Professional Documents
Culture Documents
Tampered Dat
Tampered Dat
Uploaded by
ÁngelHerreroRodríguezCopyright:
Available Formats
You might also like
- Ot Utilization Project 642Document69 pagesOt Utilization Project 642Amit Pahwa100% (3)
- Wms 2014 Sma Reach PosterDocument1 pageWms 2014 Sma Reach Posterapi-253933916No ratings yet
- 2018 VentilatorbundleDocument9 pages2018 VentilatorbundleRita RangelNo ratings yet
- Santosetal.2015.Diagnosisofasthma ThechallengegoesonDocument2 pagesSantosetal.2015.Diagnosisofasthma ThechallengegoesonMila KaniyaNo ratings yet
- Thesun 2009-04-27 Page01 Mexican Flu AlertDocument1 pageThesun 2009-04-27 Page01 Mexican Flu AlertImpulsive collectorNo ratings yet
- Acupuncture-Related Adverse Events A Systematic ReviewDocument7 pagesAcupuncture-Related Adverse Events A Systematic ReviewdanuwinataNo ratings yet
- Adverse Events of TDCS and tACS: A ReviewDocument7 pagesAdverse Events of TDCS and tACS: A ReviewCinzia MarinaroNo ratings yet
- Castien 2013 Working Mechanism M Tinct THDocument8 pagesCastien 2013 Working Mechanism M Tinct THAdnan Faris NaufalNo ratings yet
- Monitor: Help Wanted: Must Have The Brain of An Internist, Hands of A Surgeon, and Heart of A PsychiatristDocument4 pagesMonitor: Help Wanted: Must Have The Brain of An Internist, Hands of A Surgeon, and Heart of A Psychiatristjesus zapataNo ratings yet
- Wong YM 2018 Ryodoraku ShamDocument3 pagesWong YM 2018 Ryodoraku Shamrswongym449100% (1)
- Weintraub Report MagstepsDocument2 pagesWeintraub Report Magstepsapi-3743459100% (1)
- Marwiah 2018 J. Phys. Conf. Ser. 1114 012146Document3 pagesMarwiah 2018 J. Phys. Conf. Ser. 1114 012146chandiliongNo ratings yet
- The Stony Brook Press - Volume 29, Issue 12Document32 pagesThe Stony Brook Press - Volume 29, Issue 12The Stony Brook PressNo ratings yet
- 2013 Low DosesDocument1 page2013 Low DosesECO DynamicNo ratings yet
- Plantar Pressure Distribution MeasurementsDocument6 pagesPlantar Pressure Distribution Measurementschungkailun1No ratings yet
- Deep Learning Techniques For Electronic Health Record AnalysisDocument5 pagesDeep Learning Techniques For Electronic Health Record AnalysisJalpaMehtaNo ratings yet
- Deep Learning Techniques For Electronic Health Record AnalysisDocument5 pagesDeep Learning Techniques For Electronic Health Record Analysisabbas.fadhail5dNo ratings yet
- Programs Promise To End PDF Paper-Chase: Zoo NewsDocument1 pagePrograms Promise To End PDF Paper-Chase: Zoo NewsxishqNo ratings yet
- Barmack 1938Document10 pagesBarmack 1938Sheila M. CedeñoNo ratings yet
- Covid-19: Researcher Blows The Whistle On Data Integrity Issues in Pfizer's Vaccine TrialDocument3 pagesCovid-19: Researcher Blows The Whistle On Data Integrity Issues in Pfizer's Vaccine TrialLuxNo ratings yet
- Jaha 116 004180 PDFDocument50 pagesJaha 116 004180 PDFNi Made SuartiningsihNo ratings yet
- Diagnostic Ultrasound SafetyDocument7 pagesDiagnostic Ultrasound SafetyshadowgardenNo ratings yet
- The Use of Virtual Reality To Influence Engagement and Enjoyment During Exercise A Scoping ReviewDocument24 pagesThe Use of Virtual Reality To Influence Engagement and Enjoyment During Exercise A Scoping ReviewnpckhangNo ratings yet
- Elnahas Fraud in MedicineDocument3 pagesElnahas Fraud in MedicineAbilNo ratings yet
- Uncertainty Quantification and Sensitivity Analysis of Left Ventricle FunctionDocument19 pagesUncertainty Quantification and Sensitivity Analysis of Left Ventricle FunctionDr. Raheem GulNo ratings yet
- Opinion_ Scientific Peer Review in Crisis _ The Scientist Magazine®Document3 pagesOpinion_ Scientific Peer Review in Crisis _ The Scientist Magazine®Norma TamezNo ratings yet
