Professional Documents
Culture Documents
WS Grade 10 IG Chemistry 23-24 - Energy Changes
WS Grade 10 IG Chemistry 23-24 - Energy Changes
Uploaded by
SiyaCopyright:
Available Formats
You might also like
- O Level Biology Practice Questions And Answers EnzymesFrom EverandO Level Biology Practice Questions And Answers EnzymesRating: 5 out of 5 stars5/5 (1)
- CH 4Document51 pagesCH 4Hoi An Sze100% (31)
- Bushong: Radiologic Science For Technologists, 9th Edition: Chapter 3: The Structure of Matter Test Bank Multiple ChoiceDocument7 pagesBushong: Radiologic Science For Technologists, 9th Edition: Chapter 3: The Structure of Matter Test Bank Multiple ChoicePring FamNo ratings yet
- OCR Chemistry A: 9 Enthalpy Exam-Style QuestionsDocument6 pagesOCR Chemistry A: 9 Enthalpy Exam-Style QuestionsHazare 2004100% (1)
- Chemistry Matriculation Note SK025 by Vinarti MahmudDocument47 pagesChemistry Matriculation Note SK025 by Vinarti MahmudNurun NajwaNo ratings yet
- 2013 YJC H2 Chem Prelim P2Document15 pages2013 YJC H2 Chem Prelim P2Chow Kim WanNo ratings yet
- Unconfined Vapor Cloud ExplosionDocument5 pagesUnconfined Vapor Cloud ExplosionMDR PRAPHU50% (2)
- A2 Chemistry Assessment 1 List - REVISION RESOURCEDocument46 pagesA2 Chemistry Assessment 1 List - REVISION RESOURCEHarry BarkerNo ratings yet
- Answer ALL The Following Questions Below. Circle The Correct Letter To Indicate Your AnswerDocument4 pagesAnswer ALL The Following Questions Below. Circle The Correct Letter To Indicate Your AnswerTimothy HandokoNo ratings yet
- 2017 Y5 T4 Chem Focus - KineticsDocument4 pages2017 Y5 T4 Chem Focus - KineticsxmxmxmxmxmNo ratings yet
- 2019 H2 Chem Prelim P3 QPDocument12 pages2019 H2 Chem Prelim P3 QPFaith SeahNo ratings yet
- First Pre-Board Examination (2019-2020) Class: Xii Subject: CHEMISTRY Date: 12.12.2019Document9 pagesFirst Pre-Board Examination (2019-2020) Class: Xii Subject: CHEMISTRY Date: 12.12.2019gaming with skdNo ratings yet
- CEM1008F Class Test 2 2019Document10 pagesCEM1008F Class Test 2 2019lia lightNo ratings yet
- Y12 3.2.2 Rates QuestionsDocument16 pagesY12 3.2.2 Rates Questionsmelissafulcher1234No ratings yet
- 06 (H1) Energetics (QNS)Document9 pages06 (H1) Energetics (QNS)Amelia WongNo ratings yet
- Chemistry SK025 Pre-Lab ModuleDocument20 pagesChemistry SK025 Pre-Lab ModuleNORSHAWANI BINTI MD RADZI MoeNo ratings yet
- Final Revision WorksheetDocument26 pagesFinal Revision Worksheetawash0takuNo ratings yet
- West Bengal University of TechnologyDocument4 pagesWest Bengal University of Technologysayan_dasNo ratings yet
- Chem FormatDocument4 pagesChem FormatMarc Joshua CadizNo ratings yet
- c3.2 Exam QuestionsDocument21 pagesc3.2 Exam Questionsfletcherberryheath2006No ratings yet
- Major Test 4 2021 FinalDocument8 pagesMajor Test 4 2021 FinalLevi JohnsonNo ratings yet
- 1assignment On Rates of Reaction and Energy ChangesDocument6 pages1assignment On Rates of Reaction and Energy ChangesShehryar IftikharNo ratings yet
- C3 Exercise 3Document8 pagesC3 Exercise 3Noor Liyana Ahmad FuadNo ratings yet
- Ch10 - Chemical Reactions - P4 - L1 - WS1Document1 pageCh10 - Chemical Reactions - P4 - L1 - WS1Aminul IslamNo ratings yet
