Professional Documents
Culture Documents
When Signaling Kinases Meet Histones
When Signaling Kinases Meet Histones
Uploaded by
Dani PazOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
When Signaling Kinases Meet Histones
When Signaling Kinases Meet Histones
Uploaded by
Dani PazCopyright:
Available Formats
Molecular Cell
Review
When Signaling Kinases Meet Histones
and Histone Modifiers in the Nucleus
Sung Hee Baek1,*
1Department of Biological Sciences, Creative Research Initiative Center for Chromatin Dynamics, Seoul National University,
Seoul 151-742, South Korea
*Correspondence: sbaek@snu.ac.kr
DOI 10.1016/j.molcel.2011.03.022
Signaling pathways involve cascades of protein phosphorylation and ultimately affect regulation of transcrip-
tion in the nucleus. However, most of the kinases in these pathways have not been generally considered to
directly modulate transcription thus far. Here, recent significant progress in the field elucidating direct modi-
fications of histones and histone modifiers by upstream kinases is summarized, and future directions are dis-
cussed.
Introduction mammals. The classical histone H3 mitotic kinases functioning in
Histones are subject to diverse posttranslational modifications cell cycle, recently identified kinases and phosphorylation of
including phosphorylation, acetylation, methylation, ubiquitina- histone variants will be discussed briefly in this review; however,
tion, SUMOylation, ADP ribosylation, deimination, and proline other excellent reviews on histone H3 (Cheung et al., 2000;
isomerization (Bhaumik et al., 2007; Campos and Reinberg, Nowak and Corces, 2004) or histone variants (Elsaesser et al.,
2009; Chi et al., 2010; Jenuwein and Allis, 2001; Kouzarides, 2010; Hake and Allis, 2006; Sarma and Reinberg, 2005; Talbert
2007; Shilatifard, 2006). These diverse histone modifications and Henikoff, 2010) are available for further details. Considering
can modulate the activity of transcription factors, nucleosome re- the recent rapid progress in this field and its significant influence
modelers, histone chaperones, and other histone modifiers by in understanding the function of phosphorylation within the
altering the chromatin state for either activation or repression nucleus, it is a great time to summarize current status of the field
(Berger, 2007; Ho and Crabtree, 2010; Kubicek and Jenuwein, and discuss future directions.
2004; Lee et al., 2010; Ruthenburg et al., 2007; Suganuma and
Workman, 2008; Weake and Workman, 2010). The identification Upstream Kinases and Histone Substrates Modified
of the enzymes that directly modify histones has been during Transcription and Apoptosis: H2BS14/S36
intense focus over the last 15 years. Most of these and H3T6/S10/T11/S28/Y41/T45
modifications are dynamic, and the corresponding enzymes in- In comparison to other histone modifications, phosphorylation of
clude kinases/phosphatases, acetyltransferases/deacetylases, histones requires specific activation of upstream signaling path-
methyltransferases/demethylases, ubiquitin ligases/deubiquiti- ways (Hans and Dimitrov, 2001; Nowak and Corces, 2000, 2004;
nating enzymes, and SUMO ligases/deSUMOylating enzymes Vermeulen et al., 2009; Wyrick and Parra, 2009). Although these
(Berger, 2007; Bhaumik et al., 2007; Klose and Zhang, 2007; upstream kinases in the signaling pathways regulate transcrip-
Kouzarides, 2007; Mosammaparast and Shi, 2010; Sims and tion via activation of phosphorylation-dependent signal trans-
Reinberg, 2008; Weake and Workman, 2008; Yang and Seto, duction cascades, the central kinases are not considered to
2008). Among the enzymes that modify histones, kinases mainly directly modify histones or histone modifiers in the nucleus
depend on the activation of specific upstream signaling path- due to their major cytoplasmic localization. However, accumu-
ways leading to cascades of protein phosphorylation and regula- lating evidence has shown that the upstream kinases have
tion of transcription in the nucleus. Recently, significant progress a more profound effect on gene expression through direct phos-
in understanding the roles of this particular type of modification phorylation of the chromatin. On the basis of a genome-wide
has been made through the elucidation of mechanisms by which chromatin immunoprecipitation (ChIP)-on-chip analysis of the
gene expression is directly affected through specific kinase- yeast mitogen-activated protein kinases (MAPKs), it was pre-
dependent phosphorylation of histones (Bungard et al., 2010; dicted that the activated signal transduction kinases may
Cerutti and Casas-Mollano, 2009; Dawson et al., 2009; Metzger frequently occupy target genes by binding to transcription
et al., 2010; Pérez-Cadahı́a et al., 2009). Furthermore, accumu- factors and coregulators in the nucleus (Edmunds and Mahade-
lating evidence suggests that some kinases that modify histones van, 2004; Pokholok et al., 2006). Recently, direct colocalization
can also modify nonhistone substrates including chromatin of estrogen receptor a (ERa) and extracellular signal-regulated
remodeling factors and transcription factors (Cha et al., 2005; kinase 2 (ERK2) for regulation of target genes across the genome
Fischle et al., 2003; Huang and Chen, 2005; Huang et al., 2007; has been reported (Madak-Erdogan et al., 2011).
Lee et al., 2010; Lehtinen et al., 2006). The upstream kinases that directly modify histones are
This review will discuss some of the recent headway that has summarized (Table 1) (Anest et al., 2003; Bungard et al., 2010;
been made in understanding the role of upstream kinase-depen- Cerutti and Casas-Mollano, 2009; Cheung et al., 2003; Choi
dent phosphorylation of the histones and nonhistone proteins in et al., 2005; Dawson et al., 2009; Dyson et al., 2005; Hurd
274 Molecular Cell 42, May 6, 2011 ª2011 Elsevier Inc.
Molecular Cell
Review
Table 1. Kinases that Modify Histones and/or Histone Modifiers
Substrates Modified
Kinases that Modify Histones Transcription Factors and Functions/Downstream
and/or Histone Modifiers Histones Histone Modifiers Consequences
Haspin H3T3 n/a Chromatin Condensation (H)
VRK1 H3T3, H3S10 CREB-S133, p53-T18 Chromatin Condensation (H)/Txn Activation (TF)
PKCa H3T6 RORa-S35 Txn Activation (H)/Repression (TF)
PKCb H3T6 p65-S276/S536 Txn Activation (H/TF)
PIM1 H3S10 p21-T145 Txn Activation (H)/Apoptosis (TF)
IKKa H3S10 CBP-S1382 Txn Activation (H/TF)
Rsk2 H3S10 IkBa-S32, p53-S15 Txn Activation (H/TF)
PKB/Akt H3S10 EZH2-S21, p300-S1834 Txn Activation (H/TF)
Aurora B H3S10, H3S28 MYBBP1A-S1303 Chromatin Condensation (H/TF)
MSK1/2 H3S10, H3S28 CREB-S133, p65-S276, STAT3-S727 Txn Activation (H/TF)
JNK1 H3S28 SIRT1-S27/S47/T530 Chromatin Condensation (H)/Txn Repression (TF)
MLTKa H3S28 n/a Chromatin Condensation (H)
PRK1 H3T11 HDAC5-T292 Txn Activation (H/TF)
Chk1 H3T11 BLM-S646, p53-S313/S314/T377/S378 Txn Activation (H/TF)
Dlk/ZIP H3T11 Par-T155 Chromatin Condensation (H)/Apoptosis (TF)
PKCd H3T45 TBLR1-S123, p53-S46 Apoptosis (H)/Txn Activation (TF)
MST1 H2BS14 FOXO3-S207 Apoptosis (H/TF)
AMPK H2BS36 ChREBP-S568, p53-S15 Txn Activation (H)/ Txn Repression (TF)
JAK2 H3Y41 STAT5-Y694 Txn Activation (H/TF)
Abl n/a SRC3-Y1357 Txn Activation (TF)
BMK1 n/a PML-S403/T409 Txn Repression (TF)
CaMK n/a HDAC5-S259/S498, CBP-S301 Txn Activation (TF)
S6K1 n/a Mdm2-S163, SREBP1/2 Txn Activation (TF)
SIK1 n/a HDAC5-S259, TORC2-S171 Txn Activation (TF)
PKC, protein kinase C; PIM, proto-oncogene serine/threonine-protein kinase; IKK, IkB kinase; Rsk, p90 ribosomal S6 protein kinase; PKB, protein
kinase B; MSK, mitogen- and stress-activated protein kinase; JNK, c-Jun N-terminal protein kinase; MLTK, mixed lineage kinase-like mitogen-acti-
vated protein triple kinase; PRK, protein-kinase-C-related kinase; Chk, checkpoint kinase; AMPK, AMP-activated protein kinase; MST, mammalian
sterile twenty kinase; JAK, Janus kinase; Txn, transcription; H, histones; TF, transcription factors.
et al., 2009; Metzger et al., 2008, 2010; Pérez-Cadahı́a et al., H2BS14 phosphorylation is selectively associated with the
2009; Sassone-Corsi et al., 1999; Shimada et al., 2008; Wang apoptotic chromatin condensation. In contrast to the potentially
et al., 2002; Yamamoto et al., 2003; Zhong et al., 2001; Zippo deadly phosphorylation on S14, the phosphorylation of S36 by
et al., 2007). Some of these histone kinases also act on transcrip- AMPK is connected to a prosurvival pathway. AMPK is activated
tion factors and histone modifiers. Therefore, the unexpected by high AMP levels under conditions of energetic stress, and
recent findings shed light on this exciting link between upstream activated AMPK triggers a program of metabolic adaptation to
signaling kinases and direct phosphorylation of histones and/or conserve ATP and maintain cellular viability (Cantó and Auwerx,
histone modifiers. 2010). AMPK has been shown to activate transcription through
H2B Ser Phosphorylation direct association with chromatin both in stress-responsive
There are two serine phosphorylation sites in the tail of histone target gene promoters and transcribed regions by direct phos-
H2B in mammals (Figure 1A). Two of the kinases that can phorylation of H2BS36, revealing the first AMPK-dependent
phosphorylate H2B are MST1 and AMPK, which are responsible substrate in the nucleus (Bungard et al., 2010). Phosphorylation
for S14 and S36 phosphorylation, respectively. MST1 is of H2BS36 by AMPK may fine-tune specific transcriptional
a member of the sterile 20-like superfamily that is regarded as responses and be essential for survival in response to metabolic
upstream regulator of MAPK pathways with functions in cellular stress.
morphogenesis and apoptosis (Dan et al., 2001). Upon cleavage Given that using ChIP provided evidence for direct association
by caspase-3, MST1 directly phosphorylates H2BS14, suggest- of AMPK and H2BS36 phosphorylation both in promoters and
ing that this phosphorylation might be associated with the onset transcribed regions, it can be applied in search of other upstream
of apoptosis (Cheung et al., 2003; Ling et al., 2008). Considering kinases that target histones and/or nonhistones. Indeed, histone
that both DNA fragmentation and chromatin condensation are H2B tail contains a variety of potential phosphorylation sites,
hallmark features of apoptosis, it is worth emphasizing that including four serine and two threonine residues, compared to
Molecular Cell 42, May 6, 2011 ª2011 Elsevier Inc. 275
Molecular Cell
Review
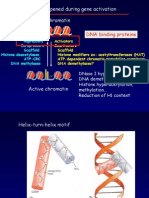
Figure 1. Kinases that Modify Histones H2B
and H3 and Residues Modified
(A) H2B phosphorylation functions in transcription
and apoptosis. MST1 and AMPK phosphorylate
H2BS14 and H2BS36 in mammals, respectively.
(B) H3 phosphorylation functions both in chro-
mosome condensation and in transcription.
Haspin, VRK1, Aurora B, and Dlk/ZIP are involved
in chromosome condensation in mitosis and
meiosis, whereas other kinases are involved in
transcriptional activation during interphase and
apoptosis in mammalian cells.
transcription or apoptosis occurs on
H3T45 in PKCd-dependent manner. This
modification on H3T45 is detected from
untreated neutrophils at basal level, but
robustly induced in the latter phases of
apoptosis, suggesting a potential link
between H3T45 phosphorylation and
apoptosis (Hurd et al., 2009). Consis-
tently, the induction of H3T45 phosphory-
lation is paralleled with caspase-3
activation, and it is increased in apoptotic
histone H2A which has two serine and one threonine residues or domain containing protein, JMJD2C, leading to the transcrip-
histone H4 which contains only one serine residue as a targeting tional activation of AR-dependent target genes. Although both
site. Considering the potential diversity of phosphorylation sites PKCb-dependent H3T6 phosphorylation and PRK1-dependent
on histone H2B, it seems that future work could yield new H3T11 phosphorylation are novel histone phosphorylation
functional connections between this histone and other signaling marks, an open question remains as to why their mode of action
pathways. appears to be limited to AR-dependent transcriptional units.
H3 Ser/Thr Phosphorylation Thus, it will be a challenging task to conduct ChIP-sequencing
There are six serine/threonine phosphorylation sites (H3T3/T6/ or ChIP-chip analysis using good quality of antibodies to search
S10/T11/S28/T45) modified by serine/threonine kinases and for other transcriptional units that are regulated by PKCb and
one site (H3Y41) phosphorylated by tyrosine kinase in the tail PRK1 as well as other H3T6 and H3T11 histone kinases depend-
of histone H3 in mammals (Figure 1B). H3T6 phosphorylation ing on cellular context. On the other hand, it will be of interest to
was newly identified to be modified by PKCb in prostate cancer find other nonhistone substrates phosphorylated by PKCb or
cells (Metzger et al., 2010). During androgen receptor (AR)-acti- PRK1. For this, mass spectrometry would be a very useful tool
vated gene expression, lysine-specific histone demethylase 1 to probe the modified resides.
(LSD1) removes mono- and dimethyl marks from H3K9 for tran- Chk1 has the same histone H3 substrate specificity as PRK1,
scriptional activation. LSD1 functions as both corepressor and H3T11 phosphorylation, but the upstream activation signal and
coactivator by demethylating mono- and dimethyl marks from mechanism of action do not overlap with PRK1. Under unper-
H3K4 and H3K9, respectively, yet it was unclear how this dual turbed conditions, Chk1 associates with chromatin and phos-
specificity might be regulated. PKCb-dependent H3T6 phos- phorylates H3T11. Phosphorylation of H3T11 results in
phorylation turned out to be the key step in preventing LSD1 enhanced recruitment of GCN5 histone acetyltransferase to
from demethylating H3K4 and in promoting demethylation of targets, perhaps best exemplified by the cyclin B1 and cdk1
H3K9 exclusively during AR-dependent gene activation, promoters, leading to the acetylation of H3K9 and H3K14
providing an intriguing example of crosstalk between the phos- (Shimada et al., 2008). Upon DNA damage, Chk1 releases from
phorylation state of H3T6 and methylation of H3K4 and H3K9. the promoters leading to dephosphorylation of H3T11, and the
Perhaps this case provides potentially plausible explanation decreased H3T11 phosphorylation leads to transcriptional
why the same modification by different kinases or the different repression due to the impaired GCN5 recruitment to its target
modifications by the same kinase might be associated with promoters. Therefore, Chk1 functions as a histone kinase
different outcomes. A more systemic approach searching for responsible for the regulation of DNA damage-induced tran-
the histone and nonhistone substrates of a particular kinase scriptional repression by inducing diminished histone acetyla-
would be valuable. In parallel, phosphorylation of H3T11 by tion. Future works will further clarify whether there is concomi-
PRK1 establishes another novel chromatin mark during tant recruitment of HDACs for transcriptional repression upon
AR-dependent transcriptional activation (Metzger et al., 2008). release of GCN5 from target promoters. H3T11-specific phos-
Phosphorylation of H3T11 by PRK1 accelerates removal of phatases might exist for counteracting function of Chk1. Finally,
repressive methyl marks from H3K9 by the Jumonji C (JmjC)- the remaining threonine phosphorylation of histone H3 during
276 Molecular Cell 42, May 6, 2011 ª2011 Elsevier Inc.
Molecular Cell
Review
cells around the time of DNA nicking. Given that the position of nucleus. JAK2 has a previously unrecognized nuclear pool in
H3T45 lies in a structurally important region involved in nucleo- hematopoietic cells (Dawson et al., 2009). Upon phosphorylation
some structure and stability (Hurd et al., 2009), it is highly of H3Y41 by JAK2, HP1a binding to the chromatin is significantly
possible that H3T45 phosphorylation facilitates activation of decreased, leading to the transcriptional activation of JAK2-
apoptotic program by inducing structural changes of nucleo- regulated genes, including the hematopoietic oncogene lmo2.
some. Further biochemical studies will be needed to test this HP1a has been shown to function as a potential tumor
idea. suppressor. Therefore, it is conceivable that the consequences
Serine phosphorylation on histone H3 includes S10 and S28 of constitutive JAK2 activation (JAK2V617F) identified in human
residues. H3S10 and H3S28 phosphorylation have a dual role myeloproliferative diseases that are exemplified by the
both in interphase where chromatin needs to be decondensed increased genetic instability are consistent with the reversal of
for transcriptional activation of genes, and in mitosis where chro- the HP1a functions. The JAK2-dependent displacement of
matin condensation is necessary. As to the upstream kinases HP1a might be tightly regulated in normal cells, whereas in
responsible for H3S10 and H3S28 phosphorylation in inter- malignancies, uncontrolled displacement of HP1a may override
phase, a variety of H3S10 kinases including PIM1, IKKa, its potential tumor suppressive functions. The HP1a recruitment
MSK1/2, PKB/Akt, and Rsk2, and for H3S28 phosphorylation mechanism has been shown to be widely used in JAK2 signaling.
kinases including MSK1/2, JNK1, and MLTKa, have been JAK2 turned out to regulate canonical JAK2-STAT target genes
reported (Anest et al., 2003; Bungard et al., 2010; Cerutti and as well as noncanonical target genes such as lmo2, confirming
Casas-Mollano, 2009; Cheung et al., 2003; Choi et al., 2005; the importance of this chromatin pathway in cancer (Rui et al.,
Dawson et al., 2009; Dyson et al., 2005; Hurd et al., 2009; 2010). Since JAK2 also phosphorylates STAT5 at Y694 as
Metzger et al., 2008, 2010; Pérez-Cadahı́a et al., 2009; a nonhistone substrate (Gouilleux et al., 1994), it will be exciting
Sassone-Corsi et al., 1999; Shimada et al., 2008; Wang et al., to study how JAK2 signals pass through STAT5 and H3Y41 and
2002; Yamamoto et al., 2003; Zhong et al., 2001; Zippo et al., converge into the regulation of downstream target genes. Impor-
2007). Compared to the mitotic kinases, these kinases are tantly, JAK2-dependent phosphorylation of STAT5 at Y694 is
activated by upstream signals including cytokines, growth necessary for STAT5 DNA binding activity and phosphorylated
factors, and ultraviolet (UV), and activated kinases directly target STAT5 functions as a transcriptional activator. In parallel,
histones in the nucleus. IKKa-dependent H3S10 phosphoryla- JAK2-dependent phosphorylation of H3Y41 results in the activa-
tion is induced by cytokines and is critical for the activation of tion of JAK2-regulated genes, including lmo2, which lacked
NF-kB-directed gene expression (Yamamoto et al., 2003). a predicted STAT5 binding site. In both cases, the outcome is
Growth factor-dependent stimulation leads to the formation of transcriptional activation of target genes.
a PIM1-MYC complex, and PIM1-dependent phosphorylation Williams-Beuren syndrome transcription factor (WSTF) has
of H3S10 is required for MYC-dependent transcriptional activa- been shown to phosphorylate Tyr142 of H2AX, and its intrinsic
tion, leading to elevated oncogenic transformation ability (Zippo tyrosine kinase activity is required for foci formation during the
et al., 2007). UV irradiation is also responsible for inducing DNA damage response in mammalian cells (Xiao et al., 2009).
H3S28 phosphorylation by JNK1 (Zhong et al., 2001). Further- As a counterpart to WSTF, a protein tyrosine phosphatase EYA
more, JIL-1 histone H3S10 kinase reported in Drosophila is has been shown to promote DNA repair rather than apoptosis
interesting due to its distinct functions in interphase, although by executing a damage signal-dependent dephosphorylation
there appears no mammalian counterpart. JIL-1 kinase activity of Tyr142 of H2AX (Cook et al., 2009). It will be of particular
functions to mark euchromatic domains and counteract interest to investigate the functional link between kinases and
heterochromatinization by Su(var)3-9-mediated histone H3K9 phosphatases and their potential interplay for the regulation of
dimethylation and heterochromatin protein 1 (HP1) recruitment targets for DNA repair and apoptosis in the cellular context.
(Zhang et al., 2006). Su(var)3-9 turns out to be a substrate phos-
phorylated by JIL-1 kinase, and the kinase activity of JIL-1 is Upstream Kinases and Histone Substrates Modified
required for the maintenance of chromatin structure in during Chromosome Condensation: H3T3/S10/T11/S28
Drosophila (Boeke et al., 2010; Wang et al., 2001). Considering Although recent studies on the specific upstream kinase-depen-
the diverse functions and sites identified for H3 Ser/Thr phos- dent direct phosphorylation of histones allowed significant
phorylation thus far in interphase, it will be of particular interest progress in this field, phosphorylation of H3 initially gained
to understand the specific mechanism regulating the dynamic considerable interest when this modification was discovered to
phosphorylation and dephosphorylation of histones in be linked to chromosome condensation and segregation during
promoter-, cell- and signal-dependent manner, in addition to mitosis and meiosis (Cheung et al., 2000; Nowak and Corces,
understanding how these phosphorylation events can ultimately 2004). Phosphorylation of H3S10 is considered to be a crucial
results in different outcomes in different contexts. event for the onset of mitosis, because this modification appears
Tyr Phosphorylation early in the G2 phase within pericentromeric heterochromatin
The first tyrosine phosphorylation site in the tail of core histone and spreads coincident with mitotic chromosome condensation
H3 identifies Y41 and the corresponding kinase turns out to be by metaphase. Aurora B, Haspin, vaccinia-related kinase 1
JAK2 (Dawson et al., 2009). Given that JAK2 is a nonreceptor (VRK1), and death-associated protein-like kinase (Dlk)/zipper-
tyrosine kinase that modulates diverse cellular process by interacting protein (ZIP) have been shown to play a role in chro-
inducing cytoplasmic signaling cascades, it was striking to mosome condensation during mitosis (Crosio et al., 2002; Dai
discover the phosphorylation of histone H3Y41 by JAK2 in the et al., 2005; Kang et al., 2007; Preuss et al., 2003), whereas
Molecular Cell 42, May 6, 2011 ª2011 Elsevier Inc. 277
Molecular Cell
Review
most of the other kinases have been shown to regulate transcrip- H3 phosphorylation sites in addition to T3, S10, T11, and S28
tion or apoptosis. residues co-occupied with corresponding new kinases and
Aurora kinases are a highly conserved family of serine/threo- phosphatases in the future. Perhaps mass spectrometry and
nine kinases consisting of Aurora A, B, and C, and Aurora B is ChIP sequencing approaches will be useful techniques along
a well-known primary mitotic H3S10 and S28 kinase (Carmena with highly sensitive imaging techniques to address the issues.
et al., 2009; Crosio et al., 2002; Keen and Taylor, 2009; Kelly
and Funabiki, 2009; Vader and Lens, 2008). The Haspin Nonhistone Substrates Modified: Transcription Factors
phosphorylates H3T3 during mitosis and meiosis and is required and Histone Modifiers
for normal metaphase chromosome alignment (Dai et al., 2005; As touched on above, the enzymes that modify histones can also
Higgins, 2010). Recently, Haspin was shown to position Aurora modify nonhistone proteins, including chromatin remodeling
B at centromeres in order to regulate selected targets of Aurora factors and transcription factors/coregulators. Like histone
B during mitosis (Wang et al., 2010). Compared to Aurora B modification, it is important to determine whether there is and
having specificity on H3S10 and H3S28 phosphorylation, chro- to decipher any potential modification code of nonhistone
matin-localized VRK1 has been shown to be responsible for substrates in consideration of specific signaling pathways and
H3T3 and H3S10 phosphorylation depending on the cell-cycle cellular contexts. Some kinases exemplified by PKCs, PIM1,
phase (Kang et al., 2007). Indeed, VRK1 being a mitotic histone IKKa, Rsk2, PKB/Akt, MSK1/2, JNK1, PRK1, Chk1, Dlk/ZIP,
H3 kinase is differentially expressed during cell cycle progres- MST1, AMPK, and JAK2 that modify histones can also modify
sion and its level peaks in the G2/M phase. Dlk/ZIP phosphory- nonhistone substrates (Cha et al., 2005; Fischle et al., 2003;
lates histone H3T11 rather than H3S10, which is characteristic of Huang and Chen, 2005; Huang et al., 2007; Lee et al., 2010;
mitotic chromosome, and this H3T11 phosphorylation is Lehtinen et al., 2006). In these cases, we should be careful in
discernable from prophase to early anaphase and is particularly analyzing as the signal-dependent responses may be going
enriched at centromeres (Preuss et al., 2003). Therefore, Dlk/ZIP through unsuspected nonhistone substrates. It has been
has been shown to function as a centromere-specific histone suggested that there exist histone-like modification cassettes
kinase for subsequent mitotic processes. Although H3T11 phos- or histone mimics as a distinct class of nonhistone targets that
phorylation shows correlation with the level of chromosome might extend the principles of the proposed histone code to
condensation, the functional consequence of H3T11 phosphor- the regulation of nonhistone proteins (Boosen et al., 2009;
ylation during mitosis and meiosis is not clear and requires Fischle et al., 2003; Huang et al., 2007; Kaur et al., 2010; Peng
further investigation. et al., 2010; Takemori et al., 2009).
The histone H3 family contains three main classes of histone H3 Ser/Thr Kinases with Nonhistone Substrates
H3 genes: the canonical, replication-dependent histone H3, The Ser/Thr PKC family comprises about 2% of the kinases in
the replication-independent histone variant H3.3, and the human. Although 12 isoforms of PKC exist in mammals,
centromeric H3 variant CENP-A (Elsaesser et al., 2010; Hake increasing evidence supports individual and nonredundant roles
and Allis, 2006; Sarma and Reinberg, 2005; Talbert and Henikoff, within the members of this family (Rosse et al., 2010). It is evident
2010). In contrast to H3S10 and H3S28, which are phosphory- in the case of different PKC family members targeting different
lated in prophase, H3.3S31 phosphorylation is observed only sites on histone H3: PKCa and PKCb phosphorylate H3T6,
in late prometaphase and metaphase stages of mitosis, suggest- whereas PKCd phosphorylates H3T45. Recently, orphan nuclear
ing that H3.3S31 phosphorylation is a mitosis-specific event receptor RORa turned out to be a nonhistone substrate phos-
(Hake et al., 2005). CENP-A substitutes for histone H3 in the phorylated by PKCa in the nucleus, and the consequence of
nucleosome core of centromeric chromatin at the inner plate of RORa phosphorylation is the inhibition of canonical Wnt/b-cate-
the kinetochore, and it implicates an important function in nin target genes in colon cancer (Lee et al., 2010). A significant
kinetochore assembly. Prevention of CENP-A phosphorylation inverse correlation between reduction of RORa phosphorylation
on S7 in prophase leads to chromosome misalignment during and PKCa activation in human colorectal tissues provides the
mitosis as a result of a defect in kinetochore attachment to clinical relevance of the findings. Another PKC isoform, PKCd
microtubules (Kunitoku et al., 2003; Sullivan et al., 1994; Yoda has a nonhistone substrate transducer b-like-related protein 1
et al., 2000). (TBLR1) in addition to histone H3T45. TBLR1 phosphorylation
It can be postulated that the phosphorylation status of H3 is at S123 by PKCd is required for overcoming C-terminal binding
dynamically regulated by opposing actions of specific kinases protein (CtBP)- and nuclear receptor corepressor (NCoR)-
and phosphatases during mitosis. Compared to the known dependent transcriptional repression checkpoint (Perissi et al.,
mitotic H3 kinases, finding the counteracting phosphatases is 2008). Considering a large number of PKC family members
an emerging area of investigation. It has been shown that Aurora and the importance as key transducers in signaling networks, it
B binds to a type 1 protein phosphatase (PP1) for spatiotemporal is conceivable that many more nonhistone targets as well as
regulation of H3 phosphorylation during mitosis and is activated new histone substrates could be uncovered in the future.
by the PP1/PP2A inhibitor okadaic acid (Sugiyama et al., 2002). IKKa phosphorylates nonhistone substrate such as CREB
In mammals, four isoforms of catalytic subunit of PP1 have binding protein (CBP) at serine 1382 and 1386 as well as histone
distinct subcellular localizations. For example, during mitosis, H3S10, and phosphorylation of CBP by IKKa is responsible for
PP1a is localized to the centrosome, while PP1g associates accelerating cell growth by switching the binding preference of
with the microtubules of the mitotic spindle, and PP1d associ- CBP from p53 to NF-kB (Huang et al., 2007). CBP is also phos-
ates with chromosome. It will be challenging to search for novel phorylated by Ca2+/calmodulin-dependent protein kinase IV
278 Molecular Cell 42, May 6, 2011 ª2011 Elsevier Inc.
Molecular Cell
Review
(CaMKIV) at S301 leading to the CREB/CBP-dependent tran- that most MAPKs turn out to phosphorylate histone residues
scriptional activation in hippocampal neurons (Impey et al., directly in the nucleus, it is worthwhile to determine whether
2002). Although the transcriptional activation function of CBP there are other histone residues that are directly modified by
and p300 is overlapping, CaMKIV-mediated phosphorylation this family of kinases. SIK1 was reported to be a class II HDAC
appears to be CBP-specific. PKB/Akt is also responsible for kinase that conducts an important role in the SIK1-HDAC
phosphorylating p300 at S1884, which enhances its enzymatic pathway by promoting survival of skeletal myocytes (Berdeaux
activity (Huang and Chen, 2005). et al., 2007). SIK1 regulates activity of myocyte enhancer factor
In many cases, the functional consequence of phosphoryla- 2 (MEF2) by phosphorylating HDAC5 at S259. Recently, BMK1
tion of histone-modifying enzymes is the modulation of their was shown to interact with promyelocytic leukemia protein
enzymatic activity either positively, such as CBP and p300, as (PML) and suppress its tumor suppressor function through phos-
mentioned above, or negatively. In other cases, phosphorylation phorylation at S403 and T409, emphasizing the importance of
of histone modifiers regulates protein stability functioning as PML phosphorylation in tumor growth (Yang et al., 2010). With
a ‘‘phosphodegron’’ by inducing ubiquitin/26S proteasome- regard to the kinase-dependent tumor-related regulation, S6K1
dependent degradation process. One example is the case of is a good example for a multifaceted regulator of Mdm2 and
steroid receptor coactivator-3 (SRC-3) where a phosphodegron p53. Under genotoxic stress, S6K1 phosphorylates Mdm2 on
is crucial for both SRC-3 stability and coactivator function S163 and inhibits Mdm2-mediated p53 ubiquitination and
(Li et al., 2008). Further, it is conceivable that phosphorylation degradation (Lai et al., 2010). The Abl tyrosine kinase phosphor-
of histone modifiers can regulate protein-protein interaction by ylates SRC-3 at Y1357, which is required for the enhanced tran-
affecting recruitment of binding modules to target proteins scriptional activity of SRC-3 in cancer cells (Oh et al., 2008).
depending on cellular context. PKB/Akt has a histone modifier, Tyrosine phosphorylation of SRC-3 allows crosstalk between
enhancer of zeste homolog 2 (EZH2) as a nonhistone substrate. hormone and growth factor-related kinase signaling pathways
PKB/Akt-mediated phosphorylation of EZH2 at serine 21 has in cancer. It is tempting to speculate that the Abl tyrosine kinase
a negative effect on the methyltransferase activity of EZH2 by might use its Src homology domain 2 (SH2) to confer substrate
impeding binding of EZH2 to histone H3, which results in specificity which recognize tyrosine phosphorylated residues
a decrease of H3K27 trimethylation and derepression of silenced as well as autoregulation of kinase activity.
target genes (Cha et al., 2005). Although cyclin-dependent Many of the nonhistone substrates can be modified by the
kinase 1 (CDK1) is not categorized into H3 Ser/Thr kinases same histone-modifying enzymes. Thus, it is conceivable that
discussed here, it is notable that EZH2 is also phosphorylated modification of other substrates by the same enzyme might
by CDK1 at multiple sites and each phosphorylation confers provide other unidentified regulatory signaling pathways or
different functions. CDK1-dependent phosphorylation of EZH2 redundant signaling pathways where the biological responses
at T350 leads to the release of EZH2 from the other PRC2 may be going through or in parallel with those of histones. For
components and thereby inhibits EZH2 enzymatic activity example, JAK2-dependent phosphorylation of H3Y41 results in
(Chen et al., 2010), while phosphorylation at T487 is involved in the exclusion of HP1a from the promoter and JAK2-dependent
suppression of methylation of H3K27 and promotion of osteo- phosphorylation of STAT5 is necessary for STAT5 DNA binding
genic differentiation of human mesenchymal stem cells activity, leading to the activation of JAK2 target genes in both
(Wei et al., 2011). Further, a domain in EZH2 comprising T345 cases. Furthermore, we should keep in mind that modifications
phosphorylated by CDK1 is important for the interaction with such as phosphorylation are very dynamic and rapidly changing
HOTAIR and the 50 end of Xist (Kaneko et al., 2010). In a similar within cells, suggesting that not only signaling kinases but also
case in which enzymatic activity is modulated by phosphoryla- corresponding phosphatases will conduct a balancing role for
tion, JNK1 leads to the phosphorylation of silent mating type timely regulated programs.
information regulation 2 homolog 1 (SIRT1) histone deacetylase
at S27, S47, and T530 and the phosphorylated SIRT1 exhibits How Histone Phosphorylation Is ‘‘Read’’:
increased enzymatic activity (Nasrin et al., 2009). The SIRT1 Phosphoprotein Binding Domains
phosphorylation by oxidative stress-activated JNK1 provides Phosphorylated histone tails may act as an integrating platform
a mechanistic insight on the regulation of the enzymatic activity to receive information from upstream signals and transmit it to
of SIRT1 by oxidative stress. Although detailed molecular mech- the downstream effectors. An important aspect of signaling
anisms of how phosphorylation can enhance or inhibit enzymatic through histone phosphorylation is the presence of ‘‘reader’’
activity have not been investigated extensively, we can specu- molecules that recognize phosphorylated residues by specific
late that phosphorylation affects nucleosome accessibility, domains and thus create an attractive deciphering mechanism
protein-protein interactions, and altered nuclear localization. to respond to alterations within cells. Proteins bind phosphory-
Kinases without Known Histone Substrates lated serines via specific phosphoprotein binding domain exem-
Although salt-inducible kinase 1 (SIK1), S6K1, Abl, CaMK, and plified by 14-3-3 domain (Taverna et al., 2007). The mammalian
big mitogen-activated protein kinase 1 (BMK1) have been shown 14-3-3 family of proteins have well-conserved phosphoserine-
to phosphorylate histone modifiers such as class II histone binding modules and are involved many signal transduction
deacetylases (HDACs) and SRC-3 possessing weak histone pathways, chromosome condensation, and apoptosis (Muslin
acetyltransferase activity, direct histone substrates have not et al., 1996; Yaffe et al., 1997). 14-3-3 proteins have been shown
yet been reported (Berdeaux et al., 2007; Lai et al., 2010; McKin- to form homo- and heterodimers, allowing interaction with at
sey et al., 2000; Oh et al., 2008; Yang et al., 2010). Considering least two phosphoserine sites, but engagement of two different
Molecular Cell 42, May 6, 2011 ª2011 Elsevier Inc. 279
Molecular Cell
Review
Figure 2. Crosstalk between Histone
Phosphorylation and Other Modifications
The negative effect of phosphorylation is depicted
by a dashed line, whereas the positive influence of
phosphorylation over another is depicted by an
arrow. PKCb-dependent phosphorylation of H3T6
increases H3K4 methylation but inhibits H3K9
methylation for transcriptional activation. PRK1-
dependent phosphorylation of H3T11 blocks
H3K9 methylation, and Chk1-dependent phos-
phorylation of H3T11 increases H3K14 acetylation
for transcriptional activation. MSK1/2-mediated
phosphorylation relieves H3K27methylation for
gene activation. An asterisk (*) indicates a variety of kinases responsible for H3S10 phosphorylation leading to increase in H3K4 methylation, decrease in H3K9
methylation, or increase in H3K14 acetylation in mammalian cells. These kinases include PIM1, IKKa, PKB/Akt, Rsk2, and MSK1/2.
histone tails at the same time has not yet been reported. While the interaction of the chromodomain of HP1 proteins with the
14-3-3 proteins target phosphoserine in the core histone H3K9me3 target site. At the end of mitosis, dephosphorylation
H3S10 context, BRCA1 carboxyl-terminal (BRCT) domains of of H3S10 by phosphatase dynamically establishes HP1 binding
DNA damage checkpoint protein 1 (MDC1) target phosphoserine to the stable H3K9me3 mark during the cell cycle. It appears that
in histone variant H2AX. What happens when DNA double- this binary switch hypothesis might apply to nonhistone proteins
strand breaks are induced and DNA repair machinery needs to and future work might yield insight into the potential generality of
be activated in the context of chromatin? Histone phosphoryla- this type of mechanism.
tion may assist in the recognition and accessibility of regions
where DNA repair needs to take place. Phosphorylation of Crosstalk between Histone Phosphorylation and Other
histone variant H2AX at S139 in mammalian cells occurs rapidly Modifications
by the phosphatidylinositol 3-kinase (PI3K)-like family of kinases, Histone phosphorylation controls many important cellular
which includes ATM and AT-related (ATR) and DNA-dependent processes, including transcription, apoptosis, DNA repair, and
protein kinase (DNA-PK) for DNA repair (Burma et al., 2001; chromosome condensation. Given that the H3S10 phosphoryla-
Rogakou et al., 1998). MDC1 localizes to sites of DNA breaks tion is critical during interphase because it confers transcrip-
and associates with checkpoint kinase 2 (Chk2) after DNA tional activation on a number of genes depending on upstream
damage responses (Lou et al., 2006). signaling pathways, the location of S10 residue in close prox-
Tyrosine phosphorylation is mainly involved in signaling imity to other modifiable residues in the H3 tail enables a variety
through transmembrane receptors that regulate cell proliferation of crosstalk between phosphorylation and other modifications
and differentiation. Readers that recognize phosphotyrosine that lead to synergistic or parallel effects on transcription. In
residues are distinct from those of phosphoserine residues, addition to S10, S28, T6, and T11 residues are located in close
and the SH2 and phosphotyrosine binding (PTB) domains are proximity to other modifiable residues of the histone H3 tail,
phosphotyrosine readers (Lim and Pawson, 2010). Given that which allow various crosstalks between phosphorylation and
certain tyrosine kinases and phosphatases possess phospho- other modifications such as methylation and acetylation
binding domains exemplified by the case of Abl kinase, it is (Figure 2). For gene activation, it is well established that H3S10
plausible that perhaps they use these domains directly to bind phosphorylation inhibits H3K9 methylation, facilitates H3K4
phosphorylated tyrosines in histones and nonhistone proteins methylation, and enhances H3K14 acetylation, which allows
for more elaborate and efficient substrate recognition, binding, further chromatin decondensation. A variety of H3S10 kinases
and regulation. Bioinformatics and systems biology as large- including Aurora B, VRK1, MSK1/2, PIM1, Rsk2, PKB/Akt, and
scale studies could be used to explore phospho-binding IKKa are involved in this crosstalk. Two cases demonstrating
domains within kinases and phosphatases. strong crosstalk between phosphorylation and methylation
The binary switch hypothesis predicts that phosphorylated during AR-dependent gene activation process in prostate
histones will prevent the binding of repressive complexes from cancer include the phosphorylation of H3T6 by PKCb and the
the methylated lysine residues or rather aggressively block phosphorylation of H3T11 by PRK1 (Metzger et al., 2008;
specific lysine methylation (Fischle et al., 2003). Histone 2010). Importantly, PKCb-dependent phosphorylation of H3T6
phosphorylation can potentially regulate the binding of bromo- prevents LSD1 from demethylating H3K4 and accelerates the
domain-containing factors to the acetyl-lysine residues or the demethylation of H3K9 by altering substrate specificity of LSD1.
binding of chromodomain-containing factors to the methyl- A more recently identified link between phosphorylation and
lysine residues to establish a combinatorial regulatory code in acetylation is the crosstalk between MSK1/2 and Chk1. Methyl-
many biological processes, including regulation of gene expres- ation of H3K27 by the polycomb repressive complex 2 (PRC2) is
sion, heterochromatin formation, and developmental pathways. well established as a repression mark during differentiation.
For example, a ‘‘binary methylation-phosphorylation switch’’ MSK1/2 preferentially phosphorylates H3S28 in the context of
mechanism can explain how HP1 proteins are released from H3K27me3-marked promoters, leading to the displacement of
mitotic chromatin (Fischle et al., 2003). At the onset of mitosis, polycomb group proteins for transcriptional activation, support-
Aurora B mediates the phosphorylation of H3S10 right next to ing the binary switch hypothesis (Gehani et al., 2010). With the
H3K9me3, the HP1 binding site, leading to the disruption of aid of antibodies against the H3K27me3S28ph double mark, it
280 Molecular Cell 42, May 6, 2011 ª2011 Elsevier Inc.
Molecular Cell
Review
was successful to investigate extensively the coexisting mecha- are replaced with the ones lacking a particular phosphorylation
nism of the K27me3 and S28ph on the same histone tail H3. by point mutation, will be extremely valuable in studying the
Generation of various antibodies against the double or triple physiological meaning of a particular phosphorylation. This
mark will be very helpful in addressing the interplay and crosstalk approach is applicable to other model organisms including
between these modifications. Another example of crosstalk can Drosophila, C. elegans, and zebrafish. Some phosphorylation
be seen from Chk1-mediated phosphorylation of H3T11 regu- sites are well conserved among species, so model organism
lating DNA damage-induced transcriptional repression of cell- studies with genetic approaches allow us to search for in vivo
cycle regulatory genes (Canton and Scott, 2010; Shimada physiological functions easily and rapidly. Considering that
et al., 2008). Loss of H3T11 phosphorylation correlates with many histone genes exist in mammalian genome, it is very
reduced binding of the GCN5 histone acetyltransferase following difficult to investigate each phosphorylation of histones resulting
reduced H3K9 and H3K14 acetylation. As to the crosstalk in a biological outcome. In this case, another model organism
between histone phosphorylation and other modifications, there such as yeast is possible. The chemical-genetic methods for
is still a lot to learn. developing a specific small-molecule inhibitor of single protein
While crosstalk between histone phosphorylation and other kinase will be particularly promising for addressing the function
modifications adds another layer of complexity and a dynamic of signaling kinase-dependent phosphorylation of histones and
view of modifications, it also raises a host of interesting and nonhistone proteins.
important questions. With multiple histone modifications It is exciting to predict that there might be specific potential
including phosphorylation present at the same time on the code for modification of nonhistone proteins as in the case of
N-terminal histone tails, what kind of interplay does it exist a proposed histone code for histones. It is possible that there
between each modification? Could these modifications function- is exciting unrevealed crosstalk between histone phosphoryla-
ally affect each other? Could they work in concert to achieve tion and nonhistone phosphorylation to decipher. Given that
a synergy? There is no doubt that phosphorylation can affect histone modifications have been implicated in a number of
other modifications nearby cooperatively or antagonistically. To epigenetic phenomena, it is conceivable that modification of
this end, it will be of great interest to monitor temporal and spatial nonhistone proteins such as transcription factors and histone
dynamics in crosstalk between phosphorylation and other modi- modifiers in the nucleus may be involved in a number of biolog-
fications using real-time imaging techniques in living cells. ically important epigenetic phenomena. Only time will tell us
more exciting stories.
Future Perspectives and New Insights
There is no doubt that signaling to chromatin is an exciting ACKNOWLEDGMENTS
research area that has already provided and will continue to
provide striking discoveries. In particular, the combinatorial I would like to apologize to all scientists whose work could not be cited due to
modification of histones and nonhistone proteins offers space limitation. I would like to thank members of the Chromatin Dynamics
Research Center, Keun Il Kim, and Jongkyeong Chung for critical readings,
numerous regulatory opportunities at the level of upstream discussions, or ideas and Eun Soon Hong for manuscript preparation. This
kinases and downstream signal transduction cascades, Creative Research Initiative work was supported by the National Research
including crosstalk. Histone phosphorylation, acting sequentially Foundation of Korea grant funded by the Korean government (Chromatin
Dynamics Research Center, 2009-0081563) to S.H.B.
or in concert with other modifications, allows initiation, propaga-
tion, termination, and fine-tuning of signal transduction
REFERENCES
cascades. Systems biology approaches will help understand
the interplay of each modification at the network levels. It is of Anest, V., Hanson, J.L., Cogswell, P.C., Steinbrecher, K.A., Strahl, B.D., and
particular interest that rapid development of genomics, epige- Baldwin, A.S. (2003). A nucleosomal function for IkappaB kinase-a in
nomics, and bioinformatics technologies allow us to investigate NF-kappaB-dependent gene expression. Nature 423, 659–663.
these fields at a global level. Moreover, by using sensitive detec- Berdeaux, R., Goebel, N., Banaszynski, L., Takemori, H., Wandless, T.,
tion techniques for phosphorylation, it should be possible to Shelton, G.D., and Montminy, M. (2007). SIK1 is a class II HDAC kinase that
promotes survival of skeletal myocytes. Nat. Med. 13, 597–603.
define new histone phosphorylation sites regulated by certain
kinases that have not been considered yet to act directly in the Berger, S.L. (2007). The complex language of chromatin regulation during tran-
nucleus and attribute unidentified functions to known histone scription. Nature 447, 407–412.
phosphorylation. Development of highly sensitive detection Bhaumik, S.R., Smith, E., and Shilatifard, A. (2007). Covalent modifications of
methods for phosphorylation with advanced technologies and histones during development and disease pathogenesis. Nat. Struct. Mol. Biol.
14, 1008–1016.
instruments would be central to answer a lot of the unanswered
questions. Boeke, J., Regnard, C., Cai, W., Johansen, J., Johansen, K.M., Becker, P.B.,
Major challenges for the future will be in the establishment of and Imhof, A. (2010). Phosphorylation of SU(VAR)3-9 by the chromosomal
kinase JIL-1. PLoS ONE 5, e10042.
biological relevance for phosphorylation of histone and nonhis-
tone proteins in vivo. This would entail dissection and decipher- Boosen, M., Vetterkind, S., Kubicek, J., Scheidtmann, K.H., Illenberger, S.,
and Preuss, U. (2009). Par-4 is an essential downstream target of DAP-like
ing of signaling pathways using in vivo model animals. In doing
kinase (Dlk) in Dlk/Par-4-mediated apoptosis. Mol. Biol. Cell 20, 4010–4020.
this, genetically engineered mice to analyze the physiological
functions of kinases and phosphatases involved in adding or Bungard, D., Fuerth, B.J., Zeng, P.Y., Faubert, B., Maas, N.L., Viollet, B.,
Carling, D., Thompson, C.B., Jones, R.G., and Berger, S.L. (2010). Signaling
removing phosphorylation will be valuable. Further, knockin kinase AMPK activates stress-promoted transcription via histone H2B phos-
mice, in which endogenous genes encoding nonhistone proteins phorylation. Science 329, 1201–1205.
Molecular Cell 42, May 6, 2011 ª2011 Elsevier Inc. 281
Molecular Cell
Review
Burma, S., Chen, B.P., Murphy, M., Kurimasa, A., and Chen, D.J. (2001). ATM Gehani, S.S., Agrawal-Singh, S., Dietrich, N., Christophersen, N.S., Helin, K.,
phosphorylates histone H2AX in response to DNA double-strand breaks. J. and Hansen, K. (2010). Polycomb group protein displacement and gene acti-
Biol. Chem. 276, 42462–42467. vation through MSK-dependent H3K27me3S28 phosphorylation. Mol. Cell 39,
886–900.
Campos, E.I., and Reinberg, D. (2009). Histones: annotating chromatin. Annu.
Rev. Genet. 43, 559–599. Gouilleux, F., Wakao, H., Mundt, M., and Groner, B. (1994). Prolactin induces
phosphorylation of Tyr694 of Stat5 (MGF), a prerequisite for DNA binding and
Cantó, C., and Auwerx, J. (2010). AMP-activated protein kinase and its down- induction of transcription. EMBO J. 13, 4361–4369.
stream transcriptional pathways. Cell. Mol. Life Sci. 67, 3407–3423.
Hake, S.B., and Allis, C.D. (2006). Histone H3 variants and their potential role in
Canton, D.A., and Scott, J.D. (2010). Chk-ing in and Chk-ing out: kinase indexing mammalian genomes: the ‘‘H3 barcode hypothesis’’. Proc. Natl.
compartmentalization comes to checkpoint control. Mol. Cell 40, 1–2. Acad. Sci. USA 103, 6428–6435.
Carmena, M., Ruchaud, S., and Earnshaw, W.C. (2009). Making the Auroras Hake, S.B., Garcia, B.A., Kauer, M., Baker, S.P., Shabanowitz, J., Hunt, D.F.,
glow: regulation of Aurora A and B kinase function by interacting proteins. and Allis, C.D. (2005). Serine 31 phosphorylation of histone variant H3.3 is
Curr. Opin. Cell Biol. 21, 796–805. specific to regions bordering centromeres in metaphase chromosomes.
Proc. Natl. Acad. Sci. USA 102, 6344–6349.
Cerutti, H., and Casas-Mollano, J.A. (2009). Histone H3 phosphorylation:
universal code or lineage specific dialects? Epigenetics 4, 71–75. Hans, F., and Dimitrov, S. (2001). Histone H3 phosphorylation and cell division.
Oncogene 20, 3021–3027.
Cha, T.L., Zhou, B.P., Xia, W., Wu, Y., Yang, C.C., Chen, C.T., Ping, B., Otte,
A.P., and Hung, M.C. (2005). Akt-mediated phosphorylation of EZH2 Higgins, J.M. (2010). Haspin: a newly discovered regulator of mitotic chromo-
suppresses methylation of lysine 27 in histone H3. Science 310, 306–310. some behavior. Chromosoma 119, 137–147.
Chen, S., Bohrer, L.R., Rai, A.N., Pan, Y., Gan, L., Zhou, X., Bagchi, A., Simon, Ho, L., and Crabtree, G.R. (2010). Chromatin remodelling during development.
J.A., and Huang, H. (2010). Cyclin-dependent kinases regulate epigenetic Nature 463, 474–484.
gene silencing through phosphorylation of EZH2. Nat. Cell Biol. 12, 1108–
1114. Huang, W.C., and Chen, C.C. (2005). Akt phosphorylation of p300 at Ser-1834
is essential for its histone acetyltransferase and transcriptional activity. Mol.
Cheung, P., Allis, C.D., and Sassone-Corsi, P. (2000). Signaling to chromatin Cell. Biol. 25, 6592–6602.
through histone modifications. Cell 103, 263–271.
Huang, W.C., Ju, T.K., Hung, M.C., and Chen, C.C. (2007). Phosphorylation of
Cheung, W.L., Ajiro, K., Samejima, K., Kloc, M., Cheung, P., Mizzen, C.A., CBP by IKKalpha promotes cell growth by switching the binding preference of
Beeser, A., Etkin, L.D., Chernoff, J., Earnshaw, W.C., and Allis, C.D. (2003). CBP from p53 to NF-kappaB. Mol. Cell 26, 75–87.
Apoptotic phosphorylation of histone H2B is mediated by mammalian sterile
Hurd, P.J., Bannister, A.J., Halls, K., Dawson, M.A., Vermeulen, M., Olsen,
twenty kinase. Cell 113, 507–517.
J.V., Ismail, H., Somers, J., Mann, M., Owen-Hughes, T., et al. (2009). Phos-
Chi, P., Allis, C.D., and Wang, G.G. (2010). Covalent histone modifications— phorylation of histone H3 Thr-45 is linked to apoptosis. J. Biol. Chem. 284,
miswritten, misinterpreted and mis-erased in human cancers. Nat. Rev. 16575–16583.
Cancer 10, 457–469.
Impey, S., Fong, A.L., Wang, Y., Cardinaux, J.R., Fass, D.M., Obrietan, K.,
Wayman, G.A., Storm, D.R., Soderling, T.R., and Goodman, R.H. (2002). Phos-
Choi, H.S., Choi, B.Y., Cho, Y.Y., Zhu, F., Bode, A.M., and Dong, Z. (2005).
phorylation of CBP mediates transcriptional activation by neural activity and
Phosphorylation of Ser28 in histone H3 mediated by mixed lineage kinase-
CaM kinase IV. Neuron 34, 235–244.
like mitogen-activated protein triple kinase a. J. Biol. Chem. 280, 13545–
13553.
Jenuwein, T., and Allis, C.D. (2001). Translating the histone code. Science 293,
1074–1080.
Cook, P.J., Ju, B.G., Telese, F., Wang, X., Glass, C.K., and Rosenfeld, M.G.
(2009). Tyrosine dephosphorylation of H2AX modulates apoptosis and survival Kaneko, S., Li, G., Son, J., Xu, C.F., Margueron, R., Neubert, T.A., and
decisions. Nature 458, 591–596. Reinberg, D. (2010). Phosphorylation of the PRC2 component Ezh2 is cell
cycle-regulated and up-regulates its binding to ncRNA. Genes Dev. 24,
Crosio, C., Fimia, G.M., Loury, R., Kimura, M., Okano, Y., Zhou, H., Sen, S., Al- 2615–2620.
lis, C.D., and Sassone-Corsi, P. (2002). Mitotic phosphorylation of histone H3:
spatio-temporal regulation by mammalian Aurora kinases. Mol. Cell. Biol. 22, Kang, T.H., Park, D.Y., Choi, Y.H., Kim, K.J., Yoon, H.S., and Kim, K.T. (2007).
874–885. Mitotic histone H3 phosphorylation by vaccinia-related kinase 1 in mammalian
cells. Mol. Cell. Biol. 27, 8533–8546.
Dai, J., Sultan, S., Taylor, S.S., and Higgins, J.M. (2005). The kinase haspin is
required for mitotic histone H3 Thr 3 phosphorylation and normal metaphase Kaur, S., Modi, P., Srivastava, V., Mudgal, R., Tikoo, S., Arora, P., Mohanty, D.,
chromosome alignment. Genes Dev. 19, 472–488. and Sengupta, S. (2010). Chk1-dependent constitutive phosphorylation of
BLM helicase at serine 646 decreases after DNA damage. Mol. Cancer Res.
Dan, I., Watanabe, N.M., and Kusumi, A. (2001). The Ste20 group kinases as 8, 1234–1247.
regulators of MAP kinase cascades. Trends Cell Biol. 11, 220–230.
Keen, N., and Taylor, S. (2009). Mitotic drivers—inhibitors of the Aurora B
Dawson, M.A., Bannister, A.J., Göttgens, B., Foster, S.D., Bartke, T., Green, Kinase. Cancer Metastasis Rev. 28, 185–195.
A.R., and Kouzarides, T. (2009). JAK2 phosphorylates histone H3Y41 and
excludes HP1a from chromatin. Nature 461, 819–822. Kelly, A.E., and Funabiki, H. (2009). Correcting aberrant kinetochore microtu-
bule attachments: an Aurora B-centric view. Curr. Opin. Cell Biol. 21, 51–58.
Dyson, M.H., Thomson, S., Inagaki, M., Goto, H., Arthur, S.J., Nightingale, K.,
Iborra, F.J., and Mahadevan, L.C. (2005). MAP kinase-mediated phosphoryla- Klose, R.J., and Zhang, Y. (2007). Regulation of histone methylation by deme-
tion of distinct pools of histone H3 at S10 or S28 via mitogen- and stress-acti- thylimination and demethylation. Nat. Rev. Mol. Cell Biol. 8, 307–318.
vated kinase 1/2. J. Cell Sci. 118, 2247–2259.
Kouzarides, T. (2007). Chromatin modifications and their function. Cell 128,
Edmunds, J.W., and Mahadevan, L.C. (2004). MAP kinases as structural adap- 693–705.
tors and enzymatic activators in transcription complexes. J. Cell Sci. 117,
3715–3723. Kubicek, S., and Jenuwein, T. (2004). A crack in histone lysine methylation. Cell
119, 903–906.
Elsaesser, S.J., Goldberg, A.D., and Allis, C.D. (2010). New functions for an old
variant: no substitute for histone H3.3. Curr. Opin. Genet. Dev. 20, 110–117. Kunitoku, N., Sasayama, T., Marumoto, T., Zhang, D., Honda, S., Kobayashi,
O., Hatakeyama, K., Ushio, Y., Saya, H., and Hirota, T. (2003). CENP-A phos-
Fischle, W., Wang, Y., and Allis, C.D. (2003). Binary switches and modification phorylation by Aurora-A in prophase is required for enrichment of Aurora-B at
cassettes in histone biology and beyond. Nature 425, 475–479. inner centromeres and for kinetochore function. Dev. Cell 5, 853–864.
282 Molecular Cell 42, May 6, 2011 ª2011 Elsevier Inc.
Molecular Cell
Review
Lai, K.P., Leong, W.F., Chau, J.F., Jia, D., Zeng, L., Liu, H., He, L., Hao, A., Perissi, V., Scafoglio, C., Zhang, J., Ohgi, K.A., Rose, D.W., Glass, C.K., and
Zhang, H., Meek, D., et al. (2010). S6K1 is a multifaceted regulator of Mdm2 Rosenfeld, M.G. (2008). TBL1 and TBLR1 phosphorylation on regulated
that connects nutrient status and DNA damage response. EMBO J. 29, gene promoters overcomes dual CtBP and NCoR/SMRT transcriptional
2994–3006. repression checkpoints. Mol. Cell 29, 755–766.
Lee, J.M., Kim, I.S., Kim, H., Lee, J.S., Kim, K., Yim, H.Y., Jeong, J., Kim, J.H., Pokholok, D.K., Zeitlinger, J., Hannett, N.M., Reynolds, D.B., and Young, R.A.
Kim, J.Y., Lee, H., et al. (2010). RORalpha attenuates Wnt/b-catenin signaling (2006). Activated signal transduction kinases frequently occupy target genes.
by PKCalpha-dependent phosphorylation in colon cancer. Mol. Cell 37, Science 313, 533–536.
183–195.
Preuss, U., Landsberg, G., and Scheidtmann, K.H. (2003). Novel mitosis-
Lehtinen, M.K., Yuan, Z., Boag, P.R., Yang, Y., Villén, J., Becker, E.B., specific phosphorylation of histone H3 at Thr11 mediated by Dlk/ZIP kinase.
DiBacco, S., de la Iglesia, N., Gygi, S., Blackwell, T.K., and Bonni, A. (2006). Nucleic Acids Res. 31, 878–885.
A conserved MST-FOXO signaling pathway mediates oxidative-stress
responses and extends life span. Cell 125, 987–1001. Rogakou, E.P., Pilch, D.R., Orr, A.H., Ivanova, V.S., and Bonner, W.M. (1998).
DNA double-stranded breaks induce histone H2AX phosphorylation on serine
Li, C., Liang, Y.Y., Feng, X.H., Tsai, S.Y., Tsai, M.J., and O’Malley, B.W. (2008). 139. J. Biol. Chem. 273, 5858–5868.
Essential phosphatases and a phospho-degron are critical for regulation of
SRC-3/AIB1 coactivator function and turnover. Mol. Cell 31, 835–849. Rosse, C., Linch, M., Kermorgant, S., Cameron, A.J.M., Boeckeler, K., and
Parker, P.J. (2010). PKC and the control of localized signal dynamics. Nat.
Lim, W.A., and Pawson, T. (2010). Phosphotyrosine signaling: evolving a new Rev. Mol. Cell Biol. 11, 103–112.
cellular communication system. Cell 142, 661–667.
Rui, L., Emre, N.C., Kruhlak, M.J., Chung, H.J., Steidl, C., Slack, G., Wright,
Ling, P., Lu, T.J., Yuan, C.J., and Lai, M.D. (2008). Biosignaling of mammalian G.W., Lenz, G., Ngo, V.N., Shaffer, A.L., et al. (2010). Cooperative epigenetic
Ste20-related kinases. Cell. Signal. 20, 1237–1247. modulation by cancer amplicon genes. Cancer Cell 18, 590–605.
Lou, Z., Minter-Dykhouse, K., Franco, S., Gostissa, M., Rivera, M.A., Celeste, Ruthenburg, A.J., Li, H., Patel, D.J., and Allis, C.D. (2007). Multivalent engage-
A., Manis, J.P., van Deursen, J., Nussenzweig, A., Paull, T.T., et al. (2006). ment of chromatin modifications by linked binding modules. Nat. Rev. Mol.
MDC1 maintains genomic stability by participating in the amplification of Cell Biol. 8, 983–994.
ATM-dependent DNA damage signals. Mol. Cell 21, 187–200.
Sarma, K., and Reinberg, D. (2005). Histone variants meet their match. Nat.
Madak-Erdogan, Z., Lupien, M., Stossi, F., Brown, M., and Katzenellenbogen, Rev. Mol. Cell Biol. 6, 139–149.
B.S. (2011). Genomic collaboration of estrogen receptor alpha and extracel-
lular signal-regulated kinase 2 in regulating gene and proliferation programs. Sassone-Corsi, P., Mizzen, C.A., Cheung, P., Crosio, C., Monaco, L., Jacquot,
Mol. Cell. Biol. 31, 226–236. S., Hanauer, A., and Allis, C.D. (1999). Requirement of Rsk-2 for epidermal
growth factor-activated phosphorylation of histone H3. Science 285, 886–891.
McKinsey, T.A., Zhang, C.L., Lu, J., and Olson, E.N. (2000). Signal-dependent
Shilatifard, A. (2006). Chromatin modifications by methylation and ubiquitina-
nuclear export of a histone deacetylase regulates muscle differentiation.
tion: implications in the regulation of gene expression. Annu. Rev. Biochem.
Nature 408, 106–111.
75, 243–269.
Metzger, E., Yin, N., Wissmann, M., Kunowska, N., Fischer, K., Friedrichs, N.,
Shimada, M., Niida, H., Zineldeen, D.H., Tagami, H., Tanaka, M., Saito, H., and
Patnaik, D., Higgins, J.M., Potier, N., Scheidtmann, K.H., et al. (2008). Phos-
Nakanishi, M. (2008). Chk1 is a histone H3 threonine 11 kinase that regulates
phorylation of histone H3 at threonine 11 establishes a novel chromatin
DNA damage-induced transcriptional repression. Cell 132, 221–232.
mark for transcriptional regulation. Nat. Cell Biol. 10, 53–60.
Sims, R.J., 3rd, and Reinberg, D. (2008). Is there a code embedded in proteins
Metzger, E., Imhof, A., Patel, D., Kahl, P., Hoffmeyer, K., Friedrichs, N., Müller,
that is based on post-translational modifications? Nat. Rev. Mol. Cell Biol. 9,
J.M., Greschik, H., Kirfel, J., Ji, S., et al. (2010). Phosphorylation of histone
815–820.
H3T6 by PKCbeta(I) controls demethylation at histone H3K4. Nature 464,
792–796. Suganuma, T., and Workman, J.L. (2008). Crosstalk among Histone Modifica-
tions. Cell 135, 604–607.
Mosammaparast, N., and Shi, Y. (2010). Reversal of histone methylation:
biochemical and molecular mechanisms of histone demethylases. Annu. Sugiyama, K., Sugiura, K., Hara, T., Sugimoto, K., Shima, H., Honda, K.,
Rev. Biochem. 79, 155–179. Furukawa, K., Yamashita, S., and Urano, T. (2002). Aurora-B associated
protein phosphatases as negative regulators of kinase activation. Oncogene
Muslin, A.J., Tanner, J.W., Allen, P.M., and Shaw, A.S. (1996). Interaction of 21, 3103–3111.
14-3-3 with signaling proteins is mediated by the recognition of phosphoser-
ine. Cell 84, 889–897. Sullivan, K.F., Hechenberger, M., and Masri, K. (1994). Human CENP-A
contains a histone H3 related histone fold domain that is required for targeting
Nasrin, N., Kaushik, V.K., Fortier, E., Wall, D., Pearson, K.J., de Cabo, R., and to the centromere. J. Cell Biol. 127, 581–592.
Bordone, L. (2009). JNK1 phosphorylates SIRT1 and promotes its enzymatic
activity. PLoS ONE 4, e8414. Takemori, H., Katoh Hashimoto, Y., Nakae, J., Olson, E.N., and Okamoto, M.
(2009). Inactivation of HDAC5 by SIK1 in AICAR-treated C2C12 myoblasts. En-
Nowak, S.J., and Corces, V.G. (2000). Phosphorylation of histone H3 corre- docr. J. 56, 121–130.
lates with transcriptionally active loci. Genes Dev. 14, 3003–3013.
Talbert, P.B., and Henikoff, S. (2010). Histone variants—ancient wrap artists of
Nowak, S.J., and Corces, V.G. (2004). Phosphorylation of histone H3: the epigenome. Nat. Rev. Mol. Cell Biol. 11, 264–275.
a balancing act between chromosome condensation and transcriptional acti-
vation. Trends Genet. 20, 214–220. Taverna, S.D., Li, H., Ruthenburg, A.J., Allis, C.D., and Patel, D.J. (2007). How
chromatin-binding modules interpret histone modifications: lessons from
Oh, A.S., Lahusen, J.T., Chien, C.D., Fereshteh, M.P., Zhang, X., Dakshana- professional pocket pickers. Nat. Struct. Mol. Biol. 14, 1025–1040.
murthy, S., Xu, J., Kagan, B.L., Wellstein, A., and Riegel, A.T. (2008). Tyrosine
phosphorylation of the nuclear receptor coactivator AIB1/SRC-3 is enhanced Vader, G., and Lens, S.M. (2008). The Aurora kinase family in cell division and
by Abl kinase and is required for its activity in cancer cells. Mol. Cell. Biol. 28, cancer. Biochim. Biophys. Acta 1786, 60–72.
6580–6593.
Vermeulen, L., Vanden Berghe, W., Beck, I.M., De Bosscher, K., and Haege-
Peng, C., Cho, Y.Y., Zhu, F., Xu, Y.M., Wen, W., Ma, W.Y., Bode, A.M., and man, G. (2009). The versatile role of MSKs in transcriptional regulation. Trends
Dong, Z. (2010). RSK2 mediates NF-kB activity through the phosphorylation Biochem. Sci. 34, 311–318.
of IkappaBalpha in the TNF-R1 pathway. FASEB J. 24, 3490–3499.
Wang, Y., Zhang, W., Jin, Y., Johansen, J., and Johansen, K.M. (2001). The
Pérez-Cadahı́a, B., Drobic, B., and Davie, J.R. (2009). H3 phosphorylation: JIL-1 tandem kinase mediates histone H3 phosphorylation and is required
dual role in mitosis and interphase. Biochem. Cell Biol. 87, 695–709. for maintenance of chromatin structure in Drosophila. Cell 105, 433–443.
Molecular Cell 42, May 6, 2011 ª2011 Elsevier Inc. 283
Molecular Cell
Review
Wang, Z., Bhattacharya, N., Mixter, P.F., Wei, W., Sedivy, J., and Magnuson, Yamamoto, Y., Verma, U.N., Prajapati, S., Kwak, Y.T., and Gaynor, R.B.
N.S. (2002). Phosphorylation of the cell cycle inhibitor p21Cip1/WAF1 by Pim- (2003). Histone H3 phosphorylation by IKK-a is critical for cytokine-induced
1 kinase. Biochim. Biophys. Acta 1593, 45–55. gene expression. Nature 423, 655–659.
Wang, F., Dai, J., Daum, J.R., Niedzialkowska, E., Banerjee, B., Stukenberg,
Yang, X.J., and Seto, E. (2008). Lysine acetylation: codified crosstalk with
P.T., Gorbsky, G.J., and Higgins, J.M. (2010). Histone H3 Thr-3 phosphoryla-
other posttranslational modifications. Mol. Cell 31, 449–461.
tion by Haspin positions Aurora B at centromeres in mitosis. Science 330,
231–235.
Yang, Q., Deng, X., Lu, B., Cameron, M., Fearns, C., Patricelli, M.P., Yates,
Weake, V.M., and Workman, J.L. (2008). Histone ubiquitination: triggering J.R., 3rd, Gray, N.S., and Lee, J.D. (2010). Pharmacological inhibition of
gene activity. Mol. Cell 29, 653–663. BMK1 suppresses tumor growth through promyelocytic leukemia protein.
Cancer Cell 18, 258–267.
Weake, V.M., and Workman, J.L. (2010). Inducible gene expression: diverse
regulatory mechanisms. Nat. Rev. Genet. 11, 426–437. Yoda, K., Ando, S., Morishita, S., Houmura, K., Hashimoto, K., Takeyasu, K.,
Wei, Y., Chen, Y.-H., Li, L.-Y., Lang, J., Yeh, S.-P., Shi, B., Yang, C.-C., Yang, and Okazaki, T. (2000). Human centromere protein A (CENP-A) can replace
J.-Y., Lin, C.-Y., Lai, C.-C., and Hung, M.C. (2011). CDK1-dependent phos- histone H3 in nucleosome reconstitution in vitro. Proc. Natl. Acad. Sci. USA
97, 7266–7271.
phorylation of EZH2 suppresses methylation of H3K27 and promotes osteo-
genic differentiation of human mesenchymal stem cells. Nat. Cell Biol. 13,
87–94. Zhang, W., Deng, H., Bao, X., Lerach, S., Girton, J., Johansen, J., and Johan-
sen, K.M. (2006). The JIL-1 histone H3S10 kinase regulates dimethyl H3K9
Wyrick, J.J., and Parra, M.A. (2009). The role of histone H2A and H2B post- modifications and heterochromatic spreading in Drosophila. Development
translational modifications in transcription: a genomic perspective. Biochim. 133, 229–235.
Biophys. Acta 1789, 37–44.
Zhong, S., Zhang, Y., Jansen, C., Goto, H., Inagaki, M., and Dong, Z. (2001).
Xiao, A., Li, H., Shechter, D., Ahn, S.H., Fabrizio, L.A., Erdjument-Bromage, H.,
MAP kinases mediate UVB-induced phosphorylation of histone H3 at serine
Ishibe-Murakami, S., Wang, B., Tempst, P., Hofmann, K., et al. (2009). WSTF
28. J. Biol. Chem. 276, 12932–12937.
regulates the H2A.X DNA damage response via a novel tyrosine kinase activity.
Nature 457, 57–62.
Zippo, A., De Robertis, A., Serafini, R., and Oliviero, S. (2007). PIM1-depen-
Yaffe, M.B., Rittinger, K., Volinia, S., Caron, P.R., Aitken, A., Leffers, H., Gam- dent phosphorylation of histone H3 at serine 10 is required for MYC-depen-
blin, S.J., Smerdon, S.J., and Cantley, L.C. (1997). The structural basis for dent transcriptional activation and oncogenic transformation. Nat. Cell Biol.
14-3-3:phosphopeptide binding specificity. Cell 91, 961–971. 9, 932–944.
284 Molecular Cell 42, May 6, 2011 ª2011 Elsevier Inc.
You might also like
- Experiments in Molecular Cell Biology: A Problems Book With Multiple-Choice Question-Based TestsDocument20 pagesExperiments in Molecular Cell Biology: A Problems Book With Multiple-Choice Question-Based TestsKudumarNo ratings yet
- 10 05 2006 Lifescience 2Document60 pages10 05 2006 Lifescience 2api-3696530No ratings yet
- MMC 3Document27 pagesMMC 3oli.finetNo ratings yet
- HHS Public AccessDocument31 pagesHHS Public AccessMelanieNo ratings yet
- Emerging Issues in Receptor Protein Tyrosine Phosphatase Function: Lifting Fog or Simply Shifting?Document11 pagesEmerging Issues in Receptor Protein Tyrosine Phosphatase Function: Lifting Fog or Simply Shifting?LUCAS GONÇALVES DE OLIVEIRANo ratings yet
- Frontiers in BiologyDocument14 pagesFrontiers in BiologyAkademisyen BirisiNo ratings yet
- Lecture 9 StrahlDocument56 pagesLecture 9 StrahlChaitanya LobheNo ratings yet
- Genomic Imprinting & Epigenetics: 徐国良 研究员 细胞大楼 300 电话 E-mail: glxu@sibs.ac.cnDocument53 pagesGenomic Imprinting & Epigenetics: 徐国良 研究员 细胞大楼 300 电话 E-mail: glxu@sibs.ac.cnapi-3700537No ratings yet
- Bidir Transcr RepeatsDocument6 pagesBidir Transcr RepeatsSnezana MihajlovicNo ratings yet
- Hansen Et Al 2015 Hippo SignalingDocument15 pagesHansen Et Al 2015 Hippo SignalingAlabhya DasNo ratings yet
- Tbfkbp12 AssignDocument3 pagesTbfkbp12 AssignRodolpho Do Aido-MachadoNo ratings yet
- Baratta2002-Somatostatin LTP Dentate GyrusDocument9 pagesBaratta2002-Somatostatin LTP Dentate Gyrusiulia andreeaNo ratings yet
- Regulation of Chromatin by Histone Modifications: ReviewDocument15 pagesRegulation of Chromatin by Histone Modifications: ReviewRabiatul AdawiyahNo ratings yet
- Involvement of SIK2/TORC2 Signaling Cascade in The Regulation of Insulin-Induced PGC-1 and UCP-1 Gene Expression in Brown AdipocytesDocument10 pagesInvolvement of SIK2/TORC2 Signaling Cascade in The Regulation of Insulin-Induced PGC-1 and UCP-1 Gene Expression in Brown Adipocytesalejandra soledad alvarado neiraNo ratings yet
- The Role of JAK/STAT Signalling in The Pathogenesis, Prognosis and Treatment of Solid TumoursDocument7 pagesThe Role of JAK/STAT Signalling in The Pathogenesis, Prognosis and Treatment of Solid TumoursGauri MahalleNo ratings yet
- Thomas 2015Document7 pagesThomas 2015Mehedi HossainNo ratings yet
- Ni Hms 262290Document8 pagesNi Hms 262290Ignacio MazzitelliNo ratings yet
- 03 Tamaru Nucleocytoplasmic Shuttling of Bmal1 Regulated by Circ ClockDocument11 pages03 Tamaru Nucleocytoplasmic Shuttling of Bmal1 Regulated by Circ ClockEliNo ratings yet
- 7 Insulin Proteo Mic 13Document33 pages7 Insulin Proteo Mic 13Sameera HameedNo ratings yet
- Sawarkar2012 PDFDocument12 pagesSawarkar2012 PDFPaige MunroeNo ratings yet
- A Comprehensive View of The Epigenetic Landscape Part IIDocument26 pagesA Comprehensive View of The Epigenetic Landscape Part IIBetzabethNo ratings yet
- Regulation of Chromatin by Histone Modifications: ReviewDocument15 pagesRegulation of Chromatin by Histone Modifications: ReviewFlorencia FirenzeNo ratings yet
- Eukaryotic Transcriptional Control: Major ConsiderationsDocument40 pagesEukaryotic Transcriptional Control: Major ConsiderationsShantanu ChakrabortyNo ratings yet
- 12-Cancer-associated mutations in the p85α N-terminal SH2 domain activate a spectrum of receptor tyrosine kinasesDocument11 pages12-Cancer-associated mutations in the p85α N-terminal SH2 domain activate a spectrum of receptor tyrosine kinasesshidis1028No ratings yet
- Molecular Aspects of MedicineDocument20 pagesMolecular Aspects of MedicineLorena RamosNo ratings yet
- Phosphorylation of P-Glycoprotein by PKA and PKC Modulates Swelling-Activated CL CurrentsDocument9 pagesPhosphorylation of P-Glycoprotein by PKA and PKC Modulates Swelling-Activated CL CurrentsDr-Dalya ShakirNo ratings yet
- Blume Jensen2001Document11 pagesBlume Jensen2001yusuf eka maulanaNo ratings yet
- Notch-Dependent Regulation of Cell-Cycle Transitions in Dmel Follicle CellsDocument13 pagesNotch-Dependent Regulation of Cell-Cycle Transitions in Dmel Follicle CellsCat RudolfNo ratings yet
- Huynen1999 PDFDocument11 pagesHuynen1999 PDFLizbethNo ratings yet
- TFG-Related Neurologic Disorders: New Insights Into Relationships Between Endoplasmic Reticulum and NeurodegenerationDocument7 pagesTFG-Related Neurologic Disorders: New Insights Into Relationships Between Endoplasmic Reticulum and NeurodegenerationAlana PachecoNo ratings yet
- Appleton 2012Document112 pagesAppleton 2012Estie KiriwennoNo ratings yet
- Fosforilación Mol. Cell. Biol.-2011-Banerjee-4858-73Document16 pagesFosforilación Mol. Cell. Biol.-2011-Banerjee-4858-73planhigion06No ratings yet
- 7.isoform-Specific Regulation of Osteogenic Factors by Polypeptide N-Acetylgalactosaminyltransferases 1 and 4 3Document6 pages7.isoform-Specific Regulation of Osteogenic Factors by Polypeptide N-Acetylgalactosaminyltransferases 1 and 4 3Shawn GaoNo ratings yet
- University of GroningenDocument6 pagesUniversity of GroningenBreixo HarguindeyNo ratings yet
- AMPK and TOR (2020)Document21 pagesAMPK and TOR (2020)BastianRiverosVasquezNo ratings yet
- Nihms285753 Take No.2Document14 pagesNihms285753 Take No.2ArizonaNo ratings yet
- Metabolic Changes Associated With Tumor Metastasis, Part 2: Mitochondria, Lipid and Amino Acid MetabolismDocument17 pagesMetabolic Changes Associated With Tumor Metastasis, Part 2: Mitochondria, Lipid and Amino Acid MetabolismUlfahRamadhaniNo ratings yet
- Protein Folding in ERDocument17 pagesProtein Folding in ERAfaq AhmadNo ratings yet
- Cancer Epigenetics - From Mechanism To TherapyDocument16 pagesCancer Epigenetics - From Mechanism To TherapyMauricio DiazNo ratings yet
- 1997-Oncoprotein NetworksDocument14 pages1997-Oncoprotein NetworksyicinenNo ratings yet
- Loreto, M (2002) - Functional Cooperation Between C-CBL and Src-Like Adaptor Protein 2 in The Negative Regulation of T-Cell Receptor SignalingDocument15 pagesLoreto, M (2002) - Functional Cooperation Between C-CBL and Src-Like Adaptor Protein 2 in The Negative Regulation of T-Cell Receptor SignalingAnadahiNo ratings yet
- Spacing Palindromic Sites Determinant (Signal Activators Transcription) Binding Transcriptional ActivityDocument5 pagesSpacing Palindromic Sites Determinant (Signal Activators Transcription) Binding Transcriptional ActivityNilabh RanjanNo ratings yet
- Previews: Glucose Starvation Blocks Translation at Multiple LevelsDocument2 pagesPreviews: Glucose Starvation Blocks Translation at Multiple LevelsMonwarul AzizNo ratings yet
- Postranslational ModificationDocument78 pagesPostranslational ModificationnsjunnarkarNo ratings yet
- Hisano 2019Document17 pagesHisano 2019GHUJNo ratings yet
- Jurnal 2Document8 pagesJurnal 2Dea SyafiraNo ratings yet
- Non-Receptor Protein Tyrosine Kinases: Frontiers in Bioscience June 2003Document42 pagesNon-Receptor Protein Tyrosine Kinases: Frontiers in Bioscience June 2003Rios Lopez Juan RobertoNo ratings yet
- Exercise Induces Muscle Fiber Type SwitchingDocument10 pagesExercise Induces Muscle Fiber Type SwitchingbookNo ratings yet
- PhosphataseDocument41 pagesPhosphataseapi-3700537No ratings yet
- NHE3 ZIjian XieDocument9 pagesNHE3 ZIjian Xienilberto2No ratings yet
- Osmostress of YeastDocument17 pagesOsmostress of YeastJimmy WongNo ratings yet
- Oxidative Stress Inhibits Osteoblastic DifferentiationDocument11 pagesOxidative Stress Inhibits Osteoblastic DifferentiationSonal BaseerNo ratings yet
- Gene Regulation in Eukaryotes: Dr. Syahril Abdullah Medical Genetics Laboratory Syahril@medic - Upm.edu - MyDocument28 pagesGene Regulation in Eukaryotes: Dr. Syahril Abdullah Medical Genetics Laboratory Syahril@medic - Upm.edu - MySuren BobNo ratings yet
- Association of P450 Oxidoreductase Gene Polymorphism With Tacrolimus Pharmacokinetics in Renal Transplant Recipients - A Systematic Review and Meta-AnalysisDocument9 pagesAssociation of P450 Oxidoreductase Gene Polymorphism With Tacrolimus Pharmacokinetics in Renal Transplant Recipients - A Systematic Review and Meta-Analysis陈梓强No ratings yet
- Cocaine and HipocampoDocument7 pagesCocaine and HipocampoNico TánatosNo ratings yet
- tmp9271 TMPDocument8 pagestmp9271 TMPFrontiersNo ratings yet
- A Chloroplast Envelope-Bound PHD Transcription Factor Mediates Chloroplast Signals To The Nucleus.Document10 pagesA Chloroplast Envelope-Bound PHD Transcription Factor Mediates Chloroplast Signals To The Nucleus.pyu2No ratings yet
- Cochlear Function in Mice With Only One Copy of The Prestin GeneDocument13 pagesCochlear Function in Mice With Only One Copy of The Prestin GeneAlex WongNo ratings yet
- Protocol To Label Histone With Acceptor and DNA With DonorDocument13 pagesProtocol To Label Histone With Acceptor and DNA With DonorSandipan SahaNo ratings yet
- Pi Is 1097276514007084Document8 pagesPi Is 1097276514007084HsnNo ratings yet
- Mechanisms of DNA Methylation and Demethylation in MammalsDocument10 pagesMechanisms of DNA Methylation and Demethylation in MammalsDani PazNo ratings yet
- Sha Et Al-2004Document7 pagesSha Et Al-2004Dani PazNo ratings yet
- Inflammation and Repair Processes in Chronic Obstructive Pulmonary DiseaseDocument5 pagesInflammation and Repair Processes in Chronic Obstructive Pulmonary DiseaseDani PazNo ratings yet
- Stem-Progenitor Cells in Lung Development, InjuryDocument4 pagesStem-Progenitor Cells in Lung Development, InjuryDani PazNo ratings yet
- Biology 2 CENTRAL DOGMA OF MOLECULAR BIOLOGYDocument31 pagesBiology 2 CENTRAL DOGMA OF MOLECULAR BIOLOGYSophia UstarisNo ratings yet
- 041 LiverDocument3 pages041 Liveraistina100% (1)
- The Role of Epigenetics in Mental Disorders: Review ArticleDocument7 pagesThe Role of Epigenetics in Mental Disorders: Review ArticleBenet DhasNo ratings yet
- Glyc o MicrobiologyDocument558 pagesGlyc o MicrobiologyBryan Sanz100% (1)
- Exemplar Science Lesson Plan: Grade Level Quarter / Domain Week & Day No. Page NoDocument5 pagesExemplar Science Lesson Plan: Grade Level Quarter / Domain Week & Day No. Page NoEricha Solomon100% (2)
- (Doi 10.1002 - Cpim.40) Coligan, John E. Bierer, Barbara E. Margulies, David H. Sheva - Current Protocols in Immunology - Flow Cytometry - An OverviewDocument11 pages(Doi 10.1002 - Cpim.40) Coligan, John E. Bierer, Barbara E. Margulies, David H. Sheva - Current Protocols in Immunology - Flow Cytometry - An OverviewMunir AliNo ratings yet
- The Double Helix - Personal View Crick Nature 1974Document4 pagesThe Double Helix - Personal View Crick Nature 1974Abhay KumarNo ratings yet
- MANAS, Biochem Lab June 29 PDFDocument2 pagesMANAS, Biochem Lab June 29 PDFlovelykissNo ratings yet
- Definitions - Topic 4 Biological Molecules - CAIE Biology IGCSEDocument2 pagesDefinitions - Topic 4 Biological Molecules - CAIE Biology IGCSEG VeezNo ratings yet
- Genetic MaterialDocument2 pagesGenetic MaterialSammie GotchuNo ratings yet
- Urokinase-A Strong Plasminogen Activator PDFDocument14 pagesUrokinase-A Strong Plasminogen Activator PDFevitaNo ratings yet
- Euroline Nuclear and Cytoplasmic Antigen Profi Les: Examples of Antigen CombinationsDocument1 pageEuroline Nuclear and Cytoplasmic Antigen Profi Les: Examples of Antigen CombinationsRiskullah MakmurNo ratings yet
- Bio ProjectDocument23 pagesBio ProjectKarthika Umashankar57% (7)
- HJHJJJJJDocument91 pagesHJHJJJJJShrey SundriyalNo ratings yet
- Assignment of Pharmacology Submitted by Laiba Shah To DR MaryumDocument5 pagesAssignment of Pharmacology Submitted by Laiba Shah To DR MaryumLaiba ShahNo ratings yet
- Alternate Phosphorylation-O-GlcNAc Modification On Human Insulin IRSs - A Road Towards Impaired Insulin Signaling in Alzheimer and DiabetesDocument19 pagesAlternate Phosphorylation-O-GlcNAc Modification On Human Insulin IRSs - A Road Towards Impaired Insulin Signaling in Alzheimer and DiabeteswaqarchemistNo ratings yet
- Polymerase Chain Reaction (PCR) (Article) - Khan AcademyDocument10 pagesPolymerase Chain Reaction (PCR) (Article) - Khan AcademySelva KumarNo ratings yet
- Atp EssayDocument3 pagesAtp EssayValeria PunzoNo ratings yet
- Protein Synthesis - Activity Sheet PDFDocument2 pagesProtein Synthesis - Activity Sheet PDFjaneyzhouNo ratings yet
- Utkal University BSC Syllabus 2016-19Document555 pagesUtkal University BSC Syllabus 2016-19Ryuzaki MeitanteiNo ratings yet
- Topic 4 Unit 1 Part2Document16 pagesTopic 4 Unit 1 Part2body fayezNo ratings yet
- Journal Pre-Proofs: in Vitro Oxidation Promoted by Chlorpyrifos Residues On Myosin and ChickenDocument32 pagesJournal Pre-Proofs: in Vitro Oxidation Promoted by Chlorpyrifos Residues On Myosin and ChickenHussain Ahmad QadriNo ratings yet
- Catecholamines: (Dopamine (DA), Norepinephrine (NE), Epinephrine (EPI) )Document30 pagesCatecholamines: (Dopamine (DA), Norepinephrine (NE), Epinephrine (EPI) )Imrana AamirNo ratings yet
- 161BCHM 100a 1 - 1452614433Document2 pages161BCHM 100a 1 - 1452614433william1230No ratings yet
- Biotech GuideDocument159 pagesBiotech GuideRenata KapcevičiūtėNo ratings yet
- Introduction To Endocrinology, Hormones and Their Classification Lecture NO: 2 MbbsDocument32 pagesIntroduction To Endocrinology, Hormones and Their Classification Lecture NO: 2 MbbsOmaraye JoshuaNo ratings yet
- JasmonatesDocument38 pagesJasmonatesSpinu LiliaNo ratings yet
- LAB0016 Covid-19 Molecular Diagnostic Lab Belbas, ButwalDocument1 pageLAB0016 Covid-19 Molecular Diagnostic Lab Belbas, ButwalPoonam RanaNo ratings yet
- Biología Celular y Patogénesis de ChlamydiaDocument16 pagesBiología Celular y Patogénesis de ChlamydiaLuis MedranoNo ratings yet