- Conflict of Interest & Bias in Health Advisory Committees: A Case Study of The WHO's Electromagnetic Field (EMF) Task GroupDocument4 pagesConflict of Interest & Bias in Health Advisory Committees: A Case Study of The WHO's Electromagnetic Field (EMF) Task GroupSwissTeslaNo ratings yet
- Philippine Star, Feb. 7, 2019, House Recommends Raps Vs Moy, 2 Others Over Dengvaxia PDFDocument1 pagePhilippine Star, Feb. 7, 2019, House Recommends Raps Vs Moy, 2 Others Over Dengvaxia PDFpribhor2No ratings yet
- DNA Waves and WaterDocument11 pagesDNA Waves and WaterItay Raphael OronNo ratings yet
- Common Causes of Open-Circuit Recreational DivingDocument15 pagesCommon Causes of Open-Circuit Recreational DivingHrvoje MatakovicNo ratings yet
- Paul Et Al 1963 A Longitudinal Study of Coronary Heart DiseaseDocument12 pagesPaul Et Al 1963 A Longitudinal Study of Coronary Heart DiseaseRamadhan Yudha PratamaNo ratings yet
- FCC O Cials Denounce Lawmakers' Attempts To Censor NewsroomsDocument52 pagesFCC O Cials Denounce Lawmakers' Attempts To Censor NewsroomsKeithStewartNo ratings yet
- Montagnier - Waves, WaterDocument11 pagesMontagnier - Waves, WaterGrado ZeroNo ratings yet
- 423 FullDocument6 pages423 FullharperllcNo ratings yet
- Parkinsons Disease Pase 1Document17 pagesParkinsons Disease Pase 11MV19ET014 Divya TANo ratings yet
- Are Scientists A WorkforceDocument10 pagesAre Scientists A WorkforceAna JakovljevicNo ratings yet
- 10.1136@bmjopen 2019 033642Document10 pages10.1136@bmjopen 2019 033642Trúc NguyễnNo ratings yet
- Rebuttal JPhysiol 2021Document2 pagesRebuttal JPhysiol 2021supardiNo ratings yet
- THE Light: Masks Do More Harm Than GoodDocument24 pagesTHE Light: Masks Do More Harm Than Goodwebtrekker UKNo ratings yet
- Are Scientists A Workforce? - Or, How Dr. Frankenstein Made Biomedical Research SickDocument9 pagesAre Scientists A Workforce? - Or, How Dr. Frankenstein Made Biomedical Research SickCharlie BirtNo ratings yet
- 1 s2.0 S138824571500231X MainDocument8 pages1 s2.0 S138824571500231X MainTianyi ZhengNo ratings yet
- Prediction of Blood Donors: FaintingDocument7 pagesPrediction of Blood Donors: FaintingSree SuniNo ratings yet
- Impact Factor Mutation, Manipulation, And.5Document5 pagesImpact Factor Mutation, Manipulation, And.5Paloma RolimNo ratings yet
- Azizulkarim 2017 IOP Conf. Ser. Mater. Sci. Eng. 226 012094Document7 pagesAzizulkarim 2017 IOP Conf. Ser. Mater. Sci. Eng. 226 012094andikurniawannugroho76No ratings yet
- Fphys 11 585400Document20 pagesFphys 11 585400Chandralal SharmendraNo ratings yet
- Tiny Plastics Turn Up in Arterial Clogs: Finding Renews Concerns About The Materials' Effects On HealthDocument1 pageTiny Plastics Turn Up in Arterial Clogs: Finding Renews Concerns About The Materials' Effects On Healthzhu286910No ratings yet
- Covid-19: Researcher Blows The Whistle On Data Integrity Issues in Pfizer's Vaccine TrialDocument3 pagesCovid-19: Researcher Blows The Whistle On Data Integrity Issues in Pfizer's Vaccine TrialKrutarth PatelNo ratings yet
- Applied Tension and Blood Donation Symptoms: The Importance of Anxiety ReductionDocument6 pagesApplied Tension and Blood Donation Symptoms: The Importance of Anxiety ReductionDessy VerlaniaNo ratings yet
- Fineberg 2012 Evaluation of Computed Tomography Achievement and ChallengeDocument4 pagesFineberg 2012 Evaluation of Computed Tomography Achievement and ChallengeDaniel GrigorasNo ratings yet
- Pacemaker PamphletDocument2 pagesPacemaker Pamphletapi-355079078No ratings yet
- Bias: Linking Evidence With PracticeDocument2 pagesBias: Linking Evidence With PracticeTomáš KrajíčekNo ratings yet
- Design and Reliability Analysis of A Novel Detector For Monitoring Spine DiseaseDocument2 pagesDesign and Reliability Analysis of A Novel Detector For Monitoring Spine DiseaseebrahimpanNo ratings yet
- 10 1161@circep 118 007142Document13 pages10 1161@circep 118 007142cardionerd101No ratings yet
