Professional Documents
Culture Documents
Hematology Lab Blood Collection HGB HCT Determination Transes
Hematology Lab Blood Collection HGB HCT Determination Transes
Uploaded by
Josiah RubioOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Hematology Lab Blood Collection HGB HCT Determination Transes
Hematology Lab Blood Collection HGB HCT Determination Transes
Uploaded by
Josiah RubioCopyright:
Available Formats
HEMATOLOGY 1 BSMLS2
MIDTERMS | LABORATORY
Prof. John Daryll R. Nacua, RMT
refers to the process of obtaining a sample of blood from an
individual for diagnostic, screening, or therapeutic purposes.
It is typically performed by a healthcare professional, such as
a nurse or phlebotomist, using specialized techniques and
equipment.
The collected blood sample can be used for various medical
tests, including blood cell counts, glucose levels, cholesterol
levels, infectious disease screening, genetic testing, and many
other diagnostic procedures. Blood collection is a crucial
component of healthcare as it provides valuable information
for diagnosing and monitoring a wide range of medical VENIPUNCTURE
conditions. ● Ideal procedure is to have the patient lie down
or if not possible, the patient should sit in a
sturdy, comfortable chair and not on high stools.
THE TYPES OF BLOOD COLLECTION
● There should be nothing inside the patient's
Venipuncture mouth during the procedure.
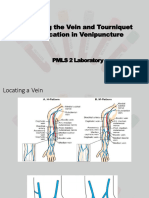
Capillary Puncture ● Ideal site for puncture: Antecubital Fossa
○ Median Cubital Vein
Arterial Puncture ○ Cephalic / Median Cephalic Vein
○ -Basilic Vein
A MUST IN PHLEBOTOMY:
1. Correct patient identification.
2. Correct specimen identification. (Proper
labeling: Patient's Full Name, Hospital
Identification Number, Location, Time and Date
and the initials of the phlebotomist)
3. Consistent with universal precautions (Gloves,
Gowns must be worn at all times and hands
must be washed in between patients)
4. Aseptic Technique (Cleaning of puncture site
“H - PATTERN”
with 70% alcohol)
1. Median Cubital Vein
2. Cephalic Vein
5. Sharp objects must be thrown to appropriate 3. Basilic
containers and must not be unsheathed or bent.
“M - PATTERN”
6. Gauze and cotton must be waste in biohazard 1. Median Vein
containers or trash bins. 2. Accessory Cephalic Vein
3. Basilic
Transcribed by: Don Filomeno Marfori B. Agus
● Assemble the materials.
● Identify and position the patient.
● Less than 30°: Angle between skin and needle
● Less than 1 minute: Tourniquet Application ● Apply a tourniquet 2-4 inches away from the
● Hemoconcentration, Hemolysis, Shortened site of the puncture.
Coagulation Time (PT/APTT) are the possible
effects of prolonged tourniquet application. ● Select the vein for puncture. Release
● Application of tourniquet must be 3-4 inches tourniquet.
above the puncture site.
● a 21 gauge (1 inch long) needle is the most ● Apply antiseptics (70% alcohol) in a circular
common needle size for adult. manner starting from inside then out. Let it
● Phlebotomist must never puncture the patient dry.
twice. ● Reapply tourniquet. Insert needle with 15-30
● Patient should not pump the fist. degrees angle
● Withdraw blood by pulling the plunger, not
too fast, not too slow
● Release tourniquet
● Withdrawing the needle. Put cotton on the
puncture site then apply pressure.
● Transfer blood sample to anticoagulated tube.
Remove needle and tube cap and let blood
flow on the sides to avoid hemolysis.
Venipuncture: Closed Method
● Prolonged tourniquet application Moisture or
contamination of blood collecting tubes.
● Needle with small bores.
● Excessive agitation.
● Frothing of the blood sample.
● Assemble vacutainer equipment.
● Identify the patient and position the patient's
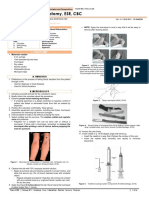
Starting from the left: (1) hemolyzed sample, (2) Lipemic forearm.
sample, (3) Normal sample, (4) Icteric sample.
● Apply a tourniquet, 2-4 inches above the
Venipuncture: Open Method puncture site.
● Select the best vein. Then release tourniquet
.
● Cleanse the area with proper aseptic
technique. Then reapply the tourniquet.
● Using a free hand, stabilize the vein.
● Advance needle through the skin (15-30
degrees angle). Stabilize the vacutainer with
- the thumb top and three fingers underneath.
Transcribed by: Don Filomeno Marfori B. Agus
● Keeping the holder steady, gently push the
tube forward.
● Release tourniquet. Remove vacutainer tube
and then withdraw the needle.
● Apply pressure on the site with a dry cotton
ball.
● Perform under supervision (students)
● Be sure that the patient is in the proper position
and be prepared that the patient will faint.
● Do not allow the tourniquet to remain in
position for more than one minute.
