Professional Documents
Culture Documents
0 ratings0% found this document useful (0 votes)
233 viewsBgcse Sda Paper 2 2001
Bgcse Sda Paper 2 2001
Uploaded by
Crystal MachipisaCopyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5834)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1093)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (852)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (903)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (541)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (349)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (824)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (405)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Bgcse Double Award Paper 3 2017 SolutionsDocument15 pagesBgcse Double Award Paper 3 2017 SolutionsCrystal Machipisa100% (4)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Bio Test F5Document5 pagesBio Test F5Crystal MachipisaNo ratings yet
- Bgcse Sda Paper 2 2004Document17 pagesBgcse Sda Paper 2 2004Crystal MachipisaNo ratings yet
- Bgcse Sda Paper 3 2006Document20 pagesBgcse Sda Paper 3 2006Crystal Machipisa100% (6)
- Double 3 2019Document20 pagesDouble 3 2019Crystal MachipisaNo ratings yet
- Double 3 2003Document16 pagesDouble 3 2003Crystal MachipisaNo ratings yet
- Bgcse Double Award 2018 SolutionsDocument16 pagesBgcse Double Award 2018 SolutionsCrystal Machipisa100% (1)
Bgcse Sda Paper 2 2001
Bgcse Sda Paper 2 2001
Uploaded by
Crystal Machipisa0 ratings0% found this document useful (0 votes)
233 views20 pagesCopyright
© © All Rights Reserved
Available Formats
PDF or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
Download as pdf
0 ratings0% found this document useful (0 votes)
233 views20 pagesBgcse Sda Paper 2 2001
Bgcse Sda Paper 2 2001
Uploaded by
Crystal MachipisaCopyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
Download as pdf
You are on page 1of 20
Candidate Name
TIME 2 hours
INSTRUCTIONS TO CANDIDATES
Contre Number
MINISTRY OF EDUCATION, BOTSWANA
in collaboration with
UNIVERSITY OF CAMBRIDGE LOCAL EXAMINATIONS SYNDICATE
Botswana General Certificate of Secondary Education
SCIENCE : DOUBLE AWARD 0569/2
PAPER 2
OCTOBER/NOVEMBER SESSION 2001 2 hours:
Candidates answer on the question paper.
No adaltional materials are required.
FOR EXAMINER'S USE
Candidate
Number
LT
1
Write your name, Centre number and candidate number in the spaces at
the top of this page. 2
‘Answer all questions.
‘Write your answers in the spaces provided on the question paper. $
INFORMATION FOR CANDIDATES ‘
The number of marks is given in brackets [ ] at the end of each question or 5
part question.
You may use a calculator. 6
A copy of the Periodic Table is printed on page 20. 7
8
8
10
"
2
13
4
18
TOTAL
This question paper consists of 18 printed pages and 2 blank pages.
unt 451 8100 S10746
‘OUCLES 2001
[Turn over
1
2
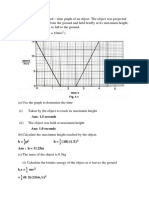
Fig. 1.1 shows a 20kg box resting on a smooth, horizontal surface and a force, F, of 15N is
applied to it. The box moves horizontally.
eter Teg Im RE
Fig.1.41
(@) Calculate the acceleration of the box. Show your working.
acceleration of box = m/s? 2]
(b) What is the advantage of moving the box alona a smooth surface?
2
3
Fig. 2.1 shows the cooling system of a refrigerator.
cooling fins _
heed
cooling pipe
‘compression
pump
Fig. 2.1
(a) The pipe and fins at the back of the refrigerator are painted black.
Explain why
())_ the pipes and the cooling fins are painted black,
(ii) there are many fins fitted along the pipe.
(b) Explain why the body of the refrigerator is painted white.
[Turn over
Fer
earners
Oso
4
3° Fig.3.1 shows a ray of light entering a rectangular glass block.
‘The refractive index of the glass is 1.5.
light ray
glass block
normal ~~:
Fig.3.4
(@) Draw the approximate path of the ray through the block and out the other side. [2]
() (i). What is meant by refractive index?
(il). The angle of incidence is 39°.