- Karoshi and ArrythmiaDocument8 pagesKaroshi and ArrythmiaPeny Ruth Jessica DamanikNo ratings yet
- BMJ h6070 FullDocument2 pagesBMJ h6070 FullAshutNo ratings yet
- Anaesthesia - 2003 - James - 1000 Anaesthetic Incidents Experience To DateDocument8 pagesAnaesthesia - 2003 - James - 1000 Anaesthetic Incidents Experience To DateBaha MirzaeifarNo ratings yet
- The Quality of Reporting of Kidney Research A.2Document2 pagesThe Quality of Reporting of Kidney Research A.2Arista RachmaNo ratings yet
- The Spookiness' of Quantum Physics Could Be IncalculableDocument2 pagesThe Spookiness' of Quantum Physics Could Be IncalculableAnahí TessaNo ratings yet
- Genexpert Error Codes:An Evaluation of Their Definitions and Its Implications On Program Strengthening EffortsDocument2 pagesGenexpert Error Codes:An Evaluation of Their Definitions and Its Implications On Program Strengthening EffortsKiem Adi BudimanNo ratings yet
- Who Counts 2Document12 pagesWho Counts 2Shairamart ABCDNo ratings yet
- Textbook Trends in The Management of Cerebrovascular Diseases Giuseppe Esposito Ebook All Chapter PDFDocument53 pagesTextbook Trends in The Management of Cerebrovascular Diseases Giuseppe Esposito Ebook All Chapter PDFjames.martin482100% (3)
- Neurogenic Bowel ManagementDocument51 pagesNeurogenic Bowel ManagementAndrei TarusNo ratings yet
- Interventional Studies 2Document28 pagesInterventional Studies 2Abdul RazzakNo ratings yet
- PH 211 Lecture NotesDocument13 pagesPH 211 Lecture NotesColata Noreen AmorNo ratings yet
- How To Write The Quality Part of An IMPD: SpeakersDocument4 pagesHow To Write The Quality Part of An IMPD: SpeakersIvana VinkovicNo ratings yet
- 119R PDFDocument49 pages119R PDFRuther Marc P. Narcida100% (1)
- NUDE MICE and Other Medical Writing Terms You Need To KnowDocument18 pagesNUDE MICE and Other Medical Writing Terms You Need To KnowThe Accidental Medical Writer75% (4)
- High Level BPM (Business Process Mapping)Document28 pagesHigh Level BPM (Business Process Mapping)Uppiliappan Gopalan50% (2)
- Presentation File 5112c3ed d0fc 4150 Bf1e 0287ac105aceDocument13 pagesPresentation File 5112c3ed d0fc 4150 Bf1e 0287ac105aceInnoVentureCommunityNo ratings yet
- Test Bank For Pharmacology 9th Edition by MccuistionDocument5 pagesTest Bank For Pharmacology 9th Edition by MccuistionRobert Allgood100% (36)
- Producing Clinical Laboratory Shift Tables From Adam Data: Rao Bingi, Octagon Research Solutions, Wayne, PADocument12 pagesProducing Clinical Laboratory Shift Tables From Adam Data: Rao Bingi, Octagon Research Solutions, Wayne, PAlenithNo ratings yet
- The Human Medicines Regulations 2012Document322 pagesThe Human Medicines Regulations 2012Fuzzy_Wood_PersonNo ratings yet
- 2017 Fish Market RFP-Final-12.1.17Document24 pages2017 Fish Market RFP-Final-12.1.17Salman DigaleNo ratings yet
- Job Description: Associate EDC Programmer / Custom Function Developer (Full Time-After Training and Evaluation) - GHANADocument2 pagesJob Description: Associate EDC Programmer / Custom Function Developer (Full Time-After Training and Evaluation) - GHANAJustice Kelvin Dzameshie KwadzoNo ratings yet
- Medicinal Cannabis The Evidence V1Document19 pagesMedicinal Cannabis The Evidence V1danisterNo ratings yet
- Zemaira Prescribing InformationDocument6 pagesZemaira Prescribing InformationArun ChadhaNo ratings yet
- Vitamin C and Cancer - Medicine or Politic - Richards, EvelleenDocument296 pagesVitamin C and Cancer - Medicine or Politic - Richards, EvelleenAnonymous gwFqQcnaX100% (1)
- Draft Conditional Use Permit For South Salt Lake Homeless Resource CenterDocument33 pagesDraft Conditional Use Permit For South Salt Lake Homeless Resource CenterThe Salt Lake TribuneNo ratings yet
