Umibilical Cord ProlapseDocument58 pagesUmibilical Cord Prolapsekhadzx100% (3)
- Fracture of The Clavicle in The Newborn Following Normal Labor and DeliveryDocument6 pagesFracture of The Clavicle in The Newborn Following Normal Labor and DeliveryAlberto OrtizNo ratings yet
- European Journal of Obstetrics & Gynecology and Reproductive BiologyDocument5 pagesEuropean Journal of Obstetrics & Gynecology and Reproductive BiologyGustina Maryanti MooyNo ratings yet
- Giagnosis and Management HipDocument4 pagesGiagnosis and Management HipSzőcs OrsolyaNo ratings yet
- Umbilical Cord ProlapseDocument19 pagesUmbilical Cord ProlapsedenekeNo ratings yet
- Umbilical Cord Prolapse - StatPearls - NCBI BookshelfDocument5 pagesUmbilical Cord Prolapse - StatPearls - NCBI Bookshelfhermalina sabruNo ratings yet
- Antepartum HaemorrhageDocument9 pagesAntepartum Haemorrhageareeb khanNo ratings yet
- Congenital Diaphragmatic Hernia (CDH) Information For Health ProfessionalsDocument5 pagesCongenital Diaphragmatic Hernia (CDH) Information For Health ProfessionalsMangku Liong GuanNo ratings yet
- 10 1097@grf 0b013e3181cd5d36Document8 pages10 1097@grf 0b013e3181cd5d36DylanNo ratings yet
- Fernandez 2021Document24 pagesFernandez 2021Waseem MoukhtarNo ratings yet
- Absolute Obstetric Anesthesia Review: The Complete Study Guide for Certification and RecertificationFrom EverandAbsolute Obstetric Anesthesia Review: The Complete Study Guide for Certification and RecertificationNo ratings yet
- Antt VenepunctureDocument1 pageAntt VenepuncturedrchiNo ratings yet
- Thomas and The BeanstalkDocument29 pagesThomas and The BeanstalkdrchiNo ratings yet
- ANTT Clinical GuidelineDocument22 pagesANTT Clinical GuidelinedrchiNo ratings yet
- Antt Indwelling Urine CatheterDocument1 pageAntt Indwelling Urine CatheterdrchiNo ratings yet
- NICU ABX ChartDocument11 pagesNICU ABX ChartdrchiNo ratings yet
- LogbookDocument25 pagesLogbookdrchiNo ratings yet
- Pediatrics Unitwise Staff ListDocument2 pagesPediatrics Unitwise Staff ListdrchiNo ratings yet
- Nicu Intravenous Drug Compatibility Chart-1 0Document1 pageNicu Intravenous Drug Compatibility Chart-1 0drchiNo ratings yet
- Morphine and Lorazepam Tapering Guidelines in The NICUDocument4 pagesMorphine and Lorazepam Tapering Guidelines in The NICUdrchiNo ratings yet
- Ambiguous Genitalia Guidelines - PAEDIATRIC INNOVATION, EDUCATION & RESEARCH NETWORKDocument10 pagesAmbiguous Genitalia Guidelines - PAEDIATRIC INNOVATION, EDUCATION & RESEARCH NETWORKdrchiNo ratings yet
- Bronchodilators For Reactive Airway DiseaseDocument1 pageBronchodilators For Reactive Airway DiseasedrchiNo ratings yet
- Quick Guide: Volume Guarantee Ventilation Prematurely Born Infants 32 Weeks Gestation Prematurely Born Infants 32 Weeks and Term InfantsDocument13 pagesQuick Guide: Volume Guarantee Ventilation Prematurely Born Infants 32 Weeks Gestation Prematurely Born Infants 32 Weeks and Term InfantsdrchiNo ratings yet
- BMJ Best Practice Guide Ambiguous Genitalia in NeonatesDocument51 pagesBMJ Best Practice Guide Ambiguous Genitalia in NeonatesdrchiNo ratings yet
- Ambiguous Genitalia in Neonates - Safer Care VictoriaDocument11 pagesAmbiguous Genitalia in Neonates - Safer Care VictoriadrchiNo ratings yet
- Picklebums FrogpuppetsDocument5 pagesPicklebums FrogpuppetsdrchiNo ratings yet
- Tongue Tie 2016Document6 pagesTongue Tie 2016drchiNo ratings yet
