Professional Documents
Culture Documents
Alberta Infant Motor Scale2
Alberta Infant Motor Scale2
Uploaded by
Gonca AriOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Alberta Infant Motor Scale2
Alberta Infant Motor Scale2
Uploaded by
Gonca AriCopyright:
Available Formats
14698749, 1998, 7, Downloaded from https://onlinelibrary.wiley.com/doi/10.1111/j.1469-8749.1998.tb15399.x by Zentralbibliothek Zuerich, Wiley Online Library on [03/03/2023].
See the Terms and Conditions (https://onlinelibrary.wiley.com/terms-and-conditions) on Wiley Online Library for rules of use; OA articles are governed by the applicable Creative Commons License
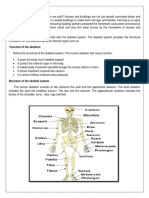
Assessment of gross Assessment for motor dysfunction or delay in at-risk infants
is an accepted practice at neonatal follow-up clinics. It is
motor skills of at-risk important to evaluatc the accuracy of the standardized mea-
sures used to evaluate infant gross motor skills for two rea-
sons. First, the evaluation of the efficacy of early treatment
infants: predictive for infants with motor dysfunction requires that screening
tests correctly identify both infants who will have persistent
validity of the Alberta .motor dysfunction, and infants who have normal gross
motor abilities. If infants with eventual normal motor abili-
ties are identified early in their lives as having motor dysfunc-
Infant Motor Scale tion, the internal validity of an efficacy study is severely
compromised. These infants may receive intervention ser-
vices and be classified as normal due to the intervention,
when in fact they would be normal without intervention.
Johanna Darrah* PhD PT, Assistant Professor, Faculty of Second, clinicians who arc administering screening tests
Rehabilitation Medicine, University ofAlbcrta, Edmonton, need to know the predictive abilities of the tests to correctly
AB, Canada, T6G 2G4; interpret the significance of an infant’s score to the parents.
Martha Piper PhD, President, University of British Columbia, Parents need to know both how accurate a test is at predict-
Vancouver, BC; ing the eventual motor abilities of infants (provided by sensi-
ManJoe Watt MB BS FHCP(C) FAAEM, Pediatric Site Chief, tivity and specificity calculations) and, given their infant’s
Glenrose Rehabilitation Site, Edmonton, AB; Canada. performance on the test, the likelihood that their infant will
encounter difficulty in their gross motor development (pro-
*Correspondence tofirst author. vided by the positive predictive value and negative predictive
value of a test). Definitions of sensitivity, spccificity, positive
predictive value, and negative predictive value are provided
in Figure 1.
Unfonunately, the accuracy of tests to identify motor
The Alberta Infant Motor Scale (AIMS) is a norm-referenced problems in both the neonatal period and in the first year of
measure of infant gross motor development. The objectives of life is disappointingly low. Investigators evaluating the pre-
this study were: (1) to establish the best cut-off scores on the dictive abilities of a variety of neonatal tests all report high
A I M S for predictive purposes, and (2) to compare the false-positive rates (Dubowitz et al. 1984. Allen and Capute
predictive abilities of the AMIS with those of the Movement 1989, Touwen 1990, Bozynski et al. 1993). That is, the
Assessment of Infants (MAI) and the Peabody neonatal assessments correctly identified many infants with
Developmental’GrossMotor Scale (PDGMS). One hundred eventual motor problems, but at the expense of incorrectly
and sisty-four infants were assessed at 4 and 8 months labeling many normally developing infants. The same trend
adjusted ages on the three measures. A pediatrician assessed applies to motor measures used by occupational and physi-
each infant’s gross motor development at 18 months as cal therapists during the first year of life. The Movement
normal, suspicious, or abnormal. For the AIMS, two different Assessment of Infants (MA) (Chandler et al. 1980) and the
cut-off points were identified: the 10th centile at 4 months Peabody Developmental Gross Motor Scale (PDGMS), a sub-
and the 5th centile at 8 months. The MAI provided the best scale of the Peabody Developmental Motor Scales (PDMS)
specificity rates at 4 months while the Aws was superior in (Folio and Fewell 1983), are two tests often used to evaluate
specificity at 8 months. Sensitivityrates were comparable . the gross motor abilities of infants at neonatal follow-up clin-
between the two tests. The PDGMS in general demonstrated ics, Poor specificity rates have been reported using the MAI at
poor predictive abilities. 4 and 8 months (Harris 1987, Swanson et al. 1992). and
Palisano (1986) reported low correlations between 12-
month and 18-month scores on both the PDMS and the
Bayley Scales of Infant Development (BSID) (Bayley 1969).
The Alberta Infant Motor Scale (AIMS) (Piper and Darrah
1994) is a recently published measure of infant gross motor
development used in neonatal follow-up clinics in the United
States and Canada.’. The AIMS demonstrates excellent inter-
rater reliability, test-retest reliability, and concurrent validity
with the PDGMS and the gross motor scale of the BSID (Piper
and Darrah 1994). As the next: step in evaluation of the AIMS,
clinicians need information regarding its predictive validity
and the cut-off points to use on the scale to-identlfy most
accurately the eventual motor abilities of at-risk infants.
The objectives of this study were: (1) to establish the scores
T h e AIMS score shcets are available from W 6.Saunders Company.
6277 Sea Harbor Dr, Orlando, FL 32821-9816, USA or. outside the
USA, from the local W B. Saunders Company representative.
DeL,elopnrentalnledicine& CbfldNeumlogy 1998,JO: 48-9 1 485
14698749, 1998, 7, Downloaded from https://onlinelibrary.wiley.com/doi/10.1111/j.1469-8749.1998.tb15399.x by Zentralbibliothek Zuerich, Wiley Online Library on [03/03/2023]. See the Terms and Conditions (https://onlinelibrary.wiley.com/terms-and-conditions) on Wiley Online Library for rules of use; OA articles are governed by the applicable Creative Commons License
on the A I M S that provided the best sensitivity and specificity MEASURES
rates, and (2) to compare the predictive abilities of the NMS Three motor measures were administered at 4 and 8months:
with the predictive abilities of the A M and the PDGMS. A the hW, the PDGMS, and the AIMS. The hW is a standard-
prospective longitudinal design was conducted. ized, criterion-referenced assessment tool developed by
physical and occupational therapists. It contains a total of 65
Method items divided into four subscales: muscle tone and posture,
SAMPLE primitive reflexes, automatic reactions, and volitional move-
Infants were recruited during their hospital stay from two ment. Muscle tone items are scored on a 6-point, bidirection-
tertiary neonatal intensive care units (NICU) in Edmonton, al scale to indicate hypotonic, normal, and hypertonic
Alberta. Any infant whose binhwcight wa.. I1500gwas eligi- muscle tone. Items in the other three subsections are scored
ble for inclusion. Pretern infants weighing > 1500g with a on a 4-point unidirectional scale, with 1 representing the
history of intraventricular hemorrhage Grades III or lv most developmentally mature response. The authors have
(Levene et al. 1982) identified by ultrasound assessment, developed 4- and 8-month risk profiles that indicate scores
were also included because of their increased risk of motor considered to be deviant for each item. Risk points are
dysfunction. Full-tern infants with hypoxic-ischemic summed to yield a total risk score. No norms are available.
encephalopathy (HIE) Stage I1 or I11 by Sarnat scoring 'I'he PDGMS, the gross motor component of thc PDMS, is
(Sarnat and Sarnat 1976) were also eligible for inclusion. a standardized assessment tool that evaluates gross motor
Infants with congenital anomalies were excluded from the development of children between birth and 83 months of
study as these infants' problems can be readily identified at age. It consists of 170 items divided into 17 age levcls and
birth. The sample recruited wa. representative of infants typ- coven five motor domains: reflexes, balance, non-locomo-
ically seen at neonatal follow-up clinics. tion mobility, locomotion, and receipt and propulsion.
Two hundred and one infants were recruited to the study. Items are scored on a 3-point ordinal scale with the middle
Twenty-six infants were lost to follow-up, and two infants score representing an emerging skill. Norms were gcnerat-
died before completing the study; one before discharge from ed using 617 children stratified o n geographical region of
the NICU, and the other from sudden infant death syn- the United States, race, sex, and age. Raw scores may be con-
drome. One hundred and seventy-three infants completed vened into age equivalents, developmental quotients, and
the study, and were assessed by the developmental pediatri- centile ranks.
cian at 18 months adjusted age. Complete data were The NMS is designed to measure motor skills from 40
obtained on 164 infants (87 male, 77 female). One hundred weeks postconception to independent walking. It contains
and fifty-six infants were born at I 3 6 weeks' gestation 58 items divided into foursubscalcs: prone, supine. sit, and
(mean=28.47 wk, SD 2.58 wk) with a mean birthweight of stand. Each item is specifically described in terms of the
1108g (SD 301.63g). Eight term infants, mean birthweight weight-bearing surface of the body, the posture necessary
31 15g (SD 748.29g) were recruited, seven of these infants to achieve the gross motor skill, and the antigravity or vol-
had Stage I I Sarnat score, and one infant had Stage 1 Sarnat untary movement performed by the infant in the position
score with a neonatal history of seizures. (Fig. 2). The AIMS has been developed as an observational
assessment requiring minimal handling and can be com-
pleted in 10 to 20 minutes. It has been normed o n 2202
infants aged 1 week to 18 months born in the province of
Alberta, Canada. Raw scores can be converted to centile
Method 1 Melhod 2 ranks and compared with the ranks of age-equivalent peers
Abnormal Normal Abnormal Normal in the normative sample.
rusDicious suspr10us
cui-on IOC
PROCEDUHES
abnormal A physical therapist, unaware of the infants' medical histories,
sospicious b
a, assessed each infant on all three measures at 4 and 8 months
of age. Corrected ages were used for preterm infants. These
cui-on C d
lor ages were selected as the MAI provides cut-off scores todistin-
normal guish infants most at risk for motor dysfunction for only these
two points in time. I'he measures were administered simulta-
neously; an infant's spontaneous movements were first
Sensitivity = a/(a+c) Specificity = dl(d+b)
Proportion of infants claksdied as Proportion of intanls classified as observed, and then specific items on the MAI and the PDGMS
abnormal or abnormaVsuspicious normal at 18 mo who were were administered. The average time to complete all three
at 18 mo who were correctly correctly identified by the cut-off assessments was 30 to 45 minutes.
identified by the cut-off
A developmental pediatrician assessed each infant blindly
Positive Predictive Vabe = &(aib) Negative Predictive Vabe = d/\d+c) at 18 months adjusted age to determine the final motor out-
Proportion of infants classified as Proportion of infants classified as come. His assessment included an evaluatip of posture,
abnormal or abnormahsp'kious by normal by the cut-off who were
the c u t d l who were classified as classified as normal at 18 mo oral-motor control, strabismus, muscle tone, evolution of
abnormal or abnormaVsuspmous at milestones, and reflexes, as described by Levine (1980).
18 mo From the results of this assessment, each infant's motor abili-
ties were classified as normal, suspicious, o r abnormal.
Criteria for suspicious and abnormal classifications are pro-
Figure 1:Two methods of calculation ofpredictive values. vided in Table 1.
486 DevelopmentalMedicine G Child Neurology 1W8.40:4 8 5 4 0 1
14698749, 1998, 7, Downloaded from https://onlinelibrary.wiley.com/doi/10.1111/j.1469-8749.1998.tb15399.x by Zentralbibliothek Zuerich, Wiley Online Library on [03/03/2023]. See the Terms and Conditions (https://onlinelibrary.wiley.com/terms-and-conditions) on Wiley Online Library for rules of use; OA articles are governed by the applicable Creative Commons License
DAlA ANALYSES athetoid quadriplegia (N=3), and severe global develop-
Sensitivity, specificity, and positive and negative predictive mental delay (N=5).
values were calculated for each of the three motor measures Table 11 provides the predictive values obtained on the
at both 4- and 8-month assessments. All values were calculat- three measures from the 4 and 8 months data when the
ed from a 2 x 2 table. To accommodate the suspicious group, intent is to identify only infants classified as abnormal at 18
which represents a third classification category, nvo methods months adjusted age. A range of centile ranks on the AiMS is
were used to calculate the values: first the infants in the sus- shown to illustrate the related changes in sensitivity and
picious category were included with the infants in the nor- specificity as the cut-offpoint increases. Selection of a cut-off
mal category; second, the values were calculated combining point must take into consideration the consequences ofbotlg
the infants in the suspicious category with the infants in the false-negative and false-positive identification. While ideally:
abnormal classification (Fig. 1). a test would provide 100%sensitivity and specificity, in reali-
ty a compromise must always be made between the two Val-
Results ues. Acut-off yielding the highest sensitivitywill result in low
At the 18-month evaluation, 128 infants (78.05%)were classi- specificity and increased false-'positive identification of nor-
fied as normal in their motor development, 14 infants mally developing infants. Because of our concern about the
(8.54%) were classified in the suspicious motor develop
ment group, and 22 infants (13.41%) were classified as
abnormal in their motor devclopment. The prevalence rate
of the suspiciousgroup is slightly lower than the 15% to 25%
usually cited in the literature (Bennett 1988). The suspicious
category typically includes children identified with commu-
nication disorders and fine motor dysfunction. The classifi-
cation of suspicious in this study was limited to mild
neuromotor abnormalities affecting only gross motor abili-
ties, and is similar to a recent study by Campbell et al. (1993)
which also defined the suspicious group only by motor
development, and reported a prevalence rate of8.496. Of the
14 infants in the suspicious category, four were not yet walk- Anhgtmty Movement
ing, nine had subtle signs of increased muscle tone, and one
demonstrated immature motor skills in association with a
hearing impairment.
The prevalence rate of 13.61% for infants classified as
abnormal in their gross motor skills is companble'with rates
cited in ocher studies (Coolrnan et al. 1985, Ornstein et al.
1991, Campbell et al. 1993). Diagnoses for children in the
abnormal category at 18 months included: spastic diplegia
( N = 6 ) , spastic triplegia (N=2), spastic quadriplegia ( N = 4 ) ,
spastic hemiplegia (N= I), hypotonic cerebral palsy ( N = l),
z'ir-
Table I Criteria for outcome classification at 18 months of age
Suspiciousclassitication
Must include one or more of the following criteria:
1. Not yet walking
2 . Asymmetry (movement and posture)
3. Retention of primitive retlexes
4. Isolated toe waikingwirh evidence of increased deep tendon
reflexes
5 . Walkingwith awide baseofsupport f
3 a
6..Severely pronated feet
7. Muscle weakness
Abnormal classification 20
Includes one or more of the following criteria:
1.Abnormal muscle tone (hypotonia or hypenonia) 0
0 1 2 3 4 5 6 7 8 D1011121314151617Ul
2. Absent or decreased deep tendon reflexes Age ImOnmS)
3. Retention ofprimitive reflexes
And
Significantly delayed motor abilities. Figure 2 Exaniple offormat ofALMS itenis. From 'Motor
If an infant has very delayed motor milestones without neurological Assessment of the Developing Infant. (Piper and Darrah
signs, the infant should be classified as abnormal in their gross 1394).Reprinted witb permissiotz of W B. Saunders
motor development. Conipany.
Motor Assessment of &-Risk InfantsJobannaDan& ef al. 487
14698749, 1998, 7, Downloaded from https://onlinelibrary.wiley.com/doi/10.1111/j.1469-8749.1998.tb15399.x by Zentralbibliothek Zuerich, Wiley Online Library on [03/03/2023]. See the Terms and Conditions (https://onlinelibrary.wiley.com/terms-and-conditions) on Wiley Online Library for rules of use; OA articles are governed by the applicable Creative Commons License
harmful effects on families of false-positive identification, we tification. The sensitivity rates for both the AlMS and MAI fall
selected cut-off points that represented the best balance at both ages compared with the values obtained when identi-
between sensitivity and specificity. Using information from fication is limited only to infants with an abnormal classifica-
evaluations at 4 months, the 10th centile rank on the AIMS tion at 18 months. The cut-off points used on the PDGMS d o
produced the best combination of sensitivity and specificity not provide an acceptable combination of sensitivity and
values. At 8 months, three cut-off points on the AIMS had specificity values: at 4 months the sensitivity is poor while at
acceptable combinations of sensitivity and specificity values. 8 months the specificity values are poor.
The 7th centile rank is optimal, but given the difficulty of Positive predictive values represent the proportion of
interpolating to this centile rank on the graph, either the 5th infants identified by the cut-off as being abnormal or suspi-
or 10th centile can be considered as an appropriate cut-off cious who actually are classified as abnormal or suspicious at
point at 8 months. 18 months. Positive predictive values are affected by the
The cut-off points selected for the MAI came from two prevalcnce of the disorder. Mausner and Kramer (1985) pro-
sources: > 9 risk points was recommended by Swanson et al. posed that it is only when the prevalence of the disorder in
(1992), and > 4 risk points by Piper et al. (1992). The cut-off the screening group reaches 15% to 20% that a 'respectable'
points selected for the PDGMS correspond to -1.0 SD (16th positive predictive value is achievable; in their exampie a
centile rank), -1.5 SD (6th centile rank), and-2 SD (2nd cen- respectable positive predictive value is between 75% and
tile rank); these cut-off points are suggested in the manual to 80%.The highest positive predictive values obtained in this
delineate between normal, suspicious, and abnormal scores. study occurred when the suspicious infants w e e included
At 4 months, the MAl cut-off of >9 risk points provided the with the abnormal infants (steTabk Ill). At 4 months the MA1
best combination of sensitivity and specificity valucs. While cut-off point of >9 risk points had the highest positive pre-
the AIMS had slightly better sensitivity (one more abnormal dictive value (69.2%), while at 8 months the highest positive
infant identifies, the MAI classified more infants in the sus- predictive value (79.3%) was obtained o n the AIMS using the
picious/normal classification. In contrast, at 8 months, the 5th centile as a cut-off point. ,
AIMS has the best specificityvalue, especially using the 5th or
7th centile as cut-off points. The only cut-off point on the Discussion
PDGMS that provided an acceptable combination of sensitiv- Three areas have been selected for discussion: (1) recom-
ity and specificity values was the 16th centile at the 4-month mended cut-off points on the AIMS, (2) comparison of the
assessment. predictive abilities of the three measures, and (3) the
Table 111 provides the predictive values when the suspi- effect of identification of suspicious infants o n predictive
cious infants are included in the abnormal category for iden- abilities.
Table Ik Sensitivity, specificity, positive predictive value and negative predictive
value far abvormal vs normal/suspicious classification (N=164)
Agekest Crct-ofl Sensitivity (%I SpeciJTcity(%) +PV V") -PV(%)
(N=22) (N=142)
4 months
AIMS centile 2nd 40.9 95.8 60.0 91.3
5th 54.5 89.4 44.4 92.7
10th 77.3 81.7 39.5 95.8
16th 77.3 77.5 34.7 95.7
25th 86.4 67.6 29.2 97.0
Icw total risk score > 4 81.8 74.6 33.3 96.3
>9 72.7 93.0 58.3 95.7
PDCMS cenrile 2nd 36.4 95.1 53.3 90.6
6th 50.0 93.0 52.4 92.3
16th 81.8 71.8 31.0 96.2
8 months
AlMS ccntilc 2nd 72.7 94.4 66.7 95.7
5th 86.4 93.0 65.5 97.8
7th 90.9 91.5 62.5 98.5
. 10th 90.9 85.9 50.0 98.4
16th 95.5 82.4 45.7 ,99.2
25th 100.0 63.4 29.7 100.0
MAI total riskscore >.I 100.0 57.0 26.5 100.0
>9 95.5 80.3 42.9 99.1
PDGMS ccntile 2nd 95.5 57.0 25.6 98.8
6th 95.5 48.6 22.3 98.6
16th 100.0 42.3 16.3 100.0
+p\! positive predictive values; -mnegative predictive values.
488 I~euelopmentalMedicineC ChildNeumlogy 1998.40: 485491
14698749, 1998, 7, Downloaded from https://onlinelibrary.wiley.com/doi/10.1111/j.1469-8749.1998.tb15399.x by Zentralbibliothek Zuerich, Wiley Online Library on [03/03/2023]. See the Terms and Conditions (https://onlinelibrary.wiley.com/terms-and-conditions) on Wiley Online Library for rules of use; OA articles are governed by the applicable Creative Commons License
'
RECOMMENDED CUT-OFF POINTS O N THE AIMS sent suspicious motor development. Of the 44 at-risk infants
Two different cut-off points are recommended for use with assessed, 10 had scores between 1and 2 SD below the mean
the AIMS to identify infants with abnormal motor develop- and three had scores that fell < 2 S D below the mean. We con-
ment. At 4 months, the 10th centile clearly provides the best cluded that these results concurred with the known preva-
combination of sensitivity and specificity values, while at 8 lence of neuromotor disturbances occurring in preterm
months the 5th centile provides the optimal values. Our infants in the first year of life. However, given the new infor-
choice of cut-off points is influenced by the conviction that mation available from this predictive study,we suggest using
false-positive identification of normally developing infants is the identified centile ranks to select infants for continued fol-
just as detrimental as false-negative identification of infants low-up. Use ofthe 16th centile (-1 SD) on the AIMS results in
with abnormal motor development. From this perspective, over-identification of normally developing infants, while use
more infants are Correctly identified at 8 months using the of the 2nd centile (-2 SD) will result in a poor rate of identifi-
5th centile rank, even though the sensitivity value obtained cation of infants with abnormal motor development. The
using the 10th centile rank is higher. The difference in sensi- results of this study question the use of the traditional stan-
tivity values between the 5th and 10th centiles represents dard deviation cut-off points in screening for motor dysfunc-
identification of one more abnormal infant using the 10th tion. Rather, the data suggest that the best centile ranks to
centile, while the specificityvalues account for 10 more nor- use as cut-off points on the AIMS are between the 16th cen-
mally developing infants correctly classified using the 5th tile and the 2nd centile.
centile. Thus there are more infants correctly classified using Unfortunately, different cut-off points on the AIMS have
the 5th centile at 8 months as a cut-off rather than using the been identified at different ages. What centile rank should
10th centile rank. However, ifthe intent is to identify as many be used as a cut-off between the two ages, or when screen-
infants with abnormal development as possible without ing infants older than 8 months? The answer must take into
regard to the concomitant decrease in specificity, then the consideration the concern regarding identification of nor-
10th centile rank should be used as a cut-off at 8 months. mally developing infants. To maintain the highest specifici-
These choices reflect the complexity of choosing cut-off ty values, the cut-off should be the 5th centile, realizing
options. that some infants with motor problems may be missed,
In the AIMS manual (Piper and Darrah 1994) we reported resulting in a lower sensitivity rate. If the intent of the
a case-validation study evaluating the ranking of infants with screening program 'is identification of the greatest number
established diagnoses and at-risk preterm infants. We used of infants with abnormal motor development and a lower
the traditional cut-off point of <-2 SD to delineate abnormal specificity rate will be tolerated, then the cut-off should be
development, and scores between -1 SD and -2 SD to repre- at the 10th centile.
Table IIk Sensitivity, specificity, positive predictive value and negative predictive
value for abnormal/suspicious vs normal classification (N=164)
Agehest Cut-ofl Sensitivity c4;) Speci@ity 6) +PV(%) -pvc36)
(N=36) (N= 128)
4 months
AIMS centile 2nd 30.6 96.9 73.3 83.2
5th 41.7 90.6 55.6 84.7
10th 58.3 82.8 48.8 87.6
16th 58.3 78.1 42.9 87.0
25th 72.2 69.5 40.0 89.9
M N total risk score >4 63.9 75.8 42.6 88.2
>9 50.0 93.8 69.2 87.0
P D C M S centile 2nd 25.0 95.3 60.0 81.9
6th 36.1 93.8 61.9 83.9
16th 63.9 72.7 39.7 87.8
8 months
AIMS centile 2nd 52.8 96. I 79.2 87.9
5th 63.9 95.3 79.3 90.4
7th 69.4 94.5 78.1 91.7
10th 72.2 89.1 65.0 91.9
16th 77.8 85.9 60.9 93.2
25th 86.1 66.4 41.9 94.4
MAI total risk score >4 88.9 60.2 38.6 95.1
>9 77.8 83.6 57.1 93.0
P D C M S centile 2nd 86.1 60.2 37.8 93.9
6th 91.7 52.3 35.1 95.7
16th 97.2 46.1 33.7 98.3
+ PV positivc predictive values; -W, negative predictive values.
M o t o r Assessment of At-Risk lnfantsjobonna Darrab el al. 489
14698749, 1998, 7, Downloaded from https://onlinelibrary.wiley.com/doi/10.1111/j.1469-8749.1998.tb15399.x by Zentralbibliothek Zuerich, Wiley Online Library on [03/03/2023]. See the Terms and Conditions (https://onlinelibrary.wiley.com/terms-and-conditions) on Wiley Online Library for rules of use; OA articles are governed by the applicable Creative Commons License
COMPARISON OF T H E PHEL)ICllVE VAI.UES 01;AIMS, M N , AND PDCMS normal gross motor abilities. This combination of difficult
Referring to Table II and the early identification of infants and eccentric itcms may contribute to the low levels ofspeci-
with definite diagnoses at 18 months, three conclusions are ficity achieved on rhe PDGMS at 8 months.
made: (1) sensitivity values of the AIMS and MAI are similar at
both ages of assessment, (2) specificity values obtained on CIASSIFICATION OF 'SUSPICIOUS' INFANTS
the AIMS and MAI are different at different ages, and (3) the Typically with screening tests, a cut-off equivalent to -2 SD is
PDGMS does not provide a very acceptable combination of recommended to identify infants with Significantly delayed
sensitivity and specificityvalues at either age. motor abilities, and a cut-off equivalent to the -1 SD is rec-
By using the recommended cut-off points for the AIMS at 4 ommended to identify infants with mild or suspicious motor
and 8 months and a cut-off of > 9 risk points for the MAI, we delays (Folio and Fewell 1983, Bayley 1993). Use ofthese cut-
obtained similar sensitivity values on both tests at 4 months off points is based on the assumption that infants with suspi-
(AIMS 77.3%versus MAI 72.7%). The MAI, however, provided cious motor development demonstrate motor abilities that
greater specificity at 4 months (MAI 93.0% versus AIMS are superior to infants with abnormal motor skills, but inferi-
81.7%). In contrast, at 8 months, while the sensitivity values or to infants with normal motorabilities. That is, their motor
again were comparable (AIMS 86.4% versus MAI 95.5%), the abilities are assumed to 'fall b e p e c n ' the two extremes o f
AIMS obtained better specificity values (AIMS 93.0% versus abnormal and normal motor abilities.
MAI 80.3%).It must be recognized that because of the small In this study, when the infants identified as suspicious in
sample o f infants classified as abnormal or suspicious, all sen- their motor development at 18 months were grouped with
sitivity values am subject to sampling error. The diffcrencc in infants with abnormal motor skills at 18 months, the sensitiv-
specificityvalues at the different ages may be explained by the ity values decreased, regardless of the cut-off used or the test
specific motor constructs emphasized by each measure. At 4 administered. In fact, the scores of infants classified as suspi-
months, the intewation ofprimitive reflexes may be the motor cious did not fall between the scores of infants classified as
construct that bcst distinguishes between abnormal and nor- normal and abnormal in their motor abilities. Instead, their
mal infants. The MI,with its emphasis on isolated testing of a scores display a large range of values. Centile ranks obtained
series of primitive reflexes, thus provides the best diSCrimind- from the raw scores of the suspicious infants on the AIMS
tion of normally developing infants. At 8 months, voluntary, ranged from 0 to the 75th centile at 4 months and from 0 to
spontaneous movement may be the motor construct that dif- the 35th centile at 8 months. The range of these scores con-
ferentiates between normal and abnormal motor abilities. firm that t o identify the majority of suspicious infants at
Thus the items on the AIMS, with their emphasis o n successful either 4 or 8 months of age, a very high cut-off point would be
execution of voluntaly movcments, may contribute to the needed. Use ofa high centile rank as a cut-off to obtain good
accurate identification of normally developing infants on the sensitivity will result in poor classification of normally devrl-
AIMS at this age. Although the MAI alsoevaluates postural con- oping infants (low specificity). The assumption that these
trol and voluntary movement, the style of assessment, which infants' motor abilities represent a continuum between
requires extensive handling of infants, may impede the obscr- those o f normal and abnormal infants may be too simplistic
vation of the infant's true spontaneous abilities. and merits re-evaluation. It appears that their pattern of
The MAI may be the best screening test to use at or before motor abilities is different but does not consistently place
4 months, given its better specificity values. After 4 months, them o n a theoretical continuum between the normal and
the AIMS provides better specificityvvalucswhile maintaining abnormal infants. These infants are heterogeneous in their
acceptable sensitivity values. Of course, choice of a test must motor abilities and may represent an artificial grouping of
also include ease of administration and scoring. 'The AIMS, infants with an array ofdifficulties.
with its observational format, is quick and easy to administer. This study exemplifies the challenges of choosing appro-
The main disadvantage of the MI is the inability to interpret priate cut-off scores on standardized tests to scrcen for
scores except at 4 and 8 months of age. motor dysfunction with at-risk infants. Choice of a cut-off
On the PDGMS, only the 16th centile at 4 months provid- must take into account the consequences of both false-nega-
ed a combination of sensitivity and specificity values that tive and False-positiveidentification of infants. We agree with
could be considered acceptable (81.8%and 7 1.8%).This rel- Allen and Alexander (1991) that false-positive identification
atively high cut-off results in a lowered specificity value. is costly both in terms of parental anxiety and the economic
Other cut-off points at 4 months produced very low sensitivi- costs of follow-up and intervention. Also, considering the
ty values and high specificity values, while at 8 months the lack ofstrong evidence of an efficacious treatment for infants
sensitivity values were high and the specificity values were with motor dysfunction, we chose to consider specificity val-
unacceptable. The contents of the items o n the PDGMS pro- ues as important as sensitivity values, and recommended cut-
vide the most likely explanation for these results. Many of the off points on the AIMS that reflected this philosophy. If
4- to 5-month items can bc accomplished by infants with clinicians using the AIMS are more concerned with the great-
impaired motor function (e.g. 'extending arms and legs', est identification ofinfants with abnormality, then the centile
'pedaling action', 'propping on extended arms'), resulting in rank chosen as a cut-off point should be increased. However,
poor sensitivity values. In contrast, the 8- to 9-month items the results of this study also suggest that arbitrarily increas-
involve complex skills (pivoting, creeping position [hands ing the value of the cut-off point does not guarantee
. and knees], crawling [hands and legs in contact with sur- improved identification of infants with suspicious motor
face]) that many normally developing infants are not yet dcvelopment at 18 months of age. Assessment of the early
practising. In addition, two items, 'raising up' (pull to sit signs of dysfunction shown by these children may need to
from supine using a chair) and 'scooting' (moves forward in include more than an evaluation oftheir gross motor abilities
a sitting position), are rarely observed even in infants with to improve identification.
490 "neveIopmenraIMedicineG- ChiIdNerrroIogy 1998,40: 48549
14698749, 1998, 7, Downloaded from https://onlinelibrary.wiley.com/doi/10.1111/j.1469-8749.1998.tb15399.x by Zentralbibliothek Zuerich, Wiley Online Library on [03/03/2023]. See the Terms and Conditions (https://onlinelibrary.wiley.com/terms-and-conditions) on Wiley Online Library for rules of use; OA articles are governed by the applicable Creative Commons License
Palmer F,Shapiro B, Wachtel R, Allen M,Hiller J, Harryman S,
Clinicians must be aware of the complexities of screening
Mosher B, Meinert C. Capute A. (1988) The effects of physical
and t h e identification of cut-off points on standardized mea- therapy on cerebral palsy. New EngIandJorcrnnlofhfedicine
sures. There is inore than one choice, d e p e n d e n t o n t h e 318: 803-8.
intent of t h e screening program and t h e a g e of t h e infant. Piper MC, D a d J. (1994) hfoforAssesnnenfof tbeDeueloping
This study provides some choices for cut-off points that c a n Infant. Philadelphia: WB Saunders.
be used with t h e AIMS. More research needs t o be done t o
--Pinnell LE, Byrne PJ, Watt MJ. (1992) Early developmental
screening: sensitivity and specificityof chronological and
identify t h e c o m p o n e n t s o f d e v e l o p m e n t that most accurate- adjusted scores.JouniafofBehaoiorufand Decelopnienfal
ly identify infants in t h c suspicious classification. Pediatrfcs13 95-10 1.
-Kunos VI, Willis DM, hlazcr BL. Ramsay $1, Silver KM. (1986) Early
Acceptedforpuhlication 9thJanuary 1998. physical therapy effects on the high-risk infant a randomked
controlled trial. Pediatrtcs 78: 2 16-24.
Rothbcrg AD, Goodman M,Jacklin IA, Cooper PA. (1991) Six-ycar
Acknoudedgenrents follow-up ofearly physiotherapy intervention in vety low birth
We thank Annette Kujda for her assistance. The research was weight infants. Pediatrics 88: 547-52.
supported by National Health Research and Development Program, Samat HB, Samat MS. (1976) Neonatal enccphalopadiy following
Health Canada. fetal distress. Archives ofNeurology33:696-705.
Swanson tMvc( Bennett FC, Shy KK. K'hitfield MF. (1992)
Identification of neumdcvelopmental abnormality at four and
eight months by the Movement Assessment of Infants.
References DeuelopmentalMedicfneundCbildh'rtrrology 34:32 1-37.
Allen MC, Alexander GK.(1991) Screening for cerebral palsy in Thelen E. (1995) Motor development. A new synthesis. Anrerican
preterm infants: delay criteria for motor milestone attainment. Psj.CbOlOgiSt 50: 79-95.
Joirrnal ofPerinatology14: 190-3. Touwen BCL. (1990) Variability and stereotypy of spontmeous
- Caputc AJ. (1989) Neonatal neurodevelopmental eltamination as motility as 3 predictor of neurological development of pretenn
a predictor of neuromotor outcome in premature infants. infants. Der*elopnietitulMedicine and Child h'eirrology
Pediatrics 83: 498-506. 3 2 501-8.
Bayley N. (1969) Manualfor the BayfeyScales of Itflatit
Developnietit.New York: Psychologicd Corporation.
- (1993)BuylqScales oflnfanfDeveloprent. 2nd edn. San
Antonio, TX:Psychological Corporation.
Bennett FC. (1988) Ncurodevelopmental outcome in low-
birthweight infants: the role ofdevelopmental intervention. In:
RB Guthrie. editor. NeonatallntensiueCare. NewYork: Churchill
Livingstone. p 22149.
Bognski MFA. Dipierro IMA. MrisclsSJ. PlunkettJW Burpee B,
Clatlin CJ. (1993) Cranial sonogrdphy and neurological
examination at term and motor pcrformance through 19 months
v f age.Joiinral ofl?e~eIopnrerital and RebnvioralYedi~i trics
1 4 1124.
Campbell MK, Halincia E, Carlyle MJ, Fox AM, Turner LA, Chance
GW (1993) Factors predictive of follow-up clinic attendance and
developmental outcome in a regional cohort ofvery low binh
weight infants. Anieric~nri.loirmnlofEpider?iioIo~ 138:70.4- 13.
Chandler L!!, Andrews MS, Swanson MW (1980) Mooernent
Assessment of hgctnts:A r\funrta/.Washington: Kolling Bay.
Coolman RB, Bennett FC, Sells CJ, Swanson M, Andrew MS,
Robinson NM. (1985) Neuromotor development of graduates of
the neonatal intensive care unit: patterns encountered in the first
two years of life.Journal of I~eilelopnreritnfarid Bcbhnuiornl
Pediatrics 6:327-33.
Dubowitz LMS, Dubowitz Y Palmer PG,hliller G , Fawer CL, Lcvene
MI. (1984 Correlation of neurologic assessment in the preterm
newborn infant with outcome at 1 y e a r . / o i m i u l o ~ ~ d j ~ f ~ c s
105 452-6.
Folio MR, Fewell RR. (1983) Peabody Developmental Motor Scales
andActivlty Curds:A hfanud. Allen: DLM leaching Resources.
Harris SR.(1987) Early detection ofcerebral palsy: sensitivity and
specificity of two motor assessment tools.JournaIof
Perfnutology7 11-5.
k v e n e M1,Fawer CL,lamont RF.(1982) Risk factors in the
development of intravenuicular haemorrhagc in the preterm
neonate.Archives ofDisease in Childhood57 4 1&7.
k v i n e MS.(1980) Cerebral palsy diagnosis in children over age 1
year: standard criteria. Arcbives of Physical Medicirie and
Rehabilitation6 1 385-9.
hlausnerJS, Knmer S. (1985) EpfdPmiofogy.an Inlroducfory Text.
Zndedn. Philadelphia: W B Saunders.
Ornstein M. Ohlsson A, EdmondsJ, hsztalos E.(1991) Neonatal
follow-up ofvery low binhweight/emmely low birthweight
infants to school age: a critical overview.Acta Puediatrica
Scandinauica80.74 1-8.
Palisano RJ. (1986) Concurrent and predictive validities of the
Bayley Motor Scale and the Peabody Developmental Motor
Scales. PhysfcalTherapy6 6 1714-9.
Motor AssessmentofAt-RiskInfantsJobunnaDam&et al. 491
You might also like
- Stonehearst AsylumDocument2 pagesStonehearst AsylumTeodora MurdzoskaNo ratings yet
- Neuro-Sensory Motor Development AssessmentDocument9 pagesNeuro-Sensory Motor Development AssessmentIin PuspariniNo ratings yet
- Clasifications/ Gordon Functional Health Patterns Cheat SheetDocument2 pagesClasifications/ Gordon Functional Health Patterns Cheat SheetRaijenne Versola100% (1)
- 3-Ascending Tracts of Spinal CordDocument28 pages3-Ascending Tracts of Spinal Cordmuhammad altaf100% (2)
- Kessler 2005Document11 pagesKessler 2005mccg1478No ratings yet
- Assessment Gross: Infants: ofDocument7 pagesAssessment Gross: Infants: ofIngrid BarkoNo ratings yet
- GMFCS Stability & ReliabilityDocument5 pagesGMFCS Stability & ReliabilityIsabela RéduaNo ratings yet
- Psychometric Properties of The Pediatric Motor Activity Log Used For Children With Cerebral PalsyDocument9 pagesPsychometric Properties of The Pediatric Motor Activity Log Used For Children With Cerebral PalsyMaria DarribaNo ratings yet
- AIMS ValidationDocument10 pagesAIMS ValidationArtur SalesNo ratings yet
- Alberta Infant Motor Scale3Document6 pagesAlberta Infant Motor Scale3Gonca AriNo ratings yet
- Prechtls Method To Assess General Movements InterDocument13 pagesPrechtls Method To Assess General Movements InterMeliana SulistioNo ratings yet
- Fosang2003 PDFDocument7 pagesFosang2003 PDFmilhimNo ratings yet
- DownloadDocument7 pagesDownloadalvinakemNo ratings yet
- Have Infant Gross Motor Abilities Changed in 20 YeDocument6 pagesHave Infant Gross Motor Abilities Changed in 20 YeMarcela Dalla PortaNo ratings yet
- The Motor Control Assessment: An Instrument To Measure Motor Control in Physically Disabled ChildrenDocument5 pagesThe Motor Control Assessment: An Instrument To Measure Motor Control in Physically Disabled Childrennandhini raguNo ratings yet
- Early Developmental Assessment With A Short Screening Test, The STEP, Predicts One-Year OutcomesDocument9 pagesEarly Developmental Assessment With A Short Screening Test, The STEP, Predicts One-Year OutcomesCaroline Abou HadirNo ratings yet
- Concurrencia PeabodyDocument8 pagesConcurrencia PeabodyIngrid BarkoNo ratings yet
- G DoxDocument7 pagesG Doxqinxiang liNo ratings yet
- Clinical Tools Used in Young Infants Born Very Preterm To Predict Motor and Cognitive Delay (Not Cerebral Palsy) - A Systematic ReviewDocument9 pagesClinical Tools Used in Young Infants Born Very Preterm To Predict Motor and Cognitive Delay (Not Cerebral Palsy) - A Systematic Reviewdra.bravodanielaNo ratings yet
- DMCN 14910Document11 pagesDMCN 14910Jem Rhod CamenseNo ratings yet
- Clinical Tools Used in Young Infants Born Very Preterm To Predict Motor and Cognitive Delay (Not Cerebral Palsy) : A Systematic ReviewDocument9 pagesClinical Tools Used in Young Infants Born Very Preterm To Predict Motor and Cognitive Delay (Not Cerebral Palsy) : A Systematic ReviewAna paula CamargoNo ratings yet
- 5a21 PDFDocument15 pages5a21 PDFekaNo ratings yet
- Development GMFCSCPDocument5 pagesDevelopment GMFCSCPPatricia González SánchezNo ratings yet
- The General Movement Checklist: A Guide To The Assessment of General Movements During Preterm and Term AgeDocument8 pagesThe General Movement Checklist: A Guide To The Assessment of General Movements During Preterm and Term AgeAndreea ManeaNo ratings yet
- The Gross Motor Function Classification System For Cerebral PalsyDocument5 pagesThe Gross Motor Function Classification System For Cerebral PalsyMarco Tulio FigueroaNo ratings yet
- Perceptual Skills With: Test-Retest Reliability of The Test of Visual Children With Learning DisabilitiesDocument6 pagesPerceptual Skills With: Test-Retest Reliability of The Test of Visual Children With Learning DisabilitiesZidna Dhiya'ul HusnaNo ratings yet
- PTJ 0393Document8 pagesPTJ 0393Leila Leandro ReisNo ratings yet
- Pedi-Cat PCDocument7 pagesPedi-Cat PCLetícia AndradeNo ratings yet
- Bosanquet Etal DMCN 2013Document9 pagesBosanquet Etal DMCN 2013ramopavelNo ratings yet
- Yabunaka 2011Document9 pagesYabunaka 2011Carolina BorchesNo ratings yet
- NeuropsicologiaDocument10 pagesNeuropsicologiaMaria Del Mar Marulanda GrizalesNo ratings yet
- Clasificationwithparents GMFCSDocument4 pagesClasificationwithparents GMFCSPatricia González SánchezNo ratings yet
- The Bruininks-Oseretsky Test of Motor Proficiency-Short Form Is Reliable in Children Living in Remote Australian Aboriginal CommunitiesDocument12 pagesThe Bruininks-Oseretsky Test of Motor Proficiency-Short Form Is Reliable in Children Living in Remote Australian Aboriginal CommunitiesCristiana FerreiraNo ratings yet
- Develop Med Child Neuro - 2013 - Bos - Development of Fine Motor Skills in Preterm InfantsDocument4 pagesDevelop Med Child Neuro - 2013 - Bos - Development of Fine Motor Skills in Preterm Infantscamilamogollon2001No ratings yet
- Test of Infant Motor Performance (TIMP)Document6 pagesTest of Infant Motor Performance (TIMP)Juan Pablo NavarroNo ratings yet
- GMA Guide 2020Document8 pagesGMA Guide 2020CamilaNo ratings yet
- Amit 2024 Oi 231496 1704211711.82967Document14 pagesAmit 2024 Oi 231496 1704211711.82967Eduardo LimaNo ratings yet
- Acurácia e Precisão PEDI-CATDocument7 pagesAcurácia e Precisão PEDI-CATLetícia AndradeNo ratings yet
- 2011 - T Han - Comparisonofthegmfm66andthepedifunctionalskillsmobretrieved-2014-07-28Document6 pages2011 - T Han - Comparisonofthegmfm66andthepedifunctionalskillsmobretrieved-2014-07-28api-260049180No ratings yet
- Denver II, Dev DelDocument11 pagesDenver II, Dev DelLilisNo ratings yet
- STEPpsychometrics JPerinatology 2018Document10 pagesSTEPpsychometrics JPerinatology 2018Maria Juliana Giron TelloNo ratings yet
- Abordagens de Reabilitação para Crianças Com Paralisia CerebralDocument10 pagesAbordagens de Reabilitação para Crianças Com Paralisia CerebralKelly AssisNo ratings yet
- What Assessments Evaluate Use of Hands in Infants? A Literature ReviewDocument5 pagesWhat Assessments Evaluate Use of Hands in Infants? A Literature ReviewMaria Camila SanabriaNo ratings yet
- Wachtel CATCLAMS CLINPED1994Document6 pagesWachtel CATCLAMS CLINPED1994Dewi Andini PutriNo ratings yet
- Valentini2016 (TGMD3)Document22 pagesValentini2016 (TGMD3)Nur SetyasihNo ratings yet
- Reliability of TGMD A Systematic ReviewDocument26 pagesReliability of TGMD A Systematic Review何阳No ratings yet
- Nihms 726255Document18 pagesNihms 726255Jem Rhod CamenseNo ratings yet
- Kinematic Cabeza Pretermino 2015Document6 pagesKinematic Cabeza Pretermino 2015LucyFloresNo ratings yet
- Alberta Infant Motor Scale (AIMS) - A Clinical Refresher and Update On Evaluation of Normative DataDocument8 pagesAlberta Infant Motor Scale (AIMS) - A Clinical Refresher and Update On Evaluation of Normative DataCamila AlmeidaNo ratings yet
- Movement-Based Interventions For Preschool-Age Children With, or at Risk Of, Motor Impairment: A Systematic ReviewDocument7 pagesMovement-Based Interventions For Preschool-Age Children With, or at Risk Of, Motor Impairment: A Systematic ReviewAlexPsrNo ratings yet
- Lin Sell 2016Document16 pagesLin Sell 2016Farin MauliaNo ratings yet
- Dewar2021 2Document10 pagesDewar2021 2Gonca AriNo ratings yet
- Develop Med Child Neuro - 2014 - Curtis - The Central Role of Trunk Control in The Gross Motor Function of Children WithDocument7 pagesDevelop Med Child Neuro - 2014 - Curtis - The Central Role of Trunk Control in The Gross Motor Function of Children WithAmr Mohamed GalalNo ratings yet
- Kinamatic Analisis Por Video Mov 2016Document7 pagesKinamatic Analisis Por Video Mov 2016LucyFloresNo ratings yet
- Evaluation of Short Term Intensive Orthotic.10Document4 pagesEvaluation of Short Term Intensive Orthotic.10silvaines06No ratings yet
- Prognosis For Gross Motor Function in CPDocument7 pagesPrognosis For Gross Motor Function in CPKamila PachecoNo ratings yet
- Parent Article - PerthDocument9 pagesParent Article - PerthKriti ShuklaNo ratings yet
- Test de Bruininks-OseretskyDocument10 pagesTest de Bruininks-OseretskyJuan Pablo NavarroNo ratings yet
- What 'S Missing in Autism Spectrum Disorder Motor Assessments?Document13 pagesWhat 'S Missing in Autism Spectrum Disorder Motor Assessments?MárciaValenteValenteNo ratings yet
- Cerebral Palsy PDFDocument12 pagesCerebral Palsy PDFapi-319493532No ratings yet
- Gross Motor Function Classification System Expanded & Revised (GMFCS E & R) - Reliability Between Therapists and Parents in BrazilDocument6 pagesGross Motor Function Classification System Expanded & Revised (GMFCS E & R) - Reliability Between Therapists and Parents in BrazilIsabela RéduaNo ratings yet
- Sensibilidad Peabody PDFDocument9 pagesSensibilidad Peabody PDFIngrid BarkoNo ratings yet
- Ccrn Certification Examination Practice Questions and Answers with Rationale: First EditionFrom EverandCcrn Certification Examination Practice Questions and Answers with Rationale: First EditionRating: 5 out of 5 stars5/5 (1)
- Logic Synthesis for Genetic Diseases: Modeling Disease Behavior Using Boolean NetworksFrom EverandLogic Synthesis for Genetic Diseases: Modeling Disease Behavior Using Boolean NetworksNo ratings yet
- My Experience With DreamsDocument7 pagesMy Experience With DreamsPamela Rivera SantosNo ratings yet
- Neuroanatomy BioDocument6 pagesNeuroanatomy BioLarry LeNo ratings yet
- Sciencedirect: Eeg Study of Emotional Intelligence Among AdolescentsDocument5 pagesSciencedirect: Eeg Study of Emotional Intelligence Among AdolescentsLupu AdrianaNo ratings yet
- States of ConsciousnessDocument87 pagesStates of ConsciousnessMegan Horswood Morrow100% (1)
- Nervous System: © 2012 Pearson Education, IncDocument185 pagesNervous System: © 2012 Pearson Education, IncCjay AdornaNo ratings yet
- Simple Guide For Vestibular Rehabilitation ExercisesDocument4 pagesSimple Guide For Vestibular Rehabilitation ExercisesNeon MercuryNo ratings yet
- Physical Therapy Progress Report1Document3 pagesPhysical Therapy Progress Report1ERAAO TagumNo ratings yet
- Differential Diagnosis of Patients Presenting With HallucinationsDocument6 pagesDifferential Diagnosis of Patients Presenting With HallucinationsJack MilesNo ratings yet
- Bufo - Rana-Wps - Office (1) - Read-OnlyDocument17 pagesBufo - Rana-Wps - Office (1) - Read-OnlyjqgjwgnnwkNo ratings yet
- Impactul Disfunctiei Articulatiei Sacroiliace in Durerea LombaraDocument6 pagesImpactul Disfunctiei Articulatiei Sacroiliace in Durerea LombaraLarisa OlaruNo ratings yet
- Assessment of Fine and Gross Motor Skills in Children: January 2018Document19 pagesAssessment of Fine and Gross Motor Skills in Children: January 2018Koiy VannakNo ratings yet
- 1.1 Sense Organ and Their FunctionDocument20 pages1.1 Sense Organ and Their FunctionReduan Ibrahim100% (3)
- NeuroestimulacionDocument5 pagesNeuroestimulacionWALTER GARCÍA TERCERONo ratings yet
- Intracranial HypotensionDocument17 pagesIntracranial HypotensionNikhil TyagiNo ratings yet
- Pathophysiology of SeizureDocument37 pagesPathophysiology of SeizureTJ ArellanoNo ratings yet
- Disorders of The Eccrine Sweat Glands and SweatingDocument8 pagesDisorders of The Eccrine Sweat Glands and SweatingAnnisa Chaerani BurhanuddinNo ratings yet
- 7' VisionDocument17 pages7' VisionZidane ZizouNo ratings yet
- Fear PsychosisDocument2 pagesFear Psychosisminty2011No ratings yet
- Adrenergic Neuron BlockersDocument3 pagesAdrenergic Neuron BlockersSyeda Nishat Fathima TajNo ratings yet
- Human Evolution of Social Temperaments: Original Human As Bipedal BonoboDocument49 pagesHuman Evolution of Social Temperaments: Original Human As Bipedal Bonobodao einsnewt100% (2)
- Movement Posture and AlignmentDocument6 pagesMovement Posture and AlignmentShivalikaNo ratings yet
- Presentasi Penjelasan 1Document17 pagesPresentasi Penjelasan 1sri wulan100% (1)
- Neuro MarketingDocument22 pagesNeuro MarketingPranav AgarwalNo ratings yet
- Problem: Pain at 6/10 For A "Sore Right Shoulder" Date Evaluated: December 14, 2020Document2 pagesProblem: Pain at 6/10 For A "Sore Right Shoulder" Date Evaluated: December 14, 2020florenzoNo ratings yet
- Skeletal SystemDocument15 pagesSkeletal SystemKarla OñasNo ratings yet
- Bioelectromagnetic Medicine - The Book - Share The WealthDocument2 pagesBioelectromagnetic Medicine - The Book - Share The WealthStellaEstelNo ratings yet