- IMSH Institute Global Oncology Trend 2015 2020 ReportDocument45 pagesIMSH Institute Global Oncology Trend 2015 2020 ReportRishabh GuptaNo ratings yet
- Guidelines For Importation and Exportation of Pharmaceutical Products in TanzaniaDocument23 pagesGuidelines For Importation and Exportation of Pharmaceutical Products in TanzaniaRajesh KatareNo ratings yet
- C. Nokes D.A.P. Bundy (1994) - Does Helminth Infection Affect Mental Processing and Educational Achievement. 10 (1), 14-18.Document5 pagesC. Nokes D.A.P. Bundy (1994) - Does Helminth Infection Affect Mental Processing and Educational Achievement. 10 (1), 14-18.Maj ImanNo ratings yet
- IFPMA Code of Practice 2012Document24 pagesIFPMA Code of Practice 2012corruptioncurrentsNo ratings yet
- Journal ObstetriDocument4 pagesJournal ObstetriDhiyah HarahapNo ratings yet
- 1 s2.0 S2352873718300052 MainDocument22 pages1 s2.0 S2352873718300052 MainDewi WulandariNo ratings yet
- Handout For Topic 1Document10 pagesHandout For Topic 1Ralph Kenneth Nastor RamosNo ratings yet
- Maiga Ayub Hussein: The African Forum For Research and Education in Health (Afrehealth)Document7 pagesMaiga Ayub Hussein: The African Forum For Research and Education in Health (Afrehealth)Maiga Ayub HusseinNo ratings yet
- Ich - Pediatric ExtrapolationDocument44 pagesIch - Pediatric ExtrapolationRizqi Dinni FauziaNo ratings yet
- Evaluation of Aromatherapy With Lavender Oil On Academic Stress - A Randomized Placebo Controlled Clinical TrialDocument9 pagesEvaluation of Aromatherapy With Lavender Oil On Academic Stress - A Randomized Placebo Controlled Clinical TrialigorfragaNo ratings yet
- Research FlowchartDocument22 pagesResearch FlowchartMuhammad ZafranNo ratings yet
Tampered Dat
Tampered Dat
Uploaded by
ÁngelHerreroRodríguezOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Tampered Dat
Tampered Dat
Uploaded by
ÁngelHerreroRodríguezCopyright:
Available Formats
NEWS & ANALYSIS NEWS & ANALYSIS
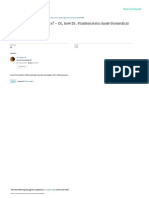
Chagrined. KPUM President Toshikazu Yoshikawa
(center) and other officials apologize after report-
ing fraud.
group. Once anomalous data were corrected,
the claim that valsartan reduces the incidence
of cardiovascular events such as angina or
stroke by about half “is not supported,” the
report says. Eight papers authored by Matsub-
ara have been retracted.
In an e-mail to Science, Matsubara
writes that he “was not involved in and gave
no instructions” for the event data analy-
sis, which he said was the responsibility of
a Novartis employee. Novartis headquarters
in Basel declined to comment on KPUM’s
Downloaded from www.sciencemag.org on May 2, 2015
J A PA N findings, stating in an e-mail to Science that
“we are unfamiliar with how the university
Tampered Data Cast conducted its review.” But the company has
confirmed that employees of its Japanese sub-
sidiary participated in the Kyoto Heart Study
Shadow on Drug Trial and several similar studies at other Japanese
institutions without noting their affiliation on
resulting papers. In a statement posted to its
TOKYO—In a scandal reverberating across The 4-year study followed 3000 patients given website on 12 July, Novartis blamed the dis-
Japan’s biomedical research landscape, a uni- valsartan or alternative medications. A main closure failures on a lack of guidelines and a
versity in Kyoto last week acknowledged data outcome, reported on 31 August 2009 in the misunderstanding of the appropriate level of
manipulation in a university-run clinical trial European Heart Journal, was that valsartan, involvement in such trials. “Preventive and
for a blockbuster hypertension drug, valsar- which reduces blood pressure by blocking the corrective measures have been implemented,”
tan. Japanese media have turned the episode receptor for the hormone angiotensin, “pre- the statement said.