- Sample Question Paper Chemistry (043) Class-XII, Session: 2021-22 TERM IIDocument5 pagesSample Question Paper Chemistry (043) Class-XII, Session: 2021-22 TERM IIShiny AlexNo ratings yet
- EnergeticsDocument6 pagesEnergeticsAmmar AbiddNo ratings yet
- CC Grade 11 Chemistry Energetics CWDocument3 pagesCC Grade 11 Chemistry Energetics CWMaliq MorrisNo ratings yet
- Ap09 Chemistry Form B q5Document10 pagesAp09 Chemistry Form B q5jessieNo ratings yet
- IB CHEM 1-4 WorksheetsDocument7 pagesIB CHEM 1-4 WorksheetsChung Khanh VUUNo ratings yet
- Che 211 Referred Eos 2022-23-2Document7 pagesChe 211 Referred Eos 2022-23-2Clevas MseluleNo ratings yet
- AS Enthalpy 3Document32 pagesAS Enthalpy 3verityfaye09No ratings yet
- Chapter 1 Reaction KineticsDocument7 pagesChapter 1 Reaction KineticsNurin QistinaNo ratings yet
- Answer All Question: Sk027 / Chapter 3: Reaction Kinetics / Exercise 1Document5 pagesAnswer All Question: Sk027 / Chapter 3: Reaction Kinetics / Exercise 1kjjkimkmkNo ratings yet
- Pre Lab Question 2022 - SK025Document18 pagesPre Lab Question 2022 - SK025HaziqahNo ratings yet
- Topic 1 Energetics WorksheetDocument5 pagesTopic 1 Energetics Worksheetindira.seebachanNo ratings yet
- Cambridge International Advanced Subsidiary and Advanced LevelDocument16 pagesCambridge International Advanced Subsidiary and Advanced LevelTrung Hoàng HuyNo ratings yet
- Class-12 Chemistry ElectroDocument4 pagesClass-12 Chemistry ElectroHemant ChaudharyNo ratings yet
- Checkup On Chapter 9: Questions Revision ChecklistDocument2 pagesCheckup On Chapter 9: Questions Revision ChecklistShahid Ur RehmanNo ratings yet
- MSS 1718MockPaper2Document8 pagesMSS 1718MockPaper2Kelvin ChowNo ratings yet
- T5 - 2017-2018 IB Chemistry SL QPDocument19 pagesT5 - 2017-2018 IB Chemistry SL QPRachelNo ratings yet
- Gr.11, Unit 3, Mod 15, L1, Reaction RatesDocument35 pagesGr.11, Unit 3, Mod 15, L1, Reaction Ratesaminbigman15No ratings yet
- National Senior Certificate: Grade 12Document21 pagesNational Senior Certificate: Grade 12MfanafuthiNo ratings yet
- Mid Term General Chem II Fall 2001Document6 pagesMid Term General Chem II Fall 2001dr.ibrahimsalemvpNo ratings yet
- Jan 2022 ExamDocument7 pagesJan 2022 ExamBeverly TshumaNo ratings yet
- IJC2009 Chem H2Prelim Paper 3Document12 pagesIJC2009 Chem H2Prelim Paper 3Bin RenNo ratings yet
- PHYS CE Tutorial QuestionsDocument3 pagesPHYS CE Tutorial QuestionsMel SalazarNo ratings yet
- Edexcel IAS Chemistry Classified Unit - 2 - Topic 1Document49 pagesEdexcel IAS Chemistry Classified Unit - 2 - Topic 1mostafa barakatNo ratings yet
- Common Foundation Organic Q in A LevelDocument21 pagesCommon Foundation Organic Q in A Level黄维燕No ratings yet
- QP Chem - XI - 2019-20 11Document5 pagesQP Chem - XI - 2019-20 11Lawrence GaikwadNo ratings yet
- AS Level Topic 8 TestDocument11 pagesAS Level Topic 8 TestMorvan BarnesNo ratings yet
- CCO 2013 Problems EnglishDocument12 pagesCCO 2013 Problems EnglishmarcusmaaaaaNo ratings yet
- Energy & Speed of RexDocument22 pagesEnergy & Speed of Rexcook n bakesNo ratings yet
- Chemistry: 2021 James Ruse Agricultural High School Year 11 Theory ExaminationDocument17 pagesChemistry: 2021 James Ruse Agricultural High School Year 11 Theory ExaminationYu-Tang LinNo ratings yet