- AB Arogyadan Group Health Insurance SchemeDocument15 pagesAB Arogyadan Group Health Insurance SchemedrchiNo ratings yet
- Phala Ghrita For Female InfertilityDocument4 pagesPhala Ghrita For Female InfertilityBuddhika Nawagamuwa100% (1)
- Development of The Learners at The Various StagesDocument32 pagesDevelopment of The Learners at The Various StagesMelanie SerquiñaNo ratings yet
- Approach To Abdominal PainDocument4 pagesApproach To Abdominal PainShamen KohNo ratings yet
- Essential Intrapartum and Newborn CareDocument2 pagesEssential Intrapartum and Newborn CareKia potz100% (1)
- SpermatogenesisDocument11 pagesSpermatogenesisSAMNo ratings yet
- De Anda - Baby Think It Over-1Document11 pagesDe Anda - Baby Think It Over-1Duyên Vũ PhươngNo ratings yet
- Mice Male and FemaleDocument6 pagesMice Male and FemaleTxemiSysTemsNo ratings yet
- HMOLEDocument34 pagesHMOLEZuellen Mae Garapan BedañoNo ratings yet
- 08 - Chapter 4 PDFDocument44 pages08 - Chapter 4 PDFDipankar SarkarNo ratings yet
- Case Studyon Neonatal JundiceDocument18 pagesCase Studyon Neonatal Jundicepayel.das0607No ratings yet
- Respiratory Physiology PregnancyDocument13 pagesRespiratory Physiology PregnancyAlejandra RequesensNo ratings yet
- Lecture 3 Associated Risk FactorsDocument55 pagesLecture 3 Associated Risk FactorsTHANISHTA KUMARNo ratings yet
- Form BDocument2 pagesForm BDoctors QuizNo ratings yet
- Anatomy 02Document90 pagesAnatomy 02Puravi SamalNo ratings yet
- Soalan Objektif Bio KSSM F4Document43 pagesSoalan Objektif Bio KSSM F4Hamirah Abd HamidNo ratings yet
- Chapter 2 Development of The Brain 2014 Clinical NeuroscienceDocument8 pagesChapter 2 Development of The Brain 2014 Clinical NeuroscienceMarta Casals CollNo ratings yet
- BJOG - 2023 - Regan - Recurrent MiscarriageGreen Top Guideline No 17Document31 pagesBJOG - 2023 - Regan - Recurrent MiscarriageGreen Top Guideline No 17Anuja RajurkarNo ratings yet
- NCP ObwardDocument3 pagesNCP ObwardWendee PadillaNo ratings yet
- MRCPI O&G Regs 2023Document17 pagesMRCPI O&G Regs 2023Qamarshahzad Joia0% (1)
- Assignment On Field Appraisal ReportDocument5 pagesAssignment On Field Appraisal ReportRenita ChrisNo ratings yet
- Cephalic Preterm Livebirth by Primary Lstcs Secondary To Hellp Syndrome Preeclampsia With Severe FeaturesDocument59 pagesCephalic Preterm Livebirth by Primary Lstcs Secondary To Hellp Syndrome Preeclampsia With Severe FeaturesAsterlyn ConiendoNo ratings yet
- Reproduction (Prashant Kirad)Document17 pagesReproduction (Prashant Kirad)sunilgarg98723No ratings yet
- GROW VP NB Combined Zs Boys enDocument2 pagesGROW VP NB Combined Zs Boys enHazel GaringoNo ratings yet
- CASE DISCUSSION Subgroup 1 1Document112 pagesCASE DISCUSSION Subgroup 1 1Kartik SharmaNo ratings yet
- Human Reproductive System: Nurturing Innovative Teachers and Education LeadersDocument25 pagesHuman Reproductive System: Nurturing Innovative Teachers and Education LeadersPhoeberly SabadoNo ratings yet
- Professional Role PaperDocument5 pagesProfessional Role Paperapi-495652873No ratings yet
- 3 - Embryology (STRATOG 2015 SBAs)Document10 pages3 - Embryology (STRATOG 2015 SBAs)w yNo ratings yet
- ETHICS 2.2 FinalsDocument36 pagesETHICS 2.2 FinalsDenisse RoblesNo ratings yet
- Breast Cancer - McMaster Pathophysiology Review PDFDocument8 pagesBreast Cancer - McMaster Pathophysiology Review PDFAprilla Ayu W.No ratings yet
- MM Kit User GuidlineDocument4 pagesMM Kit User GuidlineRussell JohnNo ratings yet