into a cause célèbre; the Yomiuri Shimbun, vented more cardiovascular events” in high- The debacle raises troubling questions
one of Japan’s biggest newspapers, labeled it a risk patients than did drugs that lower blood about research oversight. KPUM officials said
“serious betrayal” for patients. Repercussions pressure through another mechanism. that they could not probe more deeply into
could extend beyond valsartan, marketed In late 2011, bloggers started raising ques- who manipulated data because they cannot
under the trade name Diovan by the Swiss tions about alleged image manipulation in compel cooperation from Novartis employ-
pharmaceutical giant Novartis. Matsubara’s papers. Then, in an April 2012 ees. Universities in Japan have no authority
“This incident is causing a loss of confi- letter to The Lancet, Yui expressed concerns to question people outside their institution,
dence in Japan’s research internationally and about Kyoto Heart Study’s statistics and con- says Tetsuya Tanimoto, a physician at the Uni-
making Japanese patients skeptical [about clusions, writing that the effectiveness in pre- versity of Tokyo who studies pharmaceutical
treatments],” says Yoshiki Yui, a medical venting angina was not seen regulatory issues. Japan needs
doctor at Kyoto University. Health minis- in other trials of valsartanlike “Data were something like the U.S. Office
ter Norihisa Tamura has vowed to appoint a drugs or in clinical practice. of Research Integrity, he says.
committee that would propose measures to Responding to requests from manipulated.” Japan’s medical research
prevent a recurrence. journals, KPUM started its —TOSHIKAZU YOSHIKAWA establishment could come in
Sold worldwide, valsartan was approved own investigation. Matsubara KPUM PRESIDENT for broad scrutiny. KPUM
in Japan in 2000 and has become the coun- resigned from KPUM in Febru- confirmed in an e-mail to
try’s best-selling drug, pulling in roughly ary, after the European Heart Journal, citing Science that since 2008, Matsubara received
$1 billion last year, according to analysts. “[c]ritical problems … with some of the data,” about $1.4 million for his research from
A clinical trial claiming that valsartan also retracted the heart study paper. Novartis. Such grants to clinical researchers
reduces angina and stroke risk helped boost “Data were manipulated,” KPUM Presi- are not unusual, but they highlight inadequate
its popularity in Japan. That claim has fallen dent Toshikazu Yoshikawa bluntly stated at public support for clinical research in Japan,
apart, in the process raising concerns about an 11 July press conference. According to Tanimoto says. The revelations could also
cozy ties between researchers and drug com- the university’s investigative report, which erode public support for emerging plans to
CREDIT: KYODO/NEWSCOM
panies and about impediments to investigat- Science has obtained, in the Kyoto Heart shake up medical research by establishing an
ing research misconduct in Japan. Study there were 34 discrepancies between outcome-focused version of the U.S. National
At the heart of the scandal are data from the clinical medical records and the data set Institutes of Health. In the wake of the scan-
the Kyoto Heart Study, launched in 2003 by used for analysis; these overstated adverse dal, the Japanese public may question whether
Hiroaki Matsubara, a cardiologist at Kyoto cardiovascular events in the nonvalsartan the money will be well-spent and the results
Prefectural University of Medicine (KPUM). group and missed such events in the valsartan trustworthy. –DENNIS NORMILE
www.sciencemag.org SCIENCE VOL 341 19 JULY 2013 223
Published by AAAS
You might also like
- Ot Utilization Project 642Document69 pagesOt Utilization Project 642Amit Pahwa100% (3)
- Wms 2014 Sma Reach PosterDocument1 pageWms 2014 Sma Reach Posterapi-253933916No ratings yet
- 2018 VentilatorbundleDocument9 pages2018 VentilatorbundleRita RangelNo ratings yet
- Santosetal.2015.Diagnosisofasthma ThechallengegoesonDocument2 pagesSantosetal.2015.Diagnosisofasthma ThechallengegoesonMila KaniyaNo ratings yet
