Professional Documents
Culture Documents
Chapter 9 Note
Chapter 9 Note
Uploaded by
Teo Q RolOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chapter 9 Note
Chapter 9 Note
Uploaded by
Teo Q RolCopyright:
Available Formats
Chapter 9: Manufactured Substances in Industry
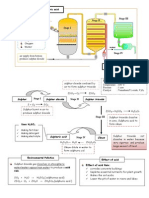
The manufacture of sulphuric acid, H2SO4 through the Contact Process Stage 1: Combustion of Sulphur In the furnace, molten sulphur is burnt in dry air to produce sulphur dioxide, SO2. The gas produced is purified and cooled. S(l) + O2(g) SO2(g) Stage 2: In the converter, sulphur dioxide, SO2 and excess oxygen gas, O2 are passed over a few plates of vanadium (V) oxide, V2O5 catalyst at 4500C to produce sulphur trioxide, SO3. 2SO2(g) + O2(g) 2SO3(g) About 99.5% of the sulphur dioxide, SO2 is converted into sulphur trioxide, SO3 through this reversible reaction. Uses of Sulphuric Acid Stage 3: In the absorber, the sulphur trioxide, SO3 is first reacted with concentrated sulphuric acid, H2SO4 to form a product called oleum, H2S2O7. SO3(g) + H2SO4(l) H2S2O7 The oleum, H2S2O7 is then diluted with water to produced concentrated sulphuric acid, H2SO4 in large quantities. H2S2O7(l) + H2O(l) 2H2SO4(l) The two reactions in the third stage are equivalent to adding sulphur trioxide, SO3 directly to water. SO3(g) + H2O(l) H2SO4(l)
Prepared by MHS 2009
The manufacture of ammonia, NH3 through the Haber Process 1. Gases mixed and scrubbed air with H2 gas from natural gas to form NH3. The two gases are mixed. The mixture is scrubbed to get rid of impurities. 2. Compressor 3. Converter through layers of iron catalyst with aluminium oxide as a promoter at a temperature of 4500C 5000C 4. Cooler Haber process combines N2 gas from the Then, it goes to the converter. It is then passed
A mixture of three gases leaves the converter. It is cooled until the ammonia condenses. The nitrogen One volume of N2 gas and three volume of and hydrogen are pumped back to the converter for H2 gas is compressed to a pressure of 200 another chance to react. 5. storage tanks 500 atm N2(g) + 3H2(g) 2NH3(g) NH3 is formed and then liquefy and separated to get a better yield. The NH3 is run into tanks and stored as a liquid under pressure.
Prepared by MHS 2009
Metals Most metals are solid Pure metals are made up of the same type of atoms and are of the same size. The arrangement of the atoms in metals gives the metals their ductile and malleable properties. The orderly arrangement of atoms in metals enables the layers of atoms to slide on one another when force is applied. Thus, metals are ductile or can be stretched. Alloy Bronze Composition 90% copper 10% tin -
Alloys A mixture of two or more elements with a certain fixed composition in which the major components is a metal. The aim of making alloys is to make them stronger, harder, resistant to corrosion, have a better furnish and luster. The presence of atoms of other metals that are of different sizes disturb the orderly arrangement of atoms in the metal. This reduces the layer of atoms from sliding. Thus, an alloy is stronger and harder than its pure metals. Uses In building of statues or
Properties Hard and strong Does not corrode easily Has shiny surface Harder than copper -
monuments In the making of medals, swords and artistic materials. In the making of musical
Brass
70% copper 30% zinc 99% iron 1% carbon
instruments and kitchenware Hard and strong In the construction of buildings and bridges In the building of the body of cars and railways tracks
Steel
Stainless Steel
74% iron 8% carbon 18% chromium 93% aluminium 3% copper 3% magnesium 1% manganese 96% tin 3% copper 1% antimony
Shiny Strong Does not rust Light Strong
In the making of cutlery In the making of surgical
instruments In the building of the body of aeroplanes and bullet train
Duralumin Pewter Prepared by MHS 2009
Luster Shiny Strong
- In the making of souvenirs
Polymers Polymers are large molecules made up of many identical repeating sub-units called monomers which are joined together by covalent bonds. Monomers are joined into chains by a process of repeated linking known as polymerization. A polymer may consists of thousands of monomers. Naturally occurring polymers: starch, cellulose, wool, protein. Silk and natural rubber Synthetic polymer Polythene Polypropene Ethene Propene Monomer
Synthetic Polymers Synthetic polymers are man-made polymers. The monomers used are usually obtained from petroleum after going through the refining cracking processes. Examples: polythene, polyvinyl chloride (PVC), polypropene, perspex, nylon and terylene. Synthetic polymers are very stable and do not corrode or decay, and also difficult to dispose. They may cause pollution, blockage of drainage systems and flash floods. Uses Plastic bags, shopping bags, plastic containers and insulation for electrical wiring Piping, bottle crates, carpets, car batteries and ropes Artificial leather, water pipes and records
Polyvinyl chloride, PVC Perspex Terylene
Chloroethene
Methylmethacrylate
Safety glass, reflectors, traffic signs and lens
Hexane-1,6-diol Clothing, sails and ropes Benzene-1,4-dicarboxylic acid Hexane-1,6-diamine Hexane-1,6-dioic acid Ropes, clothing and carpets.
Nylon Glass -
The major component of glass is silica or silicon dioxide, SiO2 which found in sand. Properties of glass: Transparent, hard but brittle, chemically inert, heat insulator, electrical insulator, impermeable to liquid
Prepared by MHS 2009
Fused glass (SiO2) Highly heat-resistant glass High transparency High melting point Resistant to chemical attack Uses Laboratory glassware, lenses, telescope, mirrors
Borosilicate glass (SiO2, Na2O, CaO, Al2O3, B2O3) - Low thermal expansion coefficient - Resistant to heat and chemical attack - Hight melting point Uses - Cooking utensuls, laboratory glassware, automobile headlights
Glass
-
Lead crystal glass (SiO2, Na2O, PbO, K2O, Al2O3) - Soft abd easy to meltn - High density - High refractive index Uses - Lead crystal glassware, art objects, lens, prisms
Soda-lime glass (SiO2, Na2O, CaO) Good chemical durability High thermal expansion coefficient Easy to make into different shapes Low melting point Uses Bottles, window panes, mirrors, electrical bulbs, flat glass and all kind of glass containers
Ceramics Ceramics are made from clay, for example kaolin, a hydrated aluminiumsilicate, Al2O3.2SiO2.2H2O. When the clay is heated to a very high temperature, they undergo a series of chemical reaction and are hardened permanently to form ceramics. Ceramics are very hard, brittle, have a very high melting point, chemically inert and do not corrode. The are good insulators of electricity and heat. Uses of ceramics: construction materials bricks, tiles, cement and pipes Ornamental articles bowls, cups, plates, vase and porcelain Electrical insulators spark plugs, fuses, insulators in electric iron and oven Superconductors
Prepared by MHS 2009
Composite Materials Composite materials is a structural material that is formed by combining two or more different substance such as metal, alloys, glass, ceramics and polymers. The resulting material has properties that are superior than those of the original components. Composite materials are created for specific application. Composite material Reinforced concrete Component Concrete Properties of component Properties of composite Uses of composites Construction of roads Rocket launching pads High-rise buildings
Steel
- Hard but - Stronger brittle - Higher tensile - Low tensile strength strength - Does not corrode easily - Strong in - Cheaper tensile - Can be moulded strength into any shape - Expensive - Can corrode - Can withsatand very high applied forces - Can support very heavy loads Insulators electricity of - Conducts electricity without resistance when cooled by liquid nitrogen
Superconductor - Copper (II) oxide - Tttrium oxide - Barium oxide Photochromic glass Glass
Magnetically levitated train Transformers Electric cable Computer parts Amplifier Information display panels Light detector devices Car windshields Optical lens
Silver chloride or silver bromide
Transparent - Reduce refraction Not sensitive of light to light - Control the amount of light passes Sensitive to light through it automatically - Has the ability to change colour and become darker when exposed to ultraviolet light -
Fibre optics
Glass with low refraction index
Transparent - Low material cost - Transmit data using Does not - Reflect light ray and light waves in reflect light allow to travel along telecommunications rays the fibre - Instruments for
Prepared by MHS 2009
Glass with higher refractive index
- Can transmit electronic data or signals, voice and images in a digital format, in the form of light along the fine glass tubes at great speeds High density - High tensile Strong but strength brittle - Moulded and Non-flexible shaped - Inert to chemicals Light - Light Flexible Inflammable - Strong Elastic but - Tough - Not inflammable weak - Impermeable to water - Resilient - Flexible -
examining internal parts of the body or inspecting the interiors of manufactured structural products
Fibre glass
Glass
Polyester plastic
Car bodies Helmets Skies Rackets Furniture Water storage tanks Small boats
Prepared by MHS 2009
Prepared by MHS 2009
You might also like
- BS en 13923-2005 PDFDocument44 pagesBS en 13923-2005 PDFBravo Rd50% (4)
- BS en 681-1-1996Document24 pagesBS en 681-1-1996Ravi Verma100% (1)
- Non Ferrous MetalsDocument7 pagesNon Ferrous MetalselleNo ratings yet
- Composite MCQDocument4 pagesComposite MCQMr. Shrikrushna Bhosale100% (1)
- Uses of H SODocument12 pagesUses of H SOFaakir GhazaliNo ratings yet
- CHEMISTRY SPM FORM 4 Short Notes Chapter 9 MANUFACTURED SUBSTANCES IN INDUSTRYDocument6 pagesCHEMISTRY SPM FORM 4 Short Notes Chapter 9 MANUFACTURED SUBSTANCES IN INDUSTRYJay Bee100% (9)
- Sulphuric Acid, H SODocument9 pagesSulphuric Acid, H SOcommen100% (3)
- Manufactured Substances in Industry2Document20 pagesManufactured Substances in Industry2Sam ZeeNo ratings yet
- Chemistry Form 4: Manufactured Substances in IndustryDocument12 pagesChemistry Form 4: Manufactured Substances in IndustryQisthina Azmina AbdullahNo ratings yet
- Chemistry Form 4 Chapter 9Document6 pagesChemistry Form 4 Chapter 9Suriati Bt A Rashid100% (1)
- Arrangement of Atoms in MetalsDocument12 pagesArrangement of Atoms in MetalsNada AliaNo ratings yet
- Manufactured Subtances in Industry: By: Nurfarahain Binti Ahmad 4ST SMK SG AbongDocument73 pagesManufactured Subtances in Industry: By: Nurfarahain Binti Ahmad 4ST SMK SG AbongSanthiya MadhavanNo ratings yet
- Chemistry SPMDocument20 pagesChemistry SPMJacob ChowNo ratings yet
- Inorganic Group 14 C, Si, Ge, SN, TBDocument12 pagesInorganic Group 14 C, Si, Ge, SN, TBLooi Chui YeanNo ratings yet
- Chemistry: Manufactured Substances in IndustriesDocument47 pagesChemistry: Manufactured Substances in IndustriesWan AkramNo ratings yet
- Key Aluminum Nitride PropertiesDocument10 pagesKey Aluminum Nitride PropertiesMuhammad TanweerNo ratings yet
- PiniSulphuric AcidDocument15 pagesPiniSulphuric AcidAsiah AsNo ratings yet
- IM - Proyecto Final InglesDocument33 pagesIM - Proyecto Final InglesLexi Noel Gutiérrez MéndezNo ratings yet
- Chapter 9: Manufactured Substances in Industry: Stage 1Document12 pagesChapter 9: Manufactured Substances in Industry: Stage 1malcovishesNo ratings yet
- Chapter 9 - NotesDocument7 pagesChapter 9 - NotesLi Lian KwangNo ratings yet
- Nonferrousmetal 171110093912Document68 pagesNonferrousmetal 171110093912maarof.emadNo ratings yet
- Ceramics Manufacturing, Properties & ApplicationsDocument6 pagesCeramics Manufacturing, Properties & ApplicationsRavi VermaNo ratings yet
- CeramicsDocument32 pagesCeramicsdicky0% (2)
- ChemistryDocument10 pagesChemistryfi3mnorNo ratings yet
- Metallic FibresDocument28 pagesMetallic Fibresmahe_ft100% (2)
- Application Notes Copper EnglishDocument6 pagesApplication Notes Copper EnglishMiguel Eduardo Cabrejos ChavezNo ratings yet
- Ch-27.9 Elastomer, Cearmic & CompositeDocument55 pagesCh-27.9 Elastomer, Cearmic & CompositeJyotilal SahuNo ratings yet
- Aluminium (Al) - Forms Casting BasedDocument13 pagesAluminium (Al) - Forms Casting BasedSansar PanchalNo ratings yet
- A. Metallic:: 3.1. Physical and Chemical PropertiesDocument7 pagesA. Metallic:: 3.1. Physical and Chemical PropertiesVinothKumarVinothNo ratings yet
- Engineering Chemistry: Manufacturing of Portland CementDocument9 pagesEngineering Chemistry: Manufacturing of Portland CementShubham KhandelwalNo ratings yet
- Ceramics 1Document39 pagesCeramics 1Pankaj YadavNo ratings yet
- Engineering Materials: Metals and Their Alloys Ceramics Polymers CompositesDocument53 pagesEngineering Materials: Metals and Their Alloys Ceramics Polymers CompositesSyed Muhammad AliNo ratings yet
- Ceramics 1Document7 pagesCeramics 1Andrea DouglasNo ratings yet
- MCR 321Document11 pagesMCR 321Rishu DeyNo ratings yet
- Manufacture D Substances in IndustryDocument53 pagesManufacture D Substances in IndustrySuriana ShamsuddinNo ratings yet
- Folio KimiaDocument12 pagesFolio KimiaAishiteru RamenNo ratings yet
- Unit303 Ceramics Callum Jakub Bronius DeclanDocument21 pagesUnit303 Ceramics Callum Jakub Bronius DeclanbroniusrupainisNo ratings yet
- Non Fe Lec 1Document41 pagesNon Fe Lec 1piks007No ratings yet
- Stage II Stage III: Sulphur Sulphur Dioxide Sulphur TrioxideDocument6 pagesStage II Stage III: Sulphur Sulphur Dioxide Sulphur TrioxideSulaiman Mohamad100% (1)
- Chemistry Form Four: Chapter 9: Manufactured Substances in IndustryDocument16 pagesChemistry Form Four: Chapter 9: Manufactured Substances in IndustrySafina IzwaniNo ratings yet
- Ferrous and Nonferrous MetalDocument45 pagesFerrous and Nonferrous MetalJad MacintoshNo ratings yet
- 11.2 Group IV CompoundsDocument7 pages11.2 Group IV CompoundsTrevor TatendaNo ratings yet
- Copper Design ManualDocument22 pagesCopper Design Manualamr_scorpion_engNo ratings yet
- Engineering Materials Metals Non MetalsDocument67 pagesEngineering Materials Metals Non MetalsVer MeoNo ratings yet
- Rautomead CC PDFDocument13 pagesRautomead CC PDFDayanand SharmaNo ratings yet
- Engineering MaterialsDocument11 pagesEngineering MaterialsThế Sự PhạmNo ratings yet
- Ceramic MaterialsDocument8 pagesCeramic MaterialsCharidBecerraNo ratings yet
- Chapter 9: Manufactured Substances in Industry: UsesDocument9 pagesChapter 9: Manufactured Substances in Industry: Useseyeda2000No ratings yet
- Chemistry Investigatory ProjectDocument17 pagesChemistry Investigatory ProjectthisismehmrNo ratings yet
- CeramicsDocument3 pagesCeramicsjannat00795No ratings yet
- Ceramics: Ceramics (Greek Keramos, "Potter's Clay"), Originally TheDocument10 pagesCeramics: Ceramics (Greek Keramos, "Potter's Clay"), Originally TheKholid FakhriyNo ratings yet
- 7 CeramicsDocument32 pages7 CeramicsSaurabh TripathiNo ratings yet
- Stuff To Remember (Chem)Document17 pagesStuff To Remember (Chem)Tamilore SobowaleNo ratings yet
- 107 1589687000 3bsc SilicatesDocument35 pages107 1589687000 3bsc SilicatesnarendradpuNo ratings yet
- Metals Problem Set 1Document5 pagesMetals Problem Set 1Rufo Daskeo Jr.No ratings yet
- Sekolah Menengah Sains Seri Puteri Kuala Lumpur: Manufactured Substances in IndustryDocument21 pagesSekolah Menengah Sains Seri Puteri Kuala Lumpur: Manufactured Substances in IndustryNur Wani Amat SujangiNo ratings yet
- BLD 121 Building Science Properties of Materials Ii1Document38 pagesBLD 121 Building Science Properties of Materials Ii1Abdulhamid KashimuNo ratings yet
- Assignment CeramicDocument5 pagesAssignment CeramicAltayeb AbdulhameedNo ratings yet
- Unit VDocument48 pagesUnit VRanjit ZendeNo ratings yet
- Iwcc Cu-Vortrag AldDocument16 pagesIwcc Cu-Vortrag Aldhadjlarbi-h100% (1)
- Evaluation of In-Plane Shear Test Methods For Composite Material LaminatesDocument6 pagesEvaluation of In-Plane Shear Test Methods For Composite Material Laminates3pherNo ratings yet
- Flatwise Compressive Properties of Sandwich Cores: Standard Test Method ForDocument8 pagesFlatwise Compressive Properties of Sandwich Cores: Standard Test Method ForEduardo SantosNo ratings yet
- Metallic MaterialsDocument3 pagesMetallic MaterialsEmre KaraNo ratings yet
- Mechanical Behaviour of y Ash/Sic Particles Reinforced Al-Zn Alloy-Based Metal Matrix Composites Fabricated by Stir Casting MethodDocument8 pagesMechanical Behaviour of y Ash/Sic Particles Reinforced Al-Zn Alloy-Based Metal Matrix Composites Fabricated by Stir Casting Methodsaeed jamalNo ratings yet
- Estruturas A340Document195 pagesEstruturas A340Jaques JaquesNo ratings yet
- FC 33Document34 pagesFC 33Pakdee KammungkunNo ratings yet
- 8th Ameron Asia Piping WorkshopDocument285 pages8th Ameron Asia Piping WorkshopHijau Auliya Keramat Al-qadiry50% (2)
- Physical and Morphological Characterisation of Typha Australis FibresDocument12 pagesPhysical and Morphological Characterisation of Typha Australis FibresIJAR JOURNALNo ratings yet
- Paek PDFDocument9 pagesPaek PDFjituni100% (1)
- A Review On NSM-CFRP Technique Using in Shear Strengthening of RC Deep BeamsDocument18 pagesA Review On NSM-CFRP Technique Using in Shear Strengthening of RC Deep Beamsbassem kooliNo ratings yet
- RT Duroid 5870 5880 Data SheetDocument2 pagesRT Duroid 5870 5880 Data SheetKingslayer RennesNo ratings yet
- MBrace Design Guide PDFDocument104 pagesMBrace Design Guide PDFjuanNo ratings yet
- Composites ASTMDocument5 pagesComposites ASTMEirick Wayne Zuñigga De-ItzelNo ratings yet
- Estado Del Arte de Las Resinas 2011Document10 pagesEstado Del Arte de Las Resinas 2011kelly johanna quintero arevaloNo ratings yet
- RMRS Rules - Part XIIIDocument169 pagesRMRS Rules - Part XIIIDaniele Di BenedettoNo ratings yet
- MWP11A - Learning Unit 8 PlasticsDocument17 pagesMWP11A - Learning Unit 8 PlasticsThapelo MokoenaNo ratings yet
- Shear Strength Analysis of Dissimilar Materials Bolted JointDocument4 pagesShear Strength Analysis of Dissimilar Materials Bolted JointSufyan Berinyuy WiysahnyuyNo ratings yet
- Mechanics of Composite MaterialsDocument4 pagesMechanics of Composite MaterialsDwi Antoro100% (1)
- Lab 4 - 3d Angle Interlock - Hannan HamimiDocument15 pagesLab 4 - 3d Angle Interlock - Hannan HamimiHannan HamimiNo ratings yet
- Construction and Building Materials: Salem Merabti, Said Kenai, Rafik Belarbi, Jamal KhatibDocument10 pagesConstruction and Building Materials: Salem Merabti, Said Kenai, Rafik Belarbi, Jamal Khatibjuan andre pariona mendozaNo ratings yet
- 107865-Aalba Dent CatDocument44 pages107865-Aalba Dent CatbuzatugeorgescuNo ratings yet
- Polymers: Fabrication and Characterization of Aluminum Nanoparticle-Reinforced CompositesDocument10 pagesPolymers: Fabrication and Characterization of Aluminum Nanoparticle-Reinforced CompositesTRNADEWNo ratings yet
- EMM ProjectDocument17 pagesEMM ProjectDHRUV SINGHALNo ratings yet
- Chapter-1: 1.1 GeneralDocument11 pagesChapter-1: 1.1 GeneralraviciviltNo ratings yet
- Multi-Criteria Decision-Making Approaches For Aircraft-Material Selection ProblemDocument24 pagesMulti-Criteria Decision-Making Approaches For Aircraft-Material Selection ProblemOmer HashimNo ratings yet
- A Study On 3D Carbon Braided CompositeDocument56 pagesA Study On 3D Carbon Braided CompositeDaryl LeeNo ratings yet
- CD 368 Design of Fibre Reinforced Polymer Bridges and Highway Structures-WebDocument55 pagesCD 368 Design of Fibre Reinforced Polymer Bridges and Highway Structures-WebRNo ratings yet