Professional Documents
Culture Documents
10 Pharma Thyroid Antithyroid
10 Pharma Thyroid Antithyroid
Uploaded by
isahCopyright:
Available Formats
You might also like
- Mcqs For Frcophth Part 2Document253 pagesMcqs For Frcophth Part 2Ophthalmology PLUS100% (4)
- Amboss ECGDocument13 pagesAmboss ECGAllysahNo ratings yet
- Gyne - Benign LesionsDocument4 pagesGyne - Benign LesionsIsabel CastilloNo ratings yet
- Thyroid Hormones & Anti Thyroid AgentsDocument3 pagesThyroid Hormones & Anti Thyroid AgentsJaybee SarmientoNo ratings yet
- 5,6-Thyroid HyperhypoDocument16 pages5,6-Thyroid Hyperhypomogesie1995No ratings yet
- 2s Finals Pharma BeshyDocument60 pages2s Finals Pharma BeshyJohanei Mae PeraltaNo ratings yet
- 1 - Pharmacology - Thyroid & Antibone DrugsDocument20 pages1 - Pharmacology - Thyroid & Antibone DrugsSoc Gerren TuasonNo ratings yet
- HormonesDocument9 pagesHormonesChandanaNo ratings yet
- PhysioLec 8 Thyroid Metabolic Hormones (Dr. Queenie)Document4 pagesPhysioLec 8 Thyroid Metabolic Hormones (Dr. Queenie)Lafayette DelantarNo ratings yet
- Thyroid Gland Clinical Chemistry 2 (Laboratory) : LessonDocument4 pagesThyroid Gland Clinical Chemistry 2 (Laboratory) : LessonCherry Ann ColechaNo ratings yet
- Endocrine Physiology) 8. Synthesis of Thyroid Hormone - KeyDocument1 pageEndocrine Physiology) 8. Synthesis of Thyroid Hormone - Keysingonstrings365No ratings yet
- Endocrine Physiology 231212 051058Document20 pagesEndocrine Physiology 231212 051058Charan SreeramdasNo ratings yet
- Topic3.3 Physiology Thyroid HormoneDocument7 pagesTopic3.3 Physiology Thyroid HormoneRen AlvNo ratings yet
- Im-Thyroid DisordersDocument9 pagesIm-Thyroid DisordersRyan F. BernalNo ratings yet
- Antithyroid DrugsDocument32 pagesAntithyroid DrugsLUMUMBA OluwatomisinNo ratings yet
- Rene Luis F. Filarca, M.D.: February 5, 2014Document8 pagesRene Luis F. Filarca, M.D.: February 5, 2014Maikka IlaganNo ratings yet
- Chapter 77Document4 pagesChapter 77Regina Ysabel SartagudaNo ratings yet
- Pharma - 4th Assessment - Thyroid Hormones - 29-30 Jan 2007Document35 pagesPharma - 4th Assessment - Thyroid Hormones - 29-30 Jan 2007api-3703352No ratings yet
- Endocrine System NotesDocument6 pagesEndocrine System NotesHannah Grace CorveraNo ratings yet
- Thyroid and Its Functions: DR Raghuveer ChoudharyDocument61 pagesThyroid and Its Functions: DR Raghuveer ChoudharyPhysiology by Dr RaghuveerNo ratings yet
- THYROID GLAND: What Happens When Something Goes Wrong?: PIT UIDocument4 pagesTHYROID GLAND: What Happens When Something Goes Wrong?: PIT UIAnonymous HH3c17osNo ratings yet
- 2 - Hyperthyroidism (Notes)Document9 pages2 - Hyperthyroidism (Notes)Rhegina SalsabilaNo ratings yet
- Subject: Pysiology Topic: Thyroid Gland and Pancreas Lecturer: Dr. Gigi Francisco Date: January 2011Document9 pagesSubject: Pysiology Topic: Thyroid Gland and Pancreas Lecturer: Dr. Gigi Francisco Date: January 2011Std Dlshsi100% (2)
- Week 11 Endocrinology Part 2 3Document12 pagesWeek 11 Endocrinology Part 2 3Toff GoyenecheaNo ratings yet
- 3ES-2 Thyroid and Antithyroid Drugs 1436Document46 pages3ES-2 Thyroid and Antithyroid Drugs 1436Muath AlqarniNo ratings yet
- Thyroid DiseasesDocument47 pagesThyroid Diseasesbiniam MesfinNo ratings yet
- HyperthyroidismDocument3 pagesHyperthyroidismGerardLum100% (3)
- 5,6-Thyroid Gland, Hypo and Hyper ThyroidismDocument23 pages5,6-Thyroid Gland, Hypo and Hyper ThyroidismMarzzzNo ratings yet
- Physiology of The Thyroid GlandDocument28 pagesPhysiology of The Thyroid GlandSecret AgentNo ratings yet
- Thyroid Gland AtfDocument43 pagesThyroid Gland AtfRana Ahsan JavedNo ratings yet
- Pharmacology Finals Lecture Thyroid DisordersDocument11 pagesPharmacology Finals Lecture Thyroid DisordersJuliann100% (1)
- The Thyroid GlandDocument74 pagesThe Thyroid GlandAboubakar Moalim Mahad moh'dNo ratings yet
- Synthesis of Thyroid HormonesDocument1 pageSynthesis of Thyroid HormonesAndioNo ratings yet
- Thyroid HormonesDocument9 pagesThyroid Hormonesftaaa oshaNo ratings yet
- Thyroid PhysiologyDocument25 pagesThyroid PhysiologyKathleen Grace ManiagoNo ratings yet
- Endocrine Physiology: ThyroidDocument49 pagesEndocrine Physiology: ThyroidriaNo ratings yet
- 2009-08-25 Nowak Thyroid PDFDocument47 pages2009-08-25 Nowak Thyroid PDFEndalew Alemu NathanNo ratings yet
- 3 - Thyroid Metabolism-OkDocument12 pages3 - Thyroid Metabolism-Okcaroline.fragniere6464No ratings yet
- Thyroidhormone 180220171148Document51 pagesThyroidhormone 180220171148Hasan Al MasudNo ratings yet
- Thyroid and Antithyroid PharmaDocument31 pagesThyroid and Antithyroid PharmaxaviermargaridaNo ratings yet
- Thyroid Hormones: Difference Between T3 and rT3 in Structure and Function?Document1 pageThyroid Hormones: Difference Between T3 and rT3 in Structure and Function?Khadijah Al HadiNo ratings yet
- Endocrinology Part 2Document4 pagesEndocrinology Part 2Alondra SagarioNo ratings yet
- Graves 3Document2 pagesGraves 3Moof06No ratings yet
- Endocrinology MergeDocument22 pagesEndocrinology MergeEricsson CarabbacanNo ratings yet
- Sam CD 2000 - EndocrinologyDocument139 pagesSam CD 2000 - EndocrinologyDr. Muha. Hasan Mahbub-Ur-RahmanNo ratings yet
- 14) Drugs For Thyroid DisordersDocument13 pages14) Drugs For Thyroid DisordersLatiNo ratings yet
- Hipertiroid 2022 UnhaluDocument59 pagesHipertiroid 2022 Unhaluramadhanadlansyah7No ratings yet
- Thyroid GlandDocument78 pagesThyroid GlandSatyam ChoubeyNo ratings yet
- Doc3 - Objetivo 3, 6 e 7-Thyroid Metabolic HormonesDocument13 pagesDoc3 - Objetivo 3, 6 e 7-Thyroid Metabolic HormonesBruna SofiaNo ratings yet
- HipertiroidDocument62 pagesHipertiroidMiftahuljjanah BaharuddinNo ratings yet
- Thyroid HormonesDocument50 pagesThyroid Hormonesanulalparayil2003No ratings yet
- The Thyroid Gland PRINT 2021Document9 pagesThe Thyroid Gland PRINT 2021abcde990075No ratings yet
- Fisiologi Hormon TiroidDocument54 pagesFisiologi Hormon Tiroidaan SyamhaNo ratings yet
- PHARMA-R4.5-Thyroid, Antithyroid, AntiosteoporosisDocument6 pagesPHARMA-R4.5-Thyroid, Antithyroid, Antiosteoporosischarmainemargaret.parreno.medNo ratings yet
- Anti-Thyroid Drugs and Thyroid HormoneDocument7 pagesAnti-Thyroid Drugs and Thyroid Hormonemicheal1960100% (1)