- Thesun 2009-04-27 Page01 Mexican Flu AlertDocument1 pageThesun 2009-04-27 Page01 Mexican Flu AlertImpulsive collectorNo ratings yet
- Acupuncture-Related Adverse Events A Systematic ReviewDocument7 pagesAcupuncture-Related Adverse Events A Systematic ReviewdanuwinataNo ratings yet
- Adverse Events of TDCS and tACS: A ReviewDocument7 pagesAdverse Events of TDCS and tACS: A ReviewCinzia MarinaroNo ratings yet
- Castien 2013 Working Mechanism M Tinct THDocument8 pagesCastien 2013 Working Mechanism M Tinct THAdnan Faris NaufalNo ratings yet
- Monitor: Help Wanted: Must Have The Brain of An Internist, Hands of A Surgeon, and Heart of A PsychiatristDocument4 pagesMonitor: Help Wanted: Must Have The Brain of An Internist, Hands of A Surgeon, and Heart of A Psychiatristjesus zapataNo ratings yet
- Wong YM 2018 Ryodoraku ShamDocument3 pagesWong YM 2018 Ryodoraku Shamrswongym449100% (1)
- Weintraub Report MagstepsDocument2 pagesWeintraub Report Magstepsapi-3743459100% (1)
- Marwiah 2018 J. Phys. Conf. Ser. 1114 012146Document3 pagesMarwiah 2018 J. Phys. Conf. Ser. 1114 012146chandiliongNo ratings yet
- The Stony Brook Press - Volume 29, Issue 12Document32 pagesThe Stony Brook Press - Volume 29, Issue 12The Stony Brook PressNo ratings yet
- 2013 Low DosesDocument1 page2013 Low DosesECO DynamicNo ratings yet
- Plantar Pressure Distribution MeasurementsDocument6 pagesPlantar Pressure Distribution Measurementschungkailun1No ratings yet
- Deep Learning Techniques For Electronic Health Record AnalysisDocument5 pagesDeep Learning Techniques For Electronic Health Record AnalysisJalpaMehtaNo ratings yet
- Deep Learning Techniques For Electronic Health Record AnalysisDocument5 pagesDeep Learning Techniques For Electronic Health Record Analysisabbas.fadhail5dNo ratings yet
- Programs Promise To End PDF Paper-Chase: Zoo NewsDocument1 pagePrograms Promise To End PDF Paper-Chase: Zoo NewsxishqNo ratings yet
- Barmack 1938Document10 pagesBarmack 1938Sheila M. CedeñoNo ratings yet
- Covid-19: Researcher Blows The Whistle On Data Integrity Issues in Pfizer's Vaccine TrialDocument3 pagesCovid-19: Researcher Blows The Whistle On Data Integrity Issues in Pfizer's Vaccine TrialLuxNo ratings yet
- Jaha 116 004180 PDFDocument50 pagesJaha 116 004180 PDFNi Made SuartiningsihNo ratings yet
- Diagnostic Ultrasound SafetyDocument7 pagesDiagnostic Ultrasound SafetyshadowgardenNo ratings yet
- The Use of Virtual Reality To Influence Engagement and Enjoyment During Exercise A Scoping ReviewDocument24 pagesThe Use of Virtual Reality To Influence Engagement and Enjoyment During Exercise A Scoping ReviewnpckhangNo ratings yet
- Elnahas Fraud in MedicineDocument3 pagesElnahas Fraud in MedicineAbilNo ratings yet
- Uncertainty Quantification and Sensitivity Analysis of Left Ventricle FunctionDocument19 pagesUncertainty Quantification and Sensitivity Analysis of Left Ventricle FunctionDr. Raheem GulNo ratings yet
- Opinion_ Scientific Peer Review in Crisis _ The Scientist Magazine®Document3 pagesOpinion_ Scientific Peer Review in Crisis _ The Scientist Magazine®Norma TamezNo ratings yet
- Conflict of Interest & Bias in Health Advisory Committees: A Case Study of The WHO's Electromagnetic Field (EMF) Task GroupDocument4 pagesConflict of Interest & Bias in Health Advisory Committees: A Case Study of The WHO's Electromagnetic Field (EMF) Task GroupSwissTeslaNo ratings yet
- Philippine Star, Feb. 7, 2019, House Recommends Raps Vs Moy, 2 Others Over Dengvaxia PDFDocument1 pagePhilippine Star, Feb. 7, 2019, House Recommends Raps Vs Moy, 2 Others Over Dengvaxia PDFpribhor2No ratings yet
- DNA Waves and WaterDocument11 pagesDNA Waves and WaterItay Raphael OronNo ratings yet
- Common Causes of Open-Circuit Recreational DivingDocument15 pagesCommon Causes of Open-Circuit Recreational DivingHrvoje MatakovicNo ratings yet
- Paul Et Al 1963 A Longitudinal Study of Coronary Heart DiseaseDocument12 pagesPaul Et Al 1963 A Longitudinal Study of Coronary Heart DiseaseRamadhan Yudha PratamaNo ratings yet
- FCC O Cials Denounce Lawmakers' Attempts To Censor NewsroomsDocument52 pagesFCC O Cials Denounce Lawmakers' Attempts To Censor NewsroomsKeithStewartNo ratings yet