● Before inserting the needle, push the plunger all
the way to expel air.
● Always remove the tourniquet before removing
the needle.
● Do not reuse needles or syringes
● Proper waste disposal.
Capillary / SKin Puncture
Capillary puncture is done if the patient is:
● Infants less than 1 year old.
● Severely burned patients.
● Patients whose veins are reserved for
therapeutic purposes.
● Extremely obese patients.
● Adult with poor veins.
Transcribed by: Don Filomeno Marfori B. Agus
● Mastectomy Patients
● Avoid applying pressure, squeezing, "milking"
○ Draw blood from the opposite of
.
mastectomy side.
● Order of Draw for
○
○ Tube for blood gas analysis
● Obesity
○ Slides
○ BP Cuff will help
○ EDTA Microcollection Tube
■ Must not be more than 40
○ Other Microcollection Tube with
mmHg and 1 minute
anticoagulants
■
○ Serum Microcollection Tube
● iatrogenic Anemia
● Discard first drop of blood ● Failure to Draw Blood
○ Discard excess tissue fluid
○ Discard dead epidermal cells ● Petechiae
○ Facilitate free flow of blood
● Nerve Damage
● Depth of Skin Puncture
● Hemolysis
○ Adult: 2.0-2.5 mm
○ Infants: <2.0 mm ● Burned, Damaged, Scarred ad Occluded Veins
● -Seizures and tremors
Materials for Capillary Puncture ● Vomiting and Choking
● Blood lancet ● Allergies
● Cotton Balls
● 70% Alcohol ● Dialysis Patients
● Capillary Tubes
● Gauze ● Edema
● Sealing Clay
● Hematoma
● Burned, damaged, occluded veins
● Intravenous catheter (IV line)
● Edema
● Post Mastectomy Side
● Skin with Tattoo
Complications during Venipuncture
● Ecchymosis (Bruise)
○ Most common
○ Leakage of small amount of blood
● Hematoma
○ Leakage of large amount of blood.
● Fainting (Syncope)
○ Short lapse in consciousness
● Hemoconcentration
○ Prolonged tourniquet application
○ Wait for 2 minutes before reapplying
the tourniquet
● Intravenous (IV) Therapy
○ Draw in the opposite side of the IV
○ Stop IV for 2 minutes
○ Discard first 5-ml of blood
Transcribed by: Don Filomeno Marfori B. Agus
● Reversible (Treated by administration of
Ascorbic Acid and Methylene Blue
Hemoglobin (HgB)
B. Sulfhemoglobin
● Also known as Respiratory Pigment.
● Addition of Hydrogen sulfide to the hemoglobin
● One of the tests used to diagnose and follow (greenish pigment)
the treatments of anemia.
● Irreversible
● Main component of the RBC (95%)
● caused by sulfonamides, phenacetin, nitrites,
● Carry oxygen to and carbon from tissues. phenylhydrazine
● Heme: has iron (in ferrous state: binds 02)
C. Carboxyhemoglobin
● Globin: portion (determines the type of
hemoglobin)Each Hgb has 4 subunits: 4 heme & ● combination of heme and carbon monoxide
4 globin
● CO has an affinity to hemoglobin of 240x than
● 1 heme is capable of carrying 1 mole of O2: 1 that of oxygen.
Hgb can carry moles of O2.
● reversible (Treated by Oxygen Saturation or
Hyperbaric oxygen therapy)
Types of Hemoglobin (Normal)
Hemoglobin A (2 alpha chains/2 beta chains) > 95%
Hemoglobin A2 (2 alpha chains/2 delta chains) < 3.5% ● Reference Method: Cyanmethemoglobin
(Hemiglobincyanide)
● Uses Drabkin's Reagent
Hemoglobin F (2 alpha chains and 2 gamma chains) 1-2%
○ Contains Potassium Cyanide, Potassium
Ferricyanide, potassium dihydrogen
● Alpha chain is coded by Chromosome 16 phosphate or sodium bicarbonate, and
● Gamma, delta and beta by Chromosome 11
a non ionic compound (improves cell
lysis)
Types of Hemoglobin (Abnormal)
● Hemoglobin S Potassium ferricyanide: hemoglobin → methemoglobin
● Hemoglobin C
● Hemoglobin E Potassium cyanide: methemoglobin → cyanmethemoglobin
● Hemoglobin D
● Hemoglobin G
● Hemoglobin Lepore ● Used EDTA Anticoagulated blood
● All hemoglobin types and variants are measured
except sulfhemoglobin.
● Read 540 nm
➢ Hemoglobin whose structures have been ● 5 ml or 5000 ul of reagent, 20 ul or 0.02 ml of
modified due to drugs or environmental whole blood.
chemicals. They are dysfunctional hemoglobin
that are unable to transport oxygen.
REFERENCE RANGE (RODAK)
A. Methemoglobin
● Male 14 - 18 g/dL
● Can cause Increased O