- The Thyroid GlandDocument40 pagesThe Thyroid Glandlodeg51699No ratings yet
- EN2 - HypothyroidismDocument17 pagesEN2 - Hypothyroidismsbobine.imsNo ratings yet
- Thyroid Gland: Ruswana Anwar, Tono DjuwantonoDocument49 pagesThyroid Gland: Ruswana Anwar, Tono Djuwantonoyuyu tuptupNo ratings yet
- Endocrinology SlidesDocument344 pagesEndocrinology SlidesleanNo ratings yet
- Thyroid DisordersDocument14 pagesThyroid DisordershaleyNo ratings yet
- Lec 10 - Thyroid Hormone PDFDocument13 pagesLec 10 - Thyroid Hormone PDFrajeshNo ratings yet
- Thyroid Disorders 1Document29 pagesThyroid Disorders 1zxcvbzaki123No ratings yet
- Overview of Postoperative Fluid Therapy in AdultsDocument27 pagesOverview of Postoperative Fluid Therapy in AdultsisahNo ratings yet
- Initial Evaluation and Management of Blunt Thoracic Trauma in AdultsDocument78 pagesInitial Evaluation and Management of Blunt Thoracic Trauma in AdultsisahNo ratings yet
- 2.13b AMP Award Notice v2Document109 pages2.13b AMP Award Notice v2isahNo ratings yet
- Ductal Carcinoma in Situ: Treatment and PrognosisDocument31 pagesDuctal Carcinoma in Situ: Treatment and PrognosisisahNo ratings yet
- Coagulation and PlateletsDocument5 pagesCoagulation and PlateletsisahNo ratings yet
- Thyroid CADocument150 pagesThyroid CAisahNo ratings yet
- Consensus On UTI in ChildrenDocument45 pagesConsensus On UTI in ChildrenisahNo ratings yet
- CT Public v131 3 4Document2 pagesCT Public v131 3 4isahNo ratings yet
- Treatment of Secondary Spontaneous Pneumothorax in AdultsDocument21 pagesTreatment of Secondary Spontaneous Pneumothorax in AdultsisahNo ratings yet
- Admitting Order FormatDocument1 pageAdmitting Order FormatisahNo ratings yet
- 8 Pharma Estrogens, Progestins, AndrogensDocument12 pages8 Pharma Estrogens, Progestins, AndrogensisahNo ratings yet
- Chap 2 and 4 Merged - 2Document14 pagesChap 2 and 4 Merged - 2isahNo ratings yet
- Anatomical Language Lab ManualDocument7 pagesAnatomical Language Lab ManualisahNo ratings yet
- OB 2.5 Intrapartum AssessmentDocument9 pagesOB 2.5 Intrapartum AssessmentisahNo ratings yet
- Surg History of SurgeryDocument85 pagesSurg History of SurgeryisahNo ratings yet
- Shipping Permit: Valid Date: Date Issued: Shipper'S Name: Shipper'S Address: Application No.: Purpose: CategoryDocument1 pageShipping Permit: Valid Date: Date Issued: Shipper'S Name: Shipper'S Address: Application No.: Purpose: CategoryisahNo ratings yet
- 9 Pharma ACTH Adrenal Steroids CortexDocument10 pages9 Pharma ACTH Adrenal Steroids CortexisahNo ratings yet
- OB 21 PHYSIOLOGY OF LABOR by KDocument7 pagesOB 21 PHYSIOLOGY OF LABOR by KisahNo ratings yet
- Monthly Trend Analysis ReportDocument1 pageMonthly Trend Analysis ReportisahNo ratings yet
- Deed of Absolute Sale: Know All Men by These PresentsDocument2 pagesDeed of Absolute Sale: Know All Men by These PresentsisahNo ratings yet
- 01 - Communication SkillsDocument2 pages01 - Communication SkillsisahNo ratings yet
- Antifungal Property of Musa Acuminata Banana Peel Ethanolic Extract AgainstDocument2 pagesAntifungal Property of Musa Acuminata Banana Peel Ethanolic Extract AgainstisahNo ratings yet
- RRLDocument11 pagesRRLisahNo ratings yet
- Course CodeDocument2 pagesCourse CodeisahNo ratings yet
- BOX 17.4 NCP Postpartum HemorrhageDocument4 pagesBOX 17.4 NCP Postpartum HemorrhageJam AliNo ratings yet
- Draft LKPDDocument34 pagesDraft LKPDaliNo ratings yet
- TASK 8 - Stanbio Glucose Oxidase MethodDocument2 pagesTASK 8 - Stanbio Glucose Oxidase MethodJ Pao Bayro - LacanilaoNo ratings yet
- Spina BifidaDocument34 pagesSpina Bifidaapi-271779479No ratings yet
- Primer: Genome-Wide Association StudiesDocument21 pagesPrimer: Genome-Wide Association Studiesrommell AlvaradoNo ratings yet
- Immunocal®Document5 pagesImmunocal®api-26034055No ratings yet
- Food Safety Is A Scientific Discipline Describing Proper HandlingDocument6 pagesFood Safety Is A Scientific Discipline Describing Proper HandlingMia ChatilaNo ratings yet
- Calcium Silicate MsdsDocument3 pagesCalcium Silicate MsdsHaniff RahimNo ratings yet
- Common NamesDocument1 pageCommon NamesPau ZaballeroNo ratings yet
- Material Safety Data Sheet Conforms With Osha Form Omb No. 1218-0072Document2 pagesMaterial Safety Data Sheet Conforms With Osha Form Omb No. 1218-0072Roseanne Park is an angelNo ratings yet
- Beginners Outline PDFDocument45 pagesBeginners Outline PDFMarginean ClaudiuNo ratings yet
- AU NZ Cortitrol PIPDocument2 pagesAU NZ Cortitrol PIPthanh.tkNo ratings yet
- PI e UREA 15Document2 pagesPI e UREA 15dewi asnaniNo ratings yet
- Gec eDocument1 pageGec eMaria Venus MontoloNo ratings yet
- International Journal of Surgery Case ReportsDocument4 pagesInternational Journal of Surgery Case ReportsAditya HendraNo ratings yet
- More Quality: Here'S How Cryopen Instruments Can Benefit YouDocument1 pageMore Quality: Here'S How Cryopen Instruments Can Benefit Youmahmoud mohamedNo ratings yet
- Natural History of Cervical CancerDocument27 pagesNatural History of Cervical CancerAri AsriniNo ratings yet
- Lesson Plan For PortfolioDocument8 pagesLesson Plan For Portfolioapi-449507744No ratings yet
- Espirometria ATS ERS 2019Document19 pagesEspirometria ATS ERS 2019Lorenzo MacoNo ratings yet
- Case Study: Spinal Cord Injury: Learning ObjectivesDocument8 pagesCase Study: Spinal Cord Injury: Learning ObjectivesMarius Clifford BilledoNo ratings yet
- BEAM 5 Unit 1 DLP 5 Taking Care of The Reproductive System PDFDocument9 pagesBEAM 5 Unit 1 DLP 5 Taking Care of The Reproductive System PDFShieyyllaMarie CasaNo ratings yet
- Lobar FunctionsDocument7 pagesLobar FunctionsSunjay Pradhep100% (2)
- Genetics in Orthodontics - 12 11 23Document88 pagesGenetics in Orthodontics - 12 11 23dr.sakshigarg0105No ratings yet
- Acj Case Diagnosis Frameworks - March 2018 - EnglishDocument14 pagesAcj Case Diagnosis Frameworks - March 2018 - EnglishKritarth Pandey0% (1)
- AluminaDocument3 pagesAluminaG AnshuNo ratings yet
- Navig8 Ship MGMT Employment FormDocument4 pagesNavig8 Ship MGMT Employment FormSrinivas PillaNo ratings yet
- Chapter 11Document11 pagesChapter 11christian anchetaNo ratings yet
10 Pharma Thyroid Antithyroid
10 Pharma Thyroid Antithyroid
Uploaded by
isahOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