- Montagnier - Waves, WaterDocument11 pagesMontagnier - Waves, WaterGrado ZeroNo ratings yet
- 423 FullDocument6 pages423 FullharperllcNo ratings yet
- Parkinsons Disease Pase 1Document17 pagesParkinsons Disease Pase 11MV19ET014 Divya TANo ratings yet
- Are Scientists A WorkforceDocument10 pagesAre Scientists A WorkforceAna JakovljevicNo ratings yet
- 10.1136@bmjopen 2019 033642Document10 pages10.1136@bmjopen 2019 033642Trúc NguyễnNo ratings yet
- Rebuttal JPhysiol 2021Document2 pagesRebuttal JPhysiol 2021supardiNo ratings yet
- THE Light: Masks Do More Harm Than GoodDocument24 pagesTHE Light: Masks Do More Harm Than Goodwebtrekker UKNo ratings yet
- Are Scientists A Workforce? - Or, How Dr. Frankenstein Made Biomedical Research SickDocument9 pagesAre Scientists A Workforce? - Or, How Dr. Frankenstein Made Biomedical Research SickCharlie BirtNo ratings yet
- 1 s2.0 S138824571500231X MainDocument8 pages1 s2.0 S138824571500231X MainTianyi ZhengNo ratings yet
- Prediction of Blood Donors: FaintingDocument7 pagesPrediction of Blood Donors: FaintingSree SuniNo ratings yet
- Impact Factor Mutation, Manipulation, And.5Document5 pagesImpact Factor Mutation, Manipulation, And.5Paloma RolimNo ratings yet
- Azizulkarim 2017 IOP Conf. Ser. Mater. Sci. Eng. 226 012094Document7 pagesAzizulkarim 2017 IOP Conf. Ser. Mater. Sci. Eng. 226 012094andikurniawannugroho76No ratings yet
- Fphys 11 585400Document20 pagesFphys 11 585400Chandralal SharmendraNo ratings yet
- Tiny Plastics Turn Up in Arterial Clogs: Finding Renews Concerns About The Materials' Effects On HealthDocument1 pageTiny Plastics Turn Up in Arterial Clogs: Finding Renews Concerns About The Materials' Effects On Healthzhu286910No ratings yet
- Covid-19: Researcher Blows The Whistle On Data Integrity Issues in Pfizer's Vaccine TrialDocument3 pagesCovid-19: Researcher Blows The Whistle On Data Integrity Issues in Pfizer's Vaccine TrialKrutarth PatelNo ratings yet
- Applied Tension and Blood Donation Symptoms: The Importance of Anxiety ReductionDocument6 pagesApplied Tension and Blood Donation Symptoms: The Importance of Anxiety ReductionDessy VerlaniaNo ratings yet
- Fineberg 2012 Evaluation of Computed Tomography Achievement and ChallengeDocument4 pagesFineberg 2012 Evaluation of Computed Tomography Achievement and ChallengeDaniel GrigorasNo ratings yet
- Pacemaker PamphletDocument2 pagesPacemaker Pamphletapi-355079078No ratings yet
- Bias: Linking Evidence With PracticeDocument2 pagesBias: Linking Evidence With PracticeTomáš KrajíčekNo ratings yet
- Design and Reliability Analysis of A Novel Detector For Monitoring Spine DiseaseDocument2 pagesDesign and Reliability Analysis of A Novel Detector For Monitoring Spine DiseaseebrahimpanNo ratings yet
- 10 1161@circep 118 007142Document13 pages10 1161@circep 118 007142cardionerd101No ratings yet
- Karoshi and ArrythmiaDocument8 pagesKaroshi and ArrythmiaPeny Ruth Jessica DamanikNo ratings yet
- BMJ h6070 FullDocument2 pagesBMJ h6070 FullAshutNo ratings yet
- Anaesthesia - 2003 - James - 1000 Anaesthetic Incidents Experience To DateDocument8 pagesAnaesthesia - 2003 - James - 1000 Anaesthetic Incidents Experience To DateBaha MirzaeifarNo ratings yet
- The Quality of Reporting of Kidney Research A.2Document2 pagesThe Quality of Reporting of Kidney Research A.2Arista RachmaNo ratings yet
- The Spookiness' of Quantum Physics Could Be IncalculableDocument2 pagesThe Spookiness' of Quantum Physics Could Be IncalculableAnahí TessaNo ratings yet
- Genexpert Error Codes:An Evaluation of Their Definitions and Its Implications On Program Strengthening EffortsDocument2 pagesGenexpert Error Codes:An Evaluation of Their Definitions and Its Implications On Program Strengthening EffortsKiem Adi BudimanNo ratings yet
- Who Counts 2Document12 pagesWho Counts 2Shairamart ABCDNo ratings yet
- Textbook Trends in The Management of Cerebrovascular Diseases Giuseppe Esposito Ebook All Chapter PDFDocument53 pagesTextbook Trends in The Management of Cerebrovascular Diseases Giuseppe Esposito Ebook All Chapter PDFjames.martin482100% (3)
- Neurogenic Bowel ManagementDocument51 pagesNeurogenic Bowel ManagementAndrei TarusNo ratings yet
- Interventional Studies 2Document28 pagesInterventional Studies 2Abdul RazzakNo ratings yet
- PH 211 Lecture NotesDocument13 pagesPH 211 Lecture NotesColata Noreen AmorNo ratings yet
- How To Write The Quality Part of An IMPD: SpeakersDocument4 pagesHow To Write The Quality Part of An IMPD: SpeakersIvana VinkovicNo ratings yet
- 119R PDFDocument49 pages119R PDFRuther Marc P. Narcida100% (1)
- NUDE MICE and Other Medical Writing Terms You Need To KnowDocument18 pagesNUDE MICE and Other Medical Writing Terms You Need To KnowThe Accidental Medical Writer75% (4)
- High Level BPM (Business Process Mapping)Document28 pagesHigh Level BPM (Business Process Mapping)Uppiliappan Gopalan50% (2)
- Presentation File 5112c3ed d0fc 4150 Bf1e 0287ac105aceDocument13 pagesPresentation File 5112c3ed d0fc 4150 Bf1e 0287ac105aceInnoVentureCommunityNo ratings yet
- Test Bank For Pharmacology 9th Edition by MccuistionDocument5 pagesTest Bank For Pharmacology 9th Edition by MccuistionRobert Allgood100% (36)
- Producing Clinical Laboratory Shift Tables From Adam Data: Rao Bingi, Octagon Research Solutions, Wayne, PADocument12 pagesProducing Clinical Laboratory Shift Tables From Adam Data: Rao Bingi, Octagon Research Solutions, Wayne, PAlenithNo ratings yet
- The Human Medicines Regulations 2012Document322 pagesThe Human Medicines Regulations 2012Fuzzy_Wood_PersonNo ratings yet
- 2017 Fish Market RFP-Final-12.1.17Document24 pages2017 Fish Market RFP-Final-12.1.17Salman DigaleNo ratings yet
- Job Description: Associate EDC Programmer / Custom Function Developer (Full Time-After Training and Evaluation) - GHANADocument2 pagesJob Description: Associate EDC Programmer / Custom Function Developer (Full Time-After Training and Evaluation) - GHANAJustice Kelvin Dzameshie KwadzoNo ratings yet
- Medicinal Cannabis The Evidence V1Document19 pagesMedicinal Cannabis The Evidence V1danisterNo ratings yet
- Zemaira Prescribing InformationDocument6 pagesZemaira Prescribing InformationArun ChadhaNo ratings yet
- Vitamin C and Cancer - Medicine or Politic - Richards, EvelleenDocument296 pagesVitamin C and Cancer - Medicine or Politic - Richards, EvelleenAnonymous gwFqQcnaX100% (1)
- Draft Conditional Use Permit For South Salt Lake Homeless Resource CenterDocument33 pagesDraft Conditional Use Permit For South Salt Lake Homeless Resource CenterThe Salt Lake TribuneNo ratings yet
- IMSH Institute Global Oncology Trend 2015 2020 ReportDocument45 pagesIMSH Institute Global Oncology Trend 2015 2020 ReportRishabh GuptaNo ratings yet
- Guidelines For Importation and Exportation of Pharmaceutical Products in TanzaniaDocument23 pagesGuidelines For Importation and Exportation of Pharmaceutical Products in TanzaniaRajesh KatareNo ratings yet
- C. Nokes D.A.P. Bundy (1994) - Does Helminth Infection Affect Mental Processing and Educational Achievement. 10 (1), 14-18.Document5 pagesC. Nokes D.A.P. Bundy (1994) - Does Helminth Infection Affect Mental Processing and Educational Achievement. 10 (1), 14-18.Maj ImanNo ratings yet
- IFPMA Code of Practice 2012Document24 pagesIFPMA Code of Practice 2012corruptioncurrentsNo ratings yet
- Journal ObstetriDocument4 pagesJournal ObstetriDhiyah HarahapNo ratings yet
- 1 s2.0 S2352873718300052 MainDocument22 pages1 s2.0 S2352873718300052 MainDewi WulandariNo ratings yet
- Handout For Topic 1Document10 pagesHandout For Topic 1Ralph Kenneth Nastor RamosNo ratings yet
- Maiga Ayub Hussein: The African Forum For Research and Education in Health (Afrehealth)Document7 pagesMaiga Ayub Hussein: The African Forum For Research and Education in Health (Afrehealth)Maiga Ayub HusseinNo ratings yet
- Ich - Pediatric ExtrapolationDocument44 pagesIch - Pediatric ExtrapolationRizqi Dinni FauziaNo ratings yet
- Evaluation of Aromatherapy With Lavender Oil On Academic Stress - A Randomized Placebo Controlled Clinical TrialDocument9 pagesEvaluation of Aromatherapy With Lavender Oil On Academic Stress - A Randomized Placebo Controlled Clinical TrialigorfragaNo ratings yet
- Research FlowchartDocument22 pagesResearch FlowchartMuhammad ZafranNo ratings yet