- Physical Sciences: Paper Ii: Please Turn OverDocument16 pagesPhysical Sciences: Paper Ii: Please Turn OverBonga DubeNo ratings yet
- Organic ChemistryDocument5 pagesOrganic ChemistryEve LeeNo ratings yet
- IBO 2020 - Theory Exam 1Document65 pagesIBO 2020 - Theory Exam 1ConorNo ratings yet
- Chemistry (Solutions) : 2021 James Ruse Agricultural High School Year 11 Theory ExaminationDocument25 pagesChemistry (Solutions) : 2021 James Ruse Agricultural High School Year 11 Theory ExaminationYu-Tang LinNo ratings yet
- ChemDocument26 pagesChemyan45411No ratings yet
- ChemistryDocument7 pagesChemistryRajesh KhannaNo ratings yet
- Unit 5 - Kinetics Free Response PracticeDocument4 pagesUnit 5 - Kinetics Free Response Practiceridhimaspam0No ratings yet
- Term Iii Mock Examination June 2019-20: Key Stage 3 (Year IX)Document9 pagesTerm Iii Mock Examination June 2019-20: Key Stage 3 (Year IX)Rashad KhanNo ratings yet
- Enthalpy Review QuestionsDocument3 pagesEnthalpy Review Questionsranjana roy100% (1)
- O Level Biology Practice Questions And Answers Plant NutritionFrom EverandO Level Biology Practice Questions And Answers Plant NutritionRating: 5 out of 5 stars5/5 (1)
- Light - Standard Proctor Compaction Test of Soil (Is-2720-Part-7-1980)Document5 pagesLight - Standard Proctor Compaction Test of Soil (Is-2720-Part-7-1980)Kantrathi BhaskarNo ratings yet
- Chapter No. 4 (MCQS)Document3 pagesChapter No. 4 (MCQS)Akkas HaiderNo ratings yet
- Invisible Solar Cell (LLADocument24 pagesInvisible Solar Cell (LLAJustin FernandezNo ratings yet
- Ozone Layer Depletion and Global WarmingDocument15 pagesOzone Layer Depletion and Global WarmingSarah ShahNo ratings yet
- 0625 m17 QP 22Document16 pages0625 m17 QP 22Yash SubraNo ratings yet
- Chapter 3 - Water Resources: CBSE Notes Class 10 Social Science GeographyDocument4 pagesChapter 3 - Water Resources: CBSE Notes Class 10 Social Science GeographyEKLABAY SONINo ratings yet
- Earth Science Geosphere and Earth Systems Key IdeasDocument92 pagesEarth Science Geosphere and Earth Systems Key Ideasroni marudutNo ratings yet
- Surface TensionpdfFilename: Surface Tensionpdf PDFDocument18 pagesSurface TensionpdfFilename: Surface Tensionpdf PDFChandra SekharNo ratings yet
- Soal Pas Bahasa Inggris Kelas XiDocument5 pagesSoal Pas Bahasa Inggris Kelas Xiayu pandini maharaniNo ratings yet
- Numerical Modelling of Brine Dispersion in Shallow Coastal WatersDocument13 pagesNumerical Modelling of Brine Dispersion in Shallow Coastal WatersIAEME PublicationNo ratings yet
- Synthesis of PtZrO2 Catalyst On Fecralloy SubstratDocument12 pagesSynthesis of PtZrO2 Catalyst On Fecralloy SubstratMohamad Ali AkbarNo ratings yet
- Calculation of Carbon EmissionDocument3 pagesCalculation of Carbon EmissionEddie TaiNo ratings yet
- Cipta Grup1Document9 pagesCipta Grup1dwi104No ratings yet
- Process Synthesis: Chemical Engineering Process DesignDocument39 pagesProcess Synthesis: Chemical Engineering Process DesignYantiNo ratings yet
- Laminar Film Condensation Heat Transfer On A Vertical, Non-Isothermal, Semi-Infinite PlateDocument13 pagesLaminar Film Condensation Heat Transfer On A Vertical, Non-Isothermal, Semi-Infinite PlatekarthikeyanNo ratings yet
- What Is The Relationship Between Wavelength and FrequencyDocument3 pagesWhat Is The Relationship Between Wavelength and FrequencyMohsin TariqNo ratings yet