10 Pharma Thyroid Antithyroid
10 Pharma Thyroid Antithyroid
Uploaded by
isahCopyright:
Available Formats
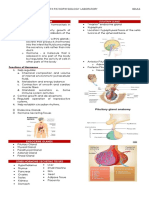
OUTLINE Biosynthesis of Thyroid Hormones
I. Thyroid Hormone IV. Iodine Uptake of Iodide Oxidation & Iodination
II. Antithyroid Drugs V. Radioactive Iodine = Iodine ingested → reaches circulation = Transport from follicular cells →
III. Ionic Inhibitors VI. Thyroid Cancer colloid via pendrin
(in the form of Iodide ion)
Legend = Iodine in blood (0.2 – 0.4 ug/dL or 15 – = Oxidation of iodide → active form via
Verbatim Book High yield thyroid peroxidase → MIT and DIT in
30 nM)
= Thyroid actively transports Iodine via thyroglobulin → Organification
I. THYROID HORMONES NIS and ↓stores of thyroid iodine
Thyroid gland → thyroid follicle & thyroid parafollicular cells enhance iodide uptake
o Follicular cells → iodothyronine hormones T3 & T4 Synthesis & Secretion of Thyroid Formation of thyroxine and
o Parafollicular cells (C cells) → Calcitonin (useful in osteoporosis and Hormones triiodothyronine from Iodotyrosines
hypercalcemia) = Proteolysis = Coupling of 2 DIT residues → T4
Chemistry of Thyroid Hormones: = Endocytosis of colloid (apical surface) = MIT + DIT → T3
o Principal hormones → Iodine-containing amino acid derivatives of + Megalin (thyroglobulin receptor) → = Reactions are catalyzed by thyroid
thyronine intracellular colloid droplets → fuse peroxidase
o T3 → much higher affinity for nuclear TR compared with T4 with lysosomes = Intrathyroidal & secreted T3 →
= TSH enhances degradation of generated by 5-deiodination of T4
thyroglobulin via ↑thiol
endopeptidase → cleaves
thyroglobulin → liberated hormones
Conversion of T4 & T3 in peripheral tissues
= Normal production of T4: bet 80 and 100 ug
= Normal production of T3: bet 30 and 40 ug
= Metabolism of T4 by 5’ or outer ring deiodination in peripheral tissues → 80%
circulating T3
Table 43-1: FACTORS THAT ALTER BINDING OF THYROXINE TO THYROXINE-BINDING GLOBULIN
INCREASE (↑) BINDING DECREASE (↓) BINDING
Drugs
Discussion Estrogens, Tamoxifen Corticosteroids, Androgens
o All of the steps are presented by the letters Selective estrogen receptor modulators L-Asparaginase, Furosemide
o Inhibitors are also present as seen in the blue box Methadone, Heroin Salicylates
o First would be the uptake of Iodine. Iodine is very little in the soil so very little in the food. Clofibrate, 5-fluorouracil Anti-seizure medications
For Iodide to be taken up by the follicular cells, it requires the NIS → Iodine transport →
Systemic Factors
through colloid via pendrin → oxidation of Iodine to more active form (Iodide) by thyroid
peroxidase → MIT, DIT → coupling of DIT-DIT → T4 then coupling of MIT-DIT → T3 → T3 Liver disease, porphyria Acute and chronic illness
and T4 will remain the thyroglobulin until there is stimulation by TSH HIV infection Inheritance
o Upon stimulation by TSH, thyroglobulin is taken up by follicular cells → interacts with Inheritance
enzymes for hydrolysis → releasing of T3 and T4 in plasma
o Deiodination of T4 → T3
Trans # 10 | TransTeam2024: shaweefa Page 1 of 8
A. Therapeutic Uses of Thyroid Hormone ▪ On weight basis: given TID, daily dose: one-third that of L-T4
❖ Major Indications ▪ Normalization of circulating TSH results in an almost 2-fold higher serum T3
▪ Hormone Replacement Therapy: Hypothyroidism compared with levothyroxine since negative feedback on TSH relies in the local
▪ TSH suppression therapy: Thyroid cancer generation of T3 from circulating T4
❖ Drugs
▪ Levothyroxine Table 43-2: FACTORS INFLUENCING ORAL LEVOTHYROXINE THERAPY
▪ Liothyronine Drugs and other factors that may increase (↑) levothyroxine dosage requirements
Impaired levothyroxine absorption
Discussion
o If there is an increase in the hormone, there is a decrease in the stimulating hormone. So Aluminum-containing antacids, PPI, sucralfate
if thyroid hormone is given to patients with thyroid cancer, there would be suppression Bile acid sequestrants (cholestyramine, colestipol, colesevelam)
of TSH. Calcium carbonate (effect generally small), phosphate binders (lanthanum
carbonate, sevelamer)
LEVOTHYROXINE Chromium picolinate, raloxifene, iron salts
Food, soy products (effect generally very small), lactose intolerance
▪ Synthetic forms of T4 (thyroxine)
▪ Preparations: Tablets, liquid-filled capsules (oral) and lyophilized powder Increased (↑) thyroxine metabolism, CYP3A4 induction
(injection) Rifampin, carbamazepine, phenytoin, sertraline
Incomplete absorption in the stomach and small intestine (~80% tablet dose) Impaired T3 → T4 conversion
Absorption slightly increased → taken on an empty stomach with less Amiodarone
variability in TSH levels Mechanisms uncertain or multifactorial
▪ If GI problems → better absorption with liquid-filled capsules Estrogen, pregnancy, lovastatin, simvastatin, ethionamide, tyrosine kinase
▪ Serum T4: peaks 2–4 h (oral) inhibitors
▪ Plasma t1/2: about 7 days
Drugs and other factors that may decrease (↓) levothyroxine dosage requirements
Omission one day’s dose → marginal effects on the serum TSH and FT4
Consistent dosing → double dose the next day. Advancing age (>65 years), androgen therapy in women
Follow-up blood tests: about 6 weeks (after initiation of treatment) due to the Drugs that may decrease (↓) TSH without changing free T4 in Levothyroxine-treated
1-week plasma t1/2 of T4 patients
If cannot take oral medications or intestinal absorption: Metformin
= IV once daily at a dose of about 80% of the patient’s daily oral
requirement B. Clinical Uses
o Thyroid hormone Replacement o Congenital Hypothyroidism
LIOTHYRONINE Therapy in Hypothyroidism o Thyroid Cancer
▪ Salt of T3 (tablets, injectable form) o Hypothyroidism in Pregnancy o Thyroid Nodules
▪ Absorption: nearly 100% o Myxedema Coma
▪ Peak serum levels 2–4 h (oral)
Used occasionally when more rapid onset of action is desired ❖ THYROID HORMONE REPLACEMENT THERAPY IN HYPOTHYROIDISM
= Rare presentation of myxedema coma ▪ Thyroxine = hormone of choice
= If rapid termination of action (preparing a patient with thyroid cancer ▪ Relies on deiodinase enzymes D1 and D2 → T4 to T3 to maintain a steady serum
for 131I therapy) level of free T3.
Less desirable for chronic replacement therapy due to: ▪ Average daily adult full replacement dose of L-T4: 1.7 μg/kg body weight (0.8
1) More-frequent dosing (plasma t ½ = 18–24 h) μg/lb) based on lean body mass.
2) Higher cost ▪ Goal of therapy:
3) Transient elevations of above T3 above the normal range. o Normalize the serum TSH (in primary hypothyroidism)
Trans # 10 | TransTeam2024: shaweefa Page 2 of 8
o Normalize free T4 (in secondary or tertiary hypothyroidism)
o Relieve symptoms of hypothyroidism ❖ CONGENITAL HYPOTHYROIDISM
▪ >60 years + known or suspected cardiac disease / with areas of autonomous ▪ Success depends on the age at which therapy is started and the speed with which
thyroid function → sub replacement dose of L-T4 (12.5–50 μg/d) hypothyroidism is corrected.
▪ Dose increased by 25 μg/d every 6 weeks until the TSH is normalized. If instituted w/in the first 2 weeks of life → normal physical and mental
▪ Monotherapy with levothyroxine most closely mimics normal physiology and development can be achieved
generally is preferred To rapidly normalize T4 concentration:
o initial daily dose of levothyroxine of 10–15 μg/kg is recommended
❖ HYPOTHYROIDISM IN PREGNANCY ▪ Goal = achieve a free T4 in the upper half of the reference range and a TSH in the
▪ Higher dose of levothyroxine due to the lower half
1) ↑serum concentration of TBG induced by estrogen ✓ Serum TSH and free T4 performed
2) expression of D3 by the placenta ✓ 2 and 4 weeks after treatment is initiated
3) small amount of transplacental passage of L-T4 from mother to fetus ✓ Every 1–2 months in the first 6 months
▪ Overt hypothyroidism → ↑risk of miscarriage, fetal distress, preterm delivery, and ✓ Every 2–3 months between 6 months and 3 years of age
impaired psychoneural and motor development in the progeny. ✓ Every 6–12 months from age 3 years until the end of growth
▪ ↑dose by about 30% as soon as pregnancy is confirmed
▪ Serum TSH: measured in the first trimester ❖ THYROID HORMONE REPLACEMENT THERAPY IN THYROID CANCER
▪ Levothyroxine dose adjusted for maintaining the TSH in the lower portion of the Papillary, Follicular type
reference range. o primary management: surgical thyroidectomy, radioiodine and
▪ Subsequent dosage adjustments: levothyroxine to maintain a low TSH
o based on serum TSH → measured 4–6 weeks after each adjustment. ▪ Rationale for TSH suppression: TSH is a growth factor for thyroid cancer, but no
▪ TSH monitoring periodically: RCT that addressed the optimal TSH target range.
1) through to 20 weeks’ gestation ▪ Reasonable approach:
2) Dose adjustment maximal 1) Adjust the levothyroxine dose to maintain a low-normal TSH value in
3) Once between weeks 26 and 32 → confirmation of adequate dose patients without persistent disease and at low risk for recurrence
▪ Dose changed back to pre-pregnancy levels the day after delivery → follow-up 2) Mildly subnormal TSH value (~0.1 mU/L) in patients at high risk for
TSH 6 weeks later. recurrence
TSH = best test during pregnancy to evaluate thyroid status in pregnancy and 3) More subnormal TSH level for patients with persistent disease.
response to treatment. ▪ Benefits of TSH suppression → need to be weighed against the risks, including
osteoporosis and atrial fibrillation.
❖ MYXEDEMA COMA
▪ Rare, extreme expression of severe, long-standing hypothyroidism. ❖ THYROID NODULES
▪ Precipitating factors: infection, congestive heart failure, and medical Most common endocrinopathy, more frequent in women
noncompliance. ▪ Usually asymptomatic, may cause discomfort, dysphagia, and choking sensation.
Cardinal features: hypothermia, respiratory depression, and decreased ▪ Risk: Exposure to ionizing radiation
consciousness. ▪ 5% that come to medical attention → malignant.
IV thyroid hormone advised ▪ Most are euthyroid
▪ Levothyroxine: loading dose of 200–400 μg → a daily full replacement dose. ▪ Diagnostic procedures:
▪ Recommend adding liothyronine (10 μg IV followed by 2.5 to 10 μg every 8 h) o ultrasound imaging and fine-needle aspiration biopsy (FNAB).
until the patient is stable and conscious. ▪ Use of levothyroxine to suppress TSH in euthyroid individuals with thyroid
▪ Ventilatory support, passive warming with blankets, correction of hyponatremia, nodules cannot be recommended as a general practice.
and treatment of the precipitating cause. ▪ If the TSH elevated:
IV glucocorticoids: recommended until coexisting adrenal insufficiency is o levothyroxine is given to bring the TSH into the lower portion of the
excluded. reference range.
Trans # 10 | TransTeam2024: shaweefa Page 3 of 8
Methimazole
C. Adverse Effects of Thyroid Hormone = 0.5 mg → ↓the organification of radioactive iodine in the thyroid gland
▪ Occur only on overtreatment and similar to the consequences of = Single dose of 10–25 mg → needed to extend the inhibition to 24 h
hyperthyroidism. = Methimazole t ½ = 4–6 h
▪ Risk *take note of the pharmacokinetics of the drugs – Doc Mau
o Atrial fibrillation (elderly)
o Osteoporosis (post- menopausal women) Table 43-4: PHARMACOKINETIC FEATURES OF ANTITHYROID DRUGS
PROPHYLTHIOURACIL METHIMAZOLE
II. ANTI-THYROID DRUGS Plasma protein binding ~75% Nil
❖ Anti-Thyroid Drugs Plasma t ½ 75 min ~4 – 6 h
❖ Therapeutic Use
Volume of distribution ~0.4 L/kg ~0.7 L/kg
❖ Response to Treatment
❖ Untoward Reactions Concentrated in thyroid Yes Yes
Metabolism of drug during
illness
ANTI-THYROID DRUGS
Severe live disease Normal Decreased (↓)
▪ Thiourelynes = family of thioamides Severe kidney disease Normal Normal
Prophylthiouracil = prototype
Dosing frequency 1 – 4x daily Once or twice daily
A. Mechanism of Action Transplacental passage Low Low
▪ inhibit the formation of thyroid Levels in breastmilk Low low
hormones by interfering with the incorporation of iodine into tyrosyl residues of
thyroglobulin C. Therapeutic Uses
▪ inhibit the coupling of these iodotyrosyl residues to form iodothyronines Definitive treatment: control the disorder in anticipation of a spontaneous
▪ Inhibit the peroxidase enzyme remission in Graves’ disease
Results in the depletion of stores of iodinated thyroglobulin as the protein is ▪ In conjunction with radioactive iodine → hasten recovery while awaiting the
hydrolyzed and the hormones are released into the circulation. effects of radiation
PTU inhibits the peripheral deiodination of T4 to T3. ▪ To control the disorder in preparation for surgical treatment
o Methimazole does not have this effect = Methimazole = drug of choice for Graves disease
o This is the rationale for the choice of PTU in the treatment of severe ▪ Effective as a single daily dose, improved adherence, less toxic
hyperthyroid states or of thyroid storm than PTU
▪ Long plasma and intra- thyroidal t ½, long duration of action.
B. ADME ▪ Starting dose = 15–40 mg per day.
▪ PTU, Methimazole = PTU starting dose = 100 mg every 8 h
▪ Drugs concentrated in the thyroid = Doses >300 mg daily → administration to every 4–6 h
▪ Drugs and metabolites appear largely in the urine ▪ Euthyroidism achieved → within 12 weeks
= dose of antithyroid drug can be reduced, but not stopped, lest an
exacerbation of Graves’ disease occur.
Carbimazole Propylthiouracil (PTU)
= carbethoxy derivative of methimazole = Absorption: w/in 20–30 min of an oral
D. Response to Treatment
= antithyroid action: conversion to dose
methimazole after absorption = 100 mg → wane in 2–3 h ▪ Thyrotoxic state improves within 3–6 weeks after therapy
= 500-mg → inhibitory for only 6–8 h. ▪ Clinical response: related to the dose of drug, size of the goiter, and pretreatment
= PTU t ½ = about 75 min serum T3 concentration.
Trans # 10 | TransTeam2024: shaweefa Page 4 of 8
▪ Rate of response determined by the ff: ❖ THYROTOXICOSIS IN PREGNANCY
1) quantity of stored hormone ▪ 0.2% occurrence → caused frequently by Graves disease
2) rate of turnover of hormone in the thyroid Antithyroid drugs = treatment of choice
3) t1/2 of the hormone in the periphery Radioactive iodine = clearly contraindicated
4) completeness of the block in synthesis imposed by the dosage given ▪ PTU and methimazole cross the placenta equally, and either may be used safely in
▪ Hypothyroidism → may develop due to overtreatment. the pregnant patient.
▪ After treatment: o Methimazole
1) Patients should be examined = avoided in the first trimester in favor of PTU due to methimazole-
2) Thyroid function tests (serum FT4 and total or free T3 concentrations) associated embryopathy
measured every 2–4 months. = used for the remainder of the pregnancy due to the concern for
3) Euthyroidism achieved → follow-up every 4–6 months PTU-associated liver failure in pregnancy.
▪ Control of the hyperthyroidism → usually associated with a ↓goiter size and o Carbimazole
normalization of serum TSH concentration. = used in the E.U. during pregnancy, rarely associated with
= dose decreased to avoid hypothyroidism congenital abnormalities.
▪ Dose minimized to keep the serum FT4 index in the upper half of the normal range
E. Untoward Reactions or slightly elevated.
▪ incidence from PTU and methimazole → relatively low. ▪ As pregnancy progresses, Graves’ disease often improves.
Agranulocytosis = absolute neutrophil count (ANC) <100 neutrophils per microlitre ▪ Relapse or worsening of Graves’ disease → common after delivery, and patients
of the blood should be monitored closely.
o Most serious reaction (first few weeks or months) ▪ In nursing mothers,
o Occurs rapidly = Methimazole is given up to 20 mg daily → no effect on thyroid function
o Not associated with a gradual reduction in granulocyte count, periodic in the infant
prospective monitoring of granulocyte counts not generally helpful. = PTU partition into breast milk even less than methimazole.
o Patients report for sore throat or fever
o Discontinue antithyroid drug G. Adjuvant Therapy
o Obtain a granulocyte count. β-adrenergic Receptor Antagonists
o Reversible on discontinuation of the offending drug ▪ Propranolol (20–40 mg qid) or Atenolol (50–100 mg OD)
o Administration of recombinant human granulocyte colony-stimulating ▪ antagonizing the sympathetic/adrenergic effects of thyrotoxicosis
factor may hasten recovery. ▪ ↓tachycardia, ↓tremor, and ↓stare
o Mild granulocytopenia = may be due to thyrotoxicosis or may be the first ▪ relieving palpitations, anxiety, and tension.
sign of this dangerous drug reaction; frequent leukocyte counts are then
required.
Ca2+-channel blockers
▪ Most common reaction = mild urticarial papular rash
o often subsides spontaneously without interrupting treatment ▪ Diltiazem (60–120 mg qid)
o sometimes requires administration of an antihistamine and ▪ Control tachycardia and decrease the incidence of supraventricular
corticosteroids and changing to another antithyroid drug tachyarrhythmias
F. Clinical Uses ✓ Short-term treatment (2–6 weeks) with β adrenergic receptor antagonists or Ca2+
❖ Thyrotoxicosis in Pregnancy channel blockers required → discontinued once the patient is euthyroid.
❖ Adjuvant Therapy ✓ Immunotherapy: used for Graves hyperthyroidism and ophthalmopathy.
❖ Thyroid Storm ✓ B-lymphocyte–depleting agent rituximab, when used with methimazole,
prolongs remission of Graves’ disease.
Trans # 10 | TransTeam2024: shaweefa Page 5 of 8
❖ THYROID STORM III. IONIC INHIBITORS
▪ uncommon but life-threatening complication of thyrotoxicosis Additive in inhibiting iodine uptake
▪ occurs in untreated or partially treated thyrotoxic patients. ▪ interfere with the concentration of iodide by the thyroid gland
▪ Precipitating factors: ▪ Thiocyanate, Perchlorate, and Fluoroborate
o Infections o Diabetic ketoacidosis
o Stress o labor Thiocyanate Perchlorate
o Trauma o heart disease = differs from the rest qualitatively = 10 times as active as thiocyanate.
o thyroidal or nonthyroidal o rare: radioactive iodine = not concentrated by the thyroid gland = blocks the entrance of iodide into the
surgery treatment. but in large amounts may inhibit the thyroid by competitively inhibiting the
▪ Clinical features = exaggerated thyrotoxicosis organification of iodine. NIS, and itself can be transported by
▪ Coma and death → 20% of patient NIS into the thyroid gland.
▪ Thyroid function abnormalities similar to in uncomplicated hyperthyroidism. = used to control hyperthyroidism;
▪ Primarily a clinical diagnosis = If in excessive amounts (2–3 g daily):
fatal aplastic anemia
= 750 mg daily = treatment of Graves’
disease
= used to “discharge” inorganic iodide
from the thyroid gland in a diagnostic
test of iodide organification.
Fluoroborate (BF4-) Lithium
= As effective as perchlorate = decreases secretion of T4 and T3,
which can cause overt
= hypothyroidism in some patients
taking Li+ for the treatment of mania
IV. IODINE
▪ Oldest remedy
▪ limits its own transport and acutely and transiently inhibits the synthesis of
iodotyrosines and iodothyronines (the Wolff-Chaikoff effect)
▪ clinical effect of high [I−] plasma → inhibition of the release of thyroid hormone.
▪ Action = rapid and efficacious in severe thyrotoxicosis
▪ Effect exerted directly on the thyroid gland and can be demonstrated in the
euthyroid subject as well as in the hyperthyroid patient.
▪ Treatment:
✓ Supportive measures such as IV fluids, antipyretics, cooling blankets, and A. Response to Iodine in Hyperthyroidism
sedation. ▪ Often striking and rapid
✓ Antithyroid drugs given in large doses: ▪ Release of thyroid hormone into the circulation is rapidly blocked, and its synthesis
o PTU preferred over methimazole → also impairs peripheral is mildly decreased.
conversion of T4 → T3. ▪ Thyroid gland:
o Oral iodides used after the first dose of an antithyroid drug has ✓ vascularity is reduced
been administered. ✓ gland becomes much firmer
✓ Treatment of the underlying precipitating illness ✓ cells become smaller
✓ colloid reaccumulates in the follicles as iodine concentration increases.
Trans # 10 | TransTeam2024: shaweefa Page 6 of 8
▪ Maximal effect = 10–15 days of continuous therapy. B. Untoward Reactions
▪ Note: Iodide therapy → does not completely control the manifestations of Angioedema: prominent symptom, and laryngeal edema may lead to suffocation.
hyperthyroidism, and the beneficial effect disappears. ▪ Multiple cutaneous hemorrhages may be present → manifestations of the serum-
▪ Uses of iodide in the treatment of sickness type of hypersensitivity
hyperthyroidism ▪ Thrombotic thrombocytopenic purpura and fatal periarteritis nodosa attributed to
1) preoperative period in hypersensitivity to iodide also have been described.
preparation for thyroidectomy ▪ Severity of symptoms of chronic intoxication with iodide (iodism) is related to the
2) in conjunction with antithyroid dose.
drugs and propranolol, in the
treatment of thyrotoxic crisis. V. RADIOACTIVE IODINE
▪ Protect the thyroid from 123
I = short-lived γ-emitter, t ½ = 13 h, used in diagnostic studies
radioactive iodine fallout following 124
a nuclear accident or military I = used with positron emission tomographic/computed
exposure. tomographic scanning for more precise dosimetry in high-risk
▪ Uptake of radioactive iodine is thyroid cancer
131
inversely proportional to the serum I = t ½ of 8 days, emits both γ rays and β particles
concentration of stable iodine = >99% of its radiation expended within 56 days.
= the administration of 30–100 = used therapeutically for thyroid destruction of an overactive
mg of iodine daily → markedly or enlarged thyroid
↓ the thyroid uptake of = in thyroid cancer for thyroid ablation and treatment of
radioisotopes. metastatic disease
= rapidly and efficiently trapped by the thyroid
❖ Strong iodine solution (Lugol solution) = incorporated into the iodoamino acids
▪ consists of 5% iodine and 10% potassium iodide, yielding a dose of about 8 mg of = deposited in the colloid of the follicles, from which it is slowly
iodine per drop. liberated.
❖ KISS (potassium iodide saturated solution) ▪ destructive β particles → originate within the follicle and act almost exclusively on
▪ available, containing 50 mg per drop the parenchymal cells of the thyroid, with little or no damage to surrounding
▪ Typical doses include 16–36 mg (2–6 drops) of Lugol solution or 50–100 mg (1–2 tissue.
drops) of KISS three times a day. ▪ γ radiation → passes through the tissue and can be quantified by external
detection.
❖ Potassium iodide ▪ Effects of the radiation depend on the dosage.
▪ over the counter drug to take in the event of a radiation emergency and block the ▪ Properly selected doses: possible to destroy the thyroid gland completely without
uptake of radioiodine into the thyroid gland. detectable injury to adjacent tissues.
= adult dose is 2 mL (130 mg) every 24 h
A. Therapeutic Uses
▪ Euthyroid patients + history of a wide variety of underlying thyroid disorders Treatment of hyperthyroidism and diagnosis of disorders of thyroid function
= may develop iodine-induced hypothyroidism when exposed to large ▪ Clearest indication hyperthyroidism in older patients and in those with heart
amounts of iodine present in many commonly pre-scribed drugs (Table disease.
43–5) ▪ Effective treatment
= these patients do not escape from the acute Wolff-Chaikoff effect ✓ when Graves disease has persisted or recurred after subtotal
thyroidectomy
✓ when prolonged treatment with antithyroid drugs has not led to
remission.
Trans # 10 | TransTeam2024: shaweefa Page 7 of 8
✓ toxic nodular goiter = not currently approved to prepare patients for radioiodine ablation of
▪ Sodium iodide 131I = available as a solution or in capsules containing carrier-free metastatic disease.
131I suitable for oral administration, available for scanning procedures.
Advantages Disadvantages
= Patient spared the risks and = Chief consequence → high incidence
discomfort of surgery of delayed hypothyroidism
= Low cost = Cancer death rate is not increased
= Hospitalization is not required after radioiodine therapy
= Patients can participate in their = Small but significant increase in
customary activities during the entire stomach, kidney, and breast cancer.
procedure, although there are o Since with expression of NIS -
recommendations to limit exposure in susceptible to effects of
young children radioactive iodine
= Radiation thyroiditis, with release of
preformed T4 and T3 into the
circulation (asymptomatic, or
worsening of symptoms of
hyperthyroidism)
o Pretreatment with antithyroid
drugs → reduce or eliminate this
complication.
o Main contraindication:
pregnancy.
= Use in children → controversial, data
insufficient, many clinics decline to
treat younger patients and reserve
radioactive iodine for patients older
than 25–30 years.
VI. THYROID CARCINOMA
▪ Well-differentiated thyroid carcinomas accumulate very little iodine
▪ Stimulation of iodine uptake with TSH = required to treat metastases effectively
▪ Endogenous TSH stimulation by withdrawal of thyroid hormone replacement
therapy in patients previously treated with near-total or total thyroidectomy.
▪ Ablative dose of 131I ranging from 30 to 150 mCi is administered + a repeat total
body scan is obtained several days to 1 week later.
▪ Recombinant thyrotropin alpha (recombinant human TSH)
= used instead of thyroid hormone withdrawal to prepare a patient for
radioiodine ablation of thyroid remnant tissue
= to test the capacity of thyroid tissue, both normal and malignant, to take
up radioactive iodine and to secrete thyroglobulin.
Trans # 10 | TransTeam2024: shaweefa Page 8 of 8
You might also like
- Mcqs For Frcophth Part 2Document253 pagesMcqs For Frcophth Part 2Ophthalmology PLUS100% (4)
- Amboss ECGDocument13 pagesAmboss ECGAllysahNo ratings yet
- Gyne - Benign LesionsDocument4 pagesGyne - Benign LesionsIsabel CastilloNo ratings yet
- Thyroid Hormones & Anti Thyroid AgentsDocument3 pagesThyroid Hormones & Anti Thyroid AgentsJaybee SarmientoNo ratings yet
- 5,6-Thyroid HyperhypoDocument16 pages5,6-Thyroid Hyperhypomogesie1995No ratings yet
- 2s Finals Pharma BeshyDocument60 pages2s Finals Pharma BeshyJohanei Mae PeraltaNo ratings yet
- 1 - Pharmacology - Thyroid & Antibone DrugsDocument20 pages1 - Pharmacology - Thyroid & Antibone DrugsSoc Gerren TuasonNo ratings yet
- HormonesDocument9 pagesHormonesChandanaNo ratings yet
- PhysioLec 8 Thyroid Metabolic Hormones (Dr. Queenie)Document4 pagesPhysioLec 8 Thyroid Metabolic Hormones (Dr. Queenie)Lafayette DelantarNo ratings yet
- Thyroid Gland Clinical Chemistry 2 (Laboratory) : LessonDocument4 pagesThyroid Gland Clinical Chemistry 2 (Laboratory) : LessonCherry Ann ColechaNo ratings yet
- Endocrine Physiology) 8. Synthesis of Thyroid Hormone - KeyDocument1 pageEndocrine Physiology) 8. Synthesis of Thyroid Hormone - Keysingonstrings365No ratings yet
- Endocrine Physiology 231212 051058Document20 pagesEndocrine Physiology 231212 051058Charan SreeramdasNo ratings yet
- Topic3.3 Physiology Thyroid HormoneDocument7 pagesTopic3.3 Physiology Thyroid HormoneRen AlvNo ratings yet
- Im-Thyroid DisordersDocument9 pagesIm-Thyroid DisordersRyan F. BernalNo ratings yet
- Antithyroid DrugsDocument32 pagesAntithyroid DrugsLUMUMBA OluwatomisinNo ratings yet
- Rene Luis F. Filarca, M.D.: February 5, 2014Document8 pagesRene Luis F. Filarca, M.D.: February 5, 2014Maikka IlaganNo ratings yet
- Chapter 77Document4 pagesChapter 77Regina Ysabel SartagudaNo ratings yet
- Pharma - 4th Assessment - Thyroid Hormones - 29-30 Jan 2007Document35 pagesPharma - 4th Assessment - Thyroid Hormones - 29-30 Jan 2007api-3703352No ratings yet
- Endocrine System NotesDocument6 pagesEndocrine System NotesHannah Grace CorveraNo ratings yet
- Thyroid and Its Functions: DR Raghuveer ChoudharyDocument61 pagesThyroid and Its Functions: DR Raghuveer ChoudharyPhysiology by Dr RaghuveerNo ratings yet
- THYROID GLAND: What Happens When Something Goes Wrong?: PIT UIDocument4 pagesTHYROID GLAND: What Happens When Something Goes Wrong?: PIT UIAnonymous HH3c17osNo ratings yet
- 2 - Hyperthyroidism (Notes)Document9 pages2 - Hyperthyroidism (Notes)Rhegina SalsabilaNo ratings yet
- Subject: Pysiology Topic: Thyroid Gland and Pancreas Lecturer: Dr. Gigi Francisco Date: January 2011Document9 pagesSubject: Pysiology Topic: Thyroid Gland and Pancreas Lecturer: Dr. Gigi Francisco Date: January 2011Std Dlshsi100% (2)
- Week 11 Endocrinology Part 2 3Document12 pagesWeek 11 Endocrinology Part 2 3Toff GoyenecheaNo ratings yet
- 3ES-2 Thyroid and Antithyroid Drugs 1436Document46 pages3ES-2 Thyroid and Antithyroid Drugs 1436Muath AlqarniNo ratings yet
- Thyroid DiseasesDocument47 pagesThyroid Diseasesbiniam MesfinNo ratings yet
- HyperthyroidismDocument3 pagesHyperthyroidismGerardLum100% (3)
- 5,6-Thyroid Gland, Hypo and Hyper ThyroidismDocument23 pages5,6-Thyroid Gland, Hypo and Hyper ThyroidismMarzzzNo ratings yet
- Physiology of The Thyroid GlandDocument28 pagesPhysiology of The Thyroid GlandSecret AgentNo ratings yet
- Thyroid Gland AtfDocument43 pagesThyroid Gland AtfRana Ahsan JavedNo ratings yet
- Pharmacology Finals Lecture Thyroid DisordersDocument11 pagesPharmacology Finals Lecture Thyroid DisordersJuliann100% (1)
- The Thyroid GlandDocument74 pagesThe Thyroid GlandAboubakar Moalim Mahad moh'dNo ratings yet
- Synthesis of Thyroid HormonesDocument1 pageSynthesis of Thyroid HormonesAndioNo ratings yet
- Thyroid HormonesDocument9 pagesThyroid Hormonesftaaa oshaNo ratings yet
- Thyroid PhysiologyDocument25 pagesThyroid PhysiologyKathleen Grace ManiagoNo ratings yet
- Endocrine Physiology: ThyroidDocument49 pagesEndocrine Physiology: ThyroidriaNo ratings yet
- 2009-08-25 Nowak Thyroid PDFDocument47 pages2009-08-25 Nowak Thyroid PDFEndalew Alemu NathanNo ratings yet
- 3 - Thyroid Metabolism-OkDocument12 pages3 - Thyroid Metabolism-Okcaroline.fragniere6464No ratings yet
- Thyroidhormone 180220171148Document51 pagesThyroidhormone 180220171148Hasan Al MasudNo ratings yet
- Thyroid and Antithyroid PharmaDocument31 pagesThyroid and Antithyroid PharmaxaviermargaridaNo ratings yet
- Thyroid Hormones: Difference Between T3 and rT3 in Structure and Function?Document1 pageThyroid Hormones: Difference Between T3 and rT3 in Structure and Function?Khadijah Al HadiNo ratings yet
- Endocrinology Part 2Document4 pagesEndocrinology Part 2Alondra SagarioNo ratings yet
- Graves 3Document2 pagesGraves 3Moof06No ratings yet
- Endocrinology MergeDocument22 pagesEndocrinology MergeEricsson CarabbacanNo ratings yet
- Sam CD 2000 - EndocrinologyDocument139 pagesSam CD 2000 - EndocrinologyDr. Muha. Hasan Mahbub-Ur-RahmanNo ratings yet
- 14) Drugs For Thyroid DisordersDocument13 pages14) Drugs For Thyroid DisordersLatiNo ratings yet
- Hipertiroid 2022 UnhaluDocument59 pagesHipertiroid 2022 Unhaluramadhanadlansyah7No ratings yet
- Thyroid GlandDocument78 pagesThyroid GlandSatyam ChoubeyNo ratings yet
- Doc3 - Objetivo 3, 6 e 7-Thyroid Metabolic HormonesDocument13 pagesDoc3 - Objetivo 3, 6 e 7-Thyroid Metabolic HormonesBruna SofiaNo ratings yet
- HipertiroidDocument62 pagesHipertiroidMiftahuljjanah BaharuddinNo ratings yet
- Thyroid HormonesDocument50 pagesThyroid Hormonesanulalparayil2003No ratings yet
- The Thyroid Gland PRINT 2021Document9 pagesThe Thyroid Gland PRINT 2021abcde990075No ratings yet
- Fisiologi Hormon TiroidDocument54 pagesFisiologi Hormon Tiroidaan SyamhaNo ratings yet
- PHARMA-R4.5-Thyroid, Antithyroid, AntiosteoporosisDocument6 pagesPHARMA-R4.5-Thyroid, Antithyroid, Antiosteoporosischarmainemargaret.parreno.medNo ratings yet
- Anti-Thyroid Drugs and Thyroid HormoneDocument7 pagesAnti-Thyroid Drugs and Thyroid Hormonemicheal1960100% (1)
- The Thyroid GlandDocument40 pagesThe Thyroid Glandlodeg51699No ratings yet
- EN2 - HypothyroidismDocument17 pagesEN2 - Hypothyroidismsbobine.imsNo ratings yet
- Thyroid Gland: Ruswana Anwar, Tono DjuwantonoDocument49 pagesThyroid Gland: Ruswana Anwar, Tono Djuwantonoyuyu tuptupNo ratings yet
- Endocrinology SlidesDocument344 pagesEndocrinology SlidesleanNo ratings yet
- Thyroid DisordersDocument14 pagesThyroid DisordershaleyNo ratings yet
- Lec 10 - Thyroid Hormone PDFDocument13 pagesLec 10 - Thyroid Hormone PDFrajeshNo ratings yet
- Thyroid Disorders 1Document29 pagesThyroid Disorders 1zxcvbzaki123No ratings yet
- Overview of Postoperative Fluid Therapy in AdultsDocument27 pagesOverview of Postoperative Fluid Therapy in AdultsisahNo ratings yet
- Initial Evaluation and Management of Blunt Thoracic Trauma in AdultsDocument78 pagesInitial Evaluation and Management of Blunt Thoracic Trauma in AdultsisahNo ratings yet
- 2.13b AMP Award Notice v2Document109 pages2.13b AMP Award Notice v2isahNo ratings yet
- Ductal Carcinoma in Situ: Treatment and PrognosisDocument31 pagesDuctal Carcinoma in Situ: Treatment and PrognosisisahNo ratings yet
- Coagulation and PlateletsDocument5 pagesCoagulation and PlateletsisahNo ratings yet
- Thyroid CADocument150 pagesThyroid CAisahNo ratings yet
- Consensus On UTI in ChildrenDocument45 pagesConsensus On UTI in ChildrenisahNo ratings yet
- CT Public v131 3 4Document2 pagesCT Public v131 3 4isahNo ratings yet
- Treatment of Secondary Spontaneous Pneumothorax in AdultsDocument21 pagesTreatment of Secondary Spontaneous Pneumothorax in AdultsisahNo ratings yet
- Admitting Order FormatDocument1 pageAdmitting Order FormatisahNo ratings yet
- 8 Pharma Estrogens, Progestins, AndrogensDocument12 pages8 Pharma Estrogens, Progestins, AndrogensisahNo ratings yet
- Chap 2 and 4 Merged - 2Document14 pagesChap 2 and 4 Merged - 2isahNo ratings yet
- Anatomical Language Lab ManualDocument7 pagesAnatomical Language Lab ManualisahNo ratings yet
- OB 2.5 Intrapartum AssessmentDocument9 pagesOB 2.5 Intrapartum AssessmentisahNo ratings yet
- Surg History of SurgeryDocument85 pagesSurg History of SurgeryisahNo ratings yet
- Shipping Permit: Valid Date: Date Issued: Shipper'S Name: Shipper'S Address: Application No.: Purpose: CategoryDocument1 pageShipping Permit: Valid Date: Date Issued: Shipper'S Name: Shipper'S Address: Application No.: Purpose: CategoryisahNo ratings yet
- 9 Pharma ACTH Adrenal Steroids CortexDocument10 pages9 Pharma ACTH Adrenal Steroids CortexisahNo ratings yet
- OB 21 PHYSIOLOGY OF LABOR by KDocument7 pagesOB 21 PHYSIOLOGY OF LABOR by KisahNo ratings yet
- Monthly Trend Analysis ReportDocument1 pageMonthly Trend Analysis ReportisahNo ratings yet
- Deed of Absolute Sale: Know All Men by These PresentsDocument2 pagesDeed of Absolute Sale: Know All Men by These PresentsisahNo ratings yet
- 01 - Communication SkillsDocument2 pages01 - Communication SkillsisahNo ratings yet
- Antifungal Property of Musa Acuminata Banana Peel Ethanolic Extract AgainstDocument2 pagesAntifungal Property of Musa Acuminata Banana Peel Ethanolic Extract AgainstisahNo ratings yet
- RRLDocument11 pagesRRLisahNo ratings yet
- Course CodeDocument2 pagesCourse CodeisahNo ratings yet
- BOX 17.4 NCP Postpartum HemorrhageDocument4 pagesBOX 17.4 NCP Postpartum HemorrhageJam AliNo ratings yet
- Draft LKPDDocument34 pagesDraft LKPDaliNo ratings yet
- TASK 8 - Stanbio Glucose Oxidase MethodDocument2 pagesTASK 8 - Stanbio Glucose Oxidase MethodJ Pao Bayro - LacanilaoNo ratings yet
- Spina BifidaDocument34 pagesSpina Bifidaapi-271779479No ratings yet
- Primer: Genome-Wide Association StudiesDocument21 pagesPrimer: Genome-Wide Association Studiesrommell AlvaradoNo ratings yet
- Immunocal®Document5 pagesImmunocal®api-26034055No ratings yet
- Food Safety Is A Scientific Discipline Describing Proper HandlingDocument6 pagesFood Safety Is A Scientific Discipline Describing Proper HandlingMia ChatilaNo ratings yet
- Calcium Silicate MsdsDocument3 pagesCalcium Silicate MsdsHaniff RahimNo ratings yet
- Common NamesDocument1 pageCommon NamesPau ZaballeroNo ratings yet
- Material Safety Data Sheet Conforms With Osha Form Omb No. 1218-0072Document2 pagesMaterial Safety Data Sheet Conforms With Osha Form Omb No. 1218-0072Roseanne Park is an angelNo ratings yet
- Beginners Outline PDFDocument45 pagesBeginners Outline PDFMarginean ClaudiuNo ratings yet
- AU NZ Cortitrol PIPDocument2 pagesAU NZ Cortitrol PIPthanh.tkNo ratings yet
- PI e UREA 15Document2 pagesPI e UREA 15dewi asnaniNo ratings yet
- Gec eDocument1 pageGec eMaria Venus MontoloNo ratings yet
- International Journal of Surgery Case ReportsDocument4 pagesInternational Journal of Surgery Case ReportsAditya HendraNo ratings yet
- More Quality: Here'S How Cryopen Instruments Can Benefit YouDocument1 pageMore Quality: Here'S How Cryopen Instruments Can Benefit Youmahmoud mohamedNo ratings yet
- Natural History of Cervical CancerDocument27 pagesNatural History of Cervical CancerAri AsriniNo ratings yet
- Lesson Plan For PortfolioDocument8 pagesLesson Plan For Portfolioapi-449507744No ratings yet
- Espirometria ATS ERS 2019Document19 pagesEspirometria ATS ERS 2019Lorenzo MacoNo ratings yet
- Case Study: Spinal Cord Injury: Learning ObjectivesDocument8 pagesCase Study: Spinal Cord Injury: Learning ObjectivesMarius Clifford BilledoNo ratings yet
- BEAM 5 Unit 1 DLP 5 Taking Care of The Reproductive System PDFDocument9 pagesBEAM 5 Unit 1 DLP 5 Taking Care of The Reproductive System PDFShieyyllaMarie CasaNo ratings yet
- Lobar FunctionsDocument7 pagesLobar FunctionsSunjay Pradhep100% (2)
- Genetics in Orthodontics - 12 11 23Document88 pagesGenetics in Orthodontics - 12 11 23dr.sakshigarg0105No ratings yet
- Acj Case Diagnosis Frameworks - March 2018 - EnglishDocument14 pagesAcj Case Diagnosis Frameworks - March 2018 - EnglishKritarth Pandey0% (1)
- AluminaDocument3 pagesAluminaG AnshuNo ratings yet
- Navig8 Ship MGMT Employment FormDocument4 pagesNavig8 Ship MGMT Employment FormSrinivas PillaNo ratings yet
- Chapter 11Document11 pagesChapter 11christian anchetaNo ratings yet