Calculate the angle of refraction. Show your working.
angle of refraction =
fe}
Q
Fie
ers
5 for
Exaners
4 Fig. 4.1 shows a person addressing a crowd using a public address system. we
microphone amplifier loudspeaker
Fig.4.1
State the main energy changes involved.
- 3)
cesrzonot [Turn over
5
6
|
Fig. 5.1 shows a negatively charged, plastic strip suspended by a thin thread and a charged |
strip R made of a different plastic material,
‘The diagram shows what happens when R is brought near end Y.
Fig.5.41
(a) Name the effect of bringing R near to Y. |
(1)
(b) Explain how the strip R was charged.
(1)
() How can the negatively charged strip be discharged?
7 For
Examiner's
se
6 _ Fig.6.1 shows an electromagnet.
electromagnet
ig.6.1
(a) State two ways by which the strength of the electromagnet can be increased.
1.
2, - 2)
(b) Sketch the voltage-time graph for two complete rotations of an a.c. generator on the
axes below.
fet tt
(3)
(c) State three ways of increasing the size of the induced electromotive force in an a.c.
generator.
13]
en0 01 [Turn over
7
(@) Complete Table 1 to show the nature of each emission and state whether the
effect of each emission is small, medium or large.
Table 1
| particle or radiation nature ionising effect
[alpha
emission
beta
emission |
gamma
emission
(4)
{b) A radioactive source Q, emits alpha, beta and gamma radiation. The radiation travels
between charged plates P, and P,. Draw in the paths followed by each radiation and
label each path accordingly.
settee | tteetet
3)
esaminer’
Use
9
8 Table 2 shows the electronic structure of six elements, U, V, W, X, Y and Z.
Table 2
element
(a) Give the letters of
) two elements in the same period of the Periodic Table,
. and
» 2]
1
t)
() (i) What is the formula of a molecule of a compound formed between hydrogen
atoms and one atom of W?
(ii) a noble gas,
i) a Group | metal.
~- ]
(ii) Draw a dot and cross diagram to show the bonding between these hydrogen
atoms and one atom of W. (Show the outer orbitals only.)
@
nese [Turn over
For
aries
10 | eat
canines
(c)_ An element T has a melting point of 30°C and a boiling point of 2440°C. It conducts we
electricity at room temperature. It burns in oxygen to form an oxide with formula T,0,,
which can react with both acids and bases. T also forms a compound with fluorine,
which has a high meiting point and conducts electricity in molten form. The
approximate relative atomic mass of T is 70.
(i) What type of oxide is T,0,? |
1]
(ii) Give two properties that indicate that T is probably a metal.
Ae
2 2)
(ili) Predict the formula for the fluoride of T.
(1)
(iv) What are the products of the electrolysis of the molten fluoride of T, using inert
electrodes?
. and ... 2)
(V) In which group and period of the Periodic Table will T be placed?
group
period fal
(vi) Write the symbol of the element in the Periodic Table which most closely |
resembles T.
m |
" For
varies
tse
(a) A and B are white powders. A is insoluble in water but B is soluble in water and its
solution has a pH value of 3.
‘A mixture of A and B bubbles or fizzes in water. A gas is given off and a clear solution. |
forms,
(i) Which powder is acidic?
‘The other powder is a carbonate,
‘What gas is given off in the reaction?
(b) Anyhdrous copper(II) sulphate is a white powder. |
(i) Write its formula... ~ (1 |
‘What happens when water is added to anhydrous copper(II) sulphate?
(c) Describe two tests to show that a given liquid is water.
ia}
creas [Tum over
2
10 Limestone, CaCO,, decomposes on heating according to the following equation.
CaCO,(s) > Ca0(s) + CO,(9)
(a) Calculate the relative molecular mass of limestone.
relative molecular mass = fo
(b) 20g of limestone was decomposed.
(Calculate the number of moles of limestone used.
number of moles a
(li) Calculate the mass of calcium oxide, CaO, formed.
mass = ~9 [2
(©) At the end of heating, the calcium oxide formed was weighed and only 10g was
collected.
(i) Calculate the percentage yield of the product
% yield = % [2]
(ii) Name a substance that would react with calcium oxide to form calcium chloride.
(1)
(iii) Write a balanced reaction equation for the reaction. include state symbols.
13
11. Fig. 11.14 shows an animal cell and Fig. 11.18 a plant cell
Fig. 11.14 Fig. 11.18
(a) State one visible similarity and one visible difference between the two cells. |
(similarity ..
= i)
(i) difference
mu)
(b) Fig. 11.2 shows how the cell in Fig. 11.1A would appear if it was left in a concentrated
solution for an hour.
Fig. 11.2
Explain the changes that have taken place in the cell while it was in the concentrated
solution.
snot [Turn over
For
varias
14
12. Fig. 12.1 shows the apparatus used to investigate a process taking place in yeast cells.
oll
yeast suspension
7 ~ + sugar
Fig. 12.1
}—limewater
(a) Name the process under investigation,
(1)
(b)_ State the word equation for this process.
1
(©) Explain why a layer of oil was put on the yeast-sugar mixture.
a
() (i) State what would be observed in the test-tube with limewater if a different
test-tube containing a boiled yeast-sugar mixture is used in the set-up.
Gil). Explain your answer in (i) above.
canines
16 for
Exaninors
Use
13 Fig. 13.1 shows the human blood circulation,
lung
z— [——w
right side left side
Y x
body cells
Fig.13.1
(a) Identify the blood vessels labelled W, X, ¥ and Z.
Ww.
x
Yu
Zz [4]
(b)_ Draw arrows in the blood vessels to show the direction of blood flow. fo}
(c)_ State two differences in structure between blood vessel X and blood vessel Y.
- 2)
(d)_ State the function of the heart in blood circulation
cnc.onet [Turn over
16
14. Fig. 14.1 shows half of a flower.
stigma
sig
Fig. 14.1
(a) Describe how this flower is pollinated
13}
(b) Give two features of a flower that is pollinated in this way.
1
ia}
(c) Pollination can tead to fertilisation and the development of fruits and seeds.
Describe the functions of the following parts of a seed.
(i) cotyledon ...
i) testa ..
~ {1
{d) Explain how the dispersal of fruits like tomatoes by animals is possible even though
there are digestive juices in the gut.
(3)
aries
7
15. Conservation of both plant and animal species is of great importance to mankind,
(a) Give two reasons why conservation of some species is of importance to Botswana.
(b) Describe how recycling of paper contributes to conservation.
(2)
. 2
18
BLANK PAGE
19
BLANK PAGE
(0:71) e1nssaid pue esnjesoduso} woot te ,uup ¥2 51 Se6 Aue Jo oj0ur ouO Jo ouINjon ous
20
so | [ene | weet | net | ee | nen” ] "| cm] wen) | | ee |e
Te) ow [pn | wa Sa" | To" | tat fa | tiv | nat | “aN | a | ea | a fon
a ee ee) 198 powNeY eO|-064
m [mw | we | ae | fa | an jp | me | us | ma | pw | vd | 20 09S PIOURYIUET 1-85.
ow ew Ad
| So | Se | we | er] a | eT) Se |
wa | “mg” | Pow” | aw! | Ge") “RS | ig” | “ae
20 ed on 40 A uo os eo | MW
on °N
| "| om’
ce | A
a 4
Co i
noi
‘squaUlo!a yp JO 121 SIP OU,
4133Hs viva
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5834)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1093)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (852)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (903)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (541)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (349)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (824)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (405)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Bgcse Double Award Paper 3 2017 SolutionsDocument15 pagesBgcse Double Award Paper 3 2017 SolutionsCrystal Machipisa100% (4)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Bio Test F5Document5 pagesBio Test F5Crystal MachipisaNo ratings yet
- Bgcse Sda Paper 2 2004Document17 pagesBgcse Sda Paper 2 2004Crystal MachipisaNo ratings yet
- Bgcse Sda Paper 3 2006Document20 pagesBgcse Sda Paper 3 2006Crystal Machipisa100% (6)
- Double 3 2019Document20 pagesDouble 3 2019Crystal MachipisaNo ratings yet
- Double 3 2003Document16 pagesDouble 3 2003Crystal MachipisaNo ratings yet
- Bgcse Double Award 2018 SolutionsDocument16 pagesBgcse Double Award 2018 SolutionsCrystal Machipisa100% (1)