- CuddaloreDocument21 pagesCuddaloreRavi KrishnanNo ratings yet
- Chapter 11Document48 pagesChapter 11Mohan LalNo ratings yet
- Worksheet-4 1Document2 pagesWorksheet-4 1alyssasc323No ratings yet
- Field Trip Guide: Point B Eaton Canyon FallsDocument2 pagesField Trip Guide: Point B Eaton Canyon Fallsapi-298647997No ratings yet
- Chemistry 11th Edition by Chang ISBN 007766695X Test BankDocument20 pagesChemistry 11th Edition by Chang ISBN 007766695X Test Bankandrea100% (27)
- Learn by Heart IB Biology A1.1 SL HL 2 Activity Bundle Matching Activity Flashcard CreatorDocument8 pagesLearn by Heart IB Biology A1.1 SL HL 2 Activity Bundle Matching Activity Flashcard Creatoranitabananita2220No ratings yet
- Front Page - Physics Lab ReportDocument7 pagesFront Page - Physics Lab ReportRalph Edrean Omadto100% (2)
- Presentation On FarakkaDocument27 pagesPresentation On Farakkanoman13bd100% (2)
- ch10 PDFDocument18 pagesch10 PDFRodrigo S QuirinoNo ratings yet
- 20121211140948-Final B&C Brochure 111212 Web Version PDFDocument16 pages20121211140948-Final B&C Brochure 111212 Web Version PDFAsh DomadoNo ratings yet
- Soil GradationDocument4 pagesSoil Gradationmypenta2008100% (1)
WS Grade 10 IG Chemistry 23-24 - Energy Changes
WS Grade 10 IG Chemistry 23-24 - Energy Changes
Uploaded by
SiyaOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
WS Grade 10 IG Chemistry 23-24 - Energy Changes
WS Grade 10 IG Chemistry 23-24 - Energy Changes
Uploaded by
SiyaCopyright:
Available Formats
NPS International School
Name: ________________ Subject: Chemistry
Date: _________________ Grade: X IGCSE
Energy Changes
1 A student investigates the energy released when zinc powder reacts with copper
sulfate solution. The student uses the apparatus shown in the figure below.
1 g zinc powder
themometer
glass beaker
100 cm3 copper(II) sulfate solution
The student:
• measured 100 cm3 copper sulfate solution into a beaker
• measured the temperature of the copper sulfate solution
• puts 1 g zinc powder into the beaker
• stirred the mixture with a thermometer
• measured the highest temperature.
The student’s results were:
Initial temperature = 21 °C; Highest temperature = 32 °C
(a) Calculate the change in temperature.
[1]
(b) From the student’s results, explain why the reaction is exothermic?
[2]
(c) The energy diagram for the reaction is shown below.
NPSI/ Version1.0/23-24/Chem./10IG/02 Page No:1
NPS International School
(i) How can you tell from the energy diagram that the reaction is
exothermic?
[1]
(iii) Which arrow, shows the activation energy, Ea?
[1]
2 Ammonia is used to make nitrogen trifluoride, NF3. Nitrogen trifluoride is essential
to the electronics industry. It is made by the following reaction.
Bond energy is the amount of energy, in kJ/mole, needed to break or make one mole
of the bond. The bonds energies are given in the table below.
Bond Bond Energy (kJ/mol)
N-H 390
N-F 155
F-F 280
H-F 565
Determine if the above reaction is exothermic or endothermic. Show all your
necessary workings clearly.
[4]
3 Methane reacts with chlorine in the presence of sunlight to form chloromethane
(CH3Cl) and hydrogen chloride
(a) Write a balanced chemical equation for the reaction.
[1]
NPSI/ Version1.0/23-24/Chem./10IG/02 Page No:2
NPS International School
(b) The bond energies of some bonds are given below.
Bond Bond Energy (kJ/mol)
C-H 412
Cl-Cl 242
C-Cl 331
H-Cl 432
Calculate the enthalpy change of the reaction.
[3]
(c) Hence, is the reaction exothermic or endothermic?
[1]
(d) Explain your answer to (c), in terms of bond breaking and bond forming.
[2]
(d) Draw a labelled energy profile diagram for the reaction.
Energy
Progress of reaction [4]
NPSI/ Version1.0/23-24/Chem./10IG/02 Page No:3
NPS International School
4 A student burned four liquid fuels in order to compare the amount of energy they
released, in the form of heat. She used the apparatus shown below.
themometer
copper can
water
spirit burner
liquid fuel
The energy released when each fuel was burned was used to raise the temperature of
100 g of water. For each fuel, the student recorded the mass of fuel burned and the
increase in temperature of the water. Her results are summarized in a table.
Fuel Average Mass of Amount of Increase in
relative fuel fuel burned temperature(°C)
molecular mass burned (g) (mole)
Diesel 170 4 0.00235 15
Ethanol 46 3 0.0652 10
Methanol 32 2 0.0625 5
Petrol 114 1 0.00877 4
The best fuel is the one that releases the most energy.
(a) The student suggested that petrol was the best fuel. Explain why, using the
information in the table.
[2]
(b) Another student suggested that diesel was the best fuel. Explain why, using
the information in the table.
[2]
(c) In another experiment, a student burned propanol and then used his results to
calculate the energy released when one mole of propanol was burned. He then
compared his result with a value from a data book. The values are shown in
the table.
Energy released per mole of propanol burned (kJ)
Student’s result 1020
Data book value 2010
NPSI/ Version1.0/23-24/Chem./10IG/02 Page No:4
NPS International School
Suggest two reasons why the student’s result is lower than the data book value.
Reason 1 :
Reason 2 :
[2]
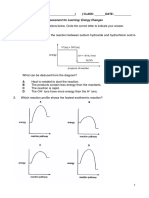
(d) The diagram shows the energy profile for burning a fuel.
Which arrow determines whether the reaction is endothermic or exothermic.
[1]
NPSI/ Version1.0/23-24/Chem./10IG/02 Page No:5
You might also like
- O Level Biology Practice Questions And Answers EnzymesFrom EverandO Level Biology Practice Questions And Answers EnzymesRating: 5 out of 5 stars5/5 (1)
- CH 4Document51 pagesCH 4Hoi An Sze100% (31)
- Bushong: Radiologic Science For Technologists, 9th Edition: Chapter 3: The Structure of Matter Test Bank Multiple ChoiceDocument7 pagesBushong: Radiologic Science For Technologists, 9th Edition: Chapter 3: The Structure of Matter Test Bank Multiple ChoicePring FamNo ratings yet
- OCR Chemistry A: 9 Enthalpy Exam-Style QuestionsDocument6 pagesOCR Chemistry A: 9 Enthalpy Exam-Style QuestionsHazare 2004100% (1)
- Chemistry Matriculation Note SK025 by Vinarti MahmudDocument47 pagesChemistry Matriculation Note SK025 by Vinarti MahmudNurun NajwaNo ratings yet
- 2013 YJC H2 Chem Prelim P2Document15 pages2013 YJC H2 Chem Prelim P2Chow Kim WanNo ratings yet
- Unconfined Vapor Cloud ExplosionDocument5 pagesUnconfined Vapor Cloud ExplosionMDR PRAPHU50% (2)
- A2 Chemistry Assessment 1 List - REVISION RESOURCEDocument46 pagesA2 Chemistry Assessment 1 List - REVISION RESOURCEHarry BarkerNo ratings yet
- Answer ALL The Following Questions Below. Circle The Correct Letter To Indicate Your AnswerDocument4 pagesAnswer ALL The Following Questions Below. Circle The Correct Letter To Indicate Your AnswerTimothy HandokoNo ratings yet
- 2017 Y5 T4 Chem Focus - KineticsDocument4 pages2017 Y5 T4 Chem Focus - KineticsxmxmxmxmxmNo ratings yet
- 2019 H2 Chem Prelim P3 QPDocument12 pages2019 H2 Chem Prelim P3 QPFaith SeahNo ratings yet
- First Pre-Board Examination (2019-2020) Class: Xii Subject: CHEMISTRY Date: 12.12.2019Document9 pagesFirst Pre-Board Examination (2019-2020) Class: Xii Subject: CHEMISTRY Date: 12.12.2019gaming with skdNo ratings yet
- CEM1008F Class Test 2 2019Document10 pagesCEM1008F Class Test 2 2019lia lightNo ratings yet
- Y12 3.2.2 Rates QuestionsDocument16 pagesY12 3.2.2 Rates Questionsmelissafulcher1234No ratings yet
- 06 (H1) Energetics (QNS)Document9 pages06 (H1) Energetics (QNS)Amelia WongNo ratings yet
- Chemistry SK025 Pre-Lab ModuleDocument20 pagesChemistry SK025 Pre-Lab ModuleNORSHAWANI BINTI MD RADZI MoeNo ratings yet
- Final Revision WorksheetDocument26 pagesFinal Revision Worksheetawash0takuNo ratings yet
- West Bengal University of TechnologyDocument4 pagesWest Bengal University of Technologysayan_dasNo ratings yet
- Chem FormatDocument4 pagesChem FormatMarc Joshua CadizNo ratings yet
- c3.2 Exam QuestionsDocument21 pagesc3.2 Exam Questionsfletcherberryheath2006No ratings yet
- Major Test 4 2021 FinalDocument8 pagesMajor Test 4 2021 FinalLevi JohnsonNo ratings yet
- 1assignment On Rates of Reaction and Energy ChangesDocument6 pages1assignment On Rates of Reaction and Energy ChangesShehryar IftikharNo ratings yet
- C3 Exercise 3Document8 pagesC3 Exercise 3Noor Liyana Ahmad FuadNo ratings yet
- Ch10 - Chemical Reactions - P4 - L1 - WS1Document1 pageCh10 - Chemical Reactions - P4 - L1 - WS1Aminul IslamNo ratings yet
- Sample Question Paper Chemistry (043) Class-XII, Session: 2021-22 TERM IIDocument5 pagesSample Question Paper Chemistry (043) Class-XII, Session: 2021-22 TERM IIShiny AlexNo ratings yet
- EnergeticsDocument6 pagesEnergeticsAmmar AbiddNo ratings yet
- CC Grade 11 Chemistry Energetics CWDocument3 pagesCC Grade 11 Chemistry Energetics CWMaliq MorrisNo ratings yet
- Ap09 Chemistry Form B q5Document10 pagesAp09 Chemistry Form B q5jessieNo ratings yet
- IB CHEM 1-4 WorksheetsDocument7 pagesIB CHEM 1-4 WorksheetsChung Khanh VUUNo ratings yet
- Che 211 Referred Eos 2022-23-2Document7 pagesChe 211 Referred Eos 2022-23-2Clevas MseluleNo ratings yet
- AS Enthalpy 3Document32 pagesAS Enthalpy 3verityfaye09No ratings yet
- Chapter 1 Reaction KineticsDocument7 pagesChapter 1 Reaction KineticsNurin QistinaNo ratings yet
- Answer All Question: Sk027 / Chapter 3: Reaction Kinetics / Exercise 1Document5 pagesAnswer All Question: Sk027 / Chapter 3: Reaction Kinetics / Exercise 1kjjkimkmkNo ratings yet
- Pre Lab Question 2022 - SK025Document18 pagesPre Lab Question 2022 - SK025HaziqahNo ratings yet
- Topic 1 Energetics WorksheetDocument5 pagesTopic 1 Energetics Worksheetindira.seebachanNo ratings yet
- Cambridge International Advanced Subsidiary and Advanced LevelDocument16 pagesCambridge International Advanced Subsidiary and Advanced LevelTrung Hoàng HuyNo ratings yet
- Class-12 Chemistry ElectroDocument4 pagesClass-12 Chemistry ElectroHemant ChaudharyNo ratings yet
- Checkup On Chapter 9: Questions Revision ChecklistDocument2 pagesCheckup On Chapter 9: Questions Revision ChecklistShahid Ur RehmanNo ratings yet
- MSS 1718MockPaper2Document8 pagesMSS 1718MockPaper2Kelvin ChowNo ratings yet
- T5 - 2017-2018 IB Chemistry SL QPDocument19 pagesT5 - 2017-2018 IB Chemistry SL QPRachelNo ratings yet
- Gr.11, Unit 3, Mod 15, L1, Reaction RatesDocument35 pagesGr.11, Unit 3, Mod 15, L1, Reaction Ratesaminbigman15No ratings yet
- National Senior Certificate: Grade 12Document21 pagesNational Senior Certificate: Grade 12MfanafuthiNo ratings yet
- Mid Term General Chem II Fall 2001Document6 pagesMid Term General Chem II Fall 2001dr.ibrahimsalemvpNo ratings yet
- Jan 2022 ExamDocument7 pagesJan 2022 ExamBeverly TshumaNo ratings yet
- IJC2009 Chem H2Prelim Paper 3Document12 pagesIJC2009 Chem H2Prelim Paper 3Bin RenNo ratings yet
- PHYS CE Tutorial QuestionsDocument3 pagesPHYS CE Tutorial QuestionsMel SalazarNo ratings yet
- Edexcel IAS Chemistry Classified Unit - 2 - Topic 1Document49 pagesEdexcel IAS Chemistry Classified Unit - 2 - Topic 1mostafa barakatNo ratings yet
- Common Foundation Organic Q in A LevelDocument21 pagesCommon Foundation Organic Q in A Level黄维燕No ratings yet
- QP Chem - XI - 2019-20 11Document5 pagesQP Chem - XI - 2019-20 11Lawrence GaikwadNo ratings yet
- AS Level Topic 8 TestDocument11 pagesAS Level Topic 8 TestMorvan BarnesNo ratings yet
- CCO 2013 Problems EnglishDocument12 pagesCCO 2013 Problems EnglishmarcusmaaaaaNo ratings yet
- Energy & Speed of RexDocument22 pagesEnergy & Speed of Rexcook n bakesNo ratings yet
- Chemistry: 2021 James Ruse Agricultural High School Year 11 Theory ExaminationDocument17 pagesChemistry: 2021 James Ruse Agricultural High School Year 11 Theory ExaminationYu-Tang LinNo ratings yet
- Physical Sciences: Paper Ii: Please Turn OverDocument16 pagesPhysical Sciences: Paper Ii: Please Turn OverBonga DubeNo ratings yet
- Organic ChemistryDocument5 pagesOrganic ChemistryEve LeeNo ratings yet
- IBO 2020 - Theory Exam 1Document65 pagesIBO 2020 - Theory Exam 1ConorNo ratings yet
- Chemistry (Solutions) : 2021 James Ruse Agricultural High School Year 11 Theory ExaminationDocument25 pagesChemistry (Solutions) : 2021 James Ruse Agricultural High School Year 11 Theory ExaminationYu-Tang LinNo ratings yet
- ChemDocument26 pagesChemyan45411No ratings yet
- ChemistryDocument7 pagesChemistryRajesh KhannaNo ratings yet
- Unit 5 - Kinetics Free Response PracticeDocument4 pagesUnit 5 - Kinetics Free Response Practiceridhimaspam0No ratings yet
- Term Iii Mock Examination June 2019-20: Key Stage 3 (Year IX)Document9 pagesTerm Iii Mock Examination June 2019-20: Key Stage 3 (Year IX)Rashad KhanNo ratings yet
- Enthalpy Review QuestionsDocument3 pagesEnthalpy Review Questionsranjana roy100% (1)
- O Level Biology Practice Questions And Answers Plant NutritionFrom EverandO Level Biology Practice Questions And Answers Plant NutritionRating: 5 out of 5 stars5/5 (1)
- Light - Standard Proctor Compaction Test of Soil (Is-2720-Part-7-1980)Document5 pagesLight - Standard Proctor Compaction Test of Soil (Is-2720-Part-7-1980)Kantrathi BhaskarNo ratings yet
- Chapter No. 4 (MCQS)Document3 pagesChapter No. 4 (MCQS)Akkas HaiderNo ratings yet
- Invisible Solar Cell (LLADocument24 pagesInvisible Solar Cell (LLAJustin FernandezNo ratings yet
- Ozone Layer Depletion and Global WarmingDocument15 pagesOzone Layer Depletion and Global WarmingSarah ShahNo ratings yet
- 0625 m17 QP 22Document16 pages0625 m17 QP 22Yash SubraNo ratings yet
- Chapter 3 - Water Resources: CBSE Notes Class 10 Social Science GeographyDocument4 pagesChapter 3 - Water Resources: CBSE Notes Class 10 Social Science GeographyEKLABAY SONINo ratings yet
- Earth Science Geosphere and Earth Systems Key IdeasDocument92 pagesEarth Science Geosphere and Earth Systems Key Ideasroni marudutNo ratings yet
- Surface TensionpdfFilename: Surface Tensionpdf PDFDocument18 pagesSurface TensionpdfFilename: Surface Tensionpdf PDFChandra SekharNo ratings yet
- Soal Pas Bahasa Inggris Kelas XiDocument5 pagesSoal Pas Bahasa Inggris Kelas Xiayu pandini maharaniNo ratings yet
- Numerical Modelling of Brine Dispersion in Shallow Coastal WatersDocument13 pagesNumerical Modelling of Brine Dispersion in Shallow Coastal WatersIAEME PublicationNo ratings yet
- Synthesis of PtZrO2 Catalyst On Fecralloy SubstratDocument12 pagesSynthesis of PtZrO2 Catalyst On Fecralloy SubstratMohamad Ali AkbarNo ratings yet
- Calculation of Carbon EmissionDocument3 pagesCalculation of Carbon EmissionEddie TaiNo ratings yet
- Cipta Grup1Document9 pagesCipta Grup1dwi104No ratings yet
- Process Synthesis: Chemical Engineering Process DesignDocument39 pagesProcess Synthesis: Chemical Engineering Process DesignYantiNo ratings yet
- Laminar Film Condensation Heat Transfer On A Vertical, Non-Isothermal, Semi-Infinite PlateDocument13 pagesLaminar Film Condensation Heat Transfer On A Vertical, Non-Isothermal, Semi-Infinite PlatekarthikeyanNo ratings yet
- What Is The Relationship Between Wavelength and FrequencyDocument3 pagesWhat Is The Relationship Between Wavelength and FrequencyMohsin TariqNo ratings yet
- CuddaloreDocument21 pagesCuddaloreRavi KrishnanNo ratings yet
- Chapter 11Document48 pagesChapter 11Mohan LalNo ratings yet
- Worksheet-4 1Document2 pagesWorksheet-4 1alyssasc323No ratings yet
- Field Trip Guide: Point B Eaton Canyon FallsDocument2 pagesField Trip Guide: Point B Eaton Canyon Fallsapi-298647997No ratings yet
- Chemistry 11th Edition by Chang ISBN 007766695X Test BankDocument20 pagesChemistry 11th Edition by Chang ISBN 007766695X Test Bankandrea100% (27)
- Learn by Heart IB Biology A1.1 SL HL 2 Activity Bundle Matching Activity Flashcard CreatorDocument8 pagesLearn by Heart IB Biology A1.1 SL HL 2 Activity Bundle Matching Activity Flashcard Creatoranitabananita2220No ratings yet
- Front Page - Physics Lab ReportDocument7 pagesFront Page - Physics Lab ReportRalph Edrean Omadto100% (2)
- Presentation On FarakkaDocument27 pagesPresentation On Farakkanoman13bd100% (2)
- ch10 PDFDocument18 pagesch10 PDFRodrigo S QuirinoNo ratings yet
- 20121211140948-Final B&C Brochure 111212 Web Version PDFDocument16 pages20121211140948-Final B&C Brochure 111212 Web Version PDFAsh DomadoNo ratings yet
- Soil GradationDocument4 pagesSoil Gradationmypenta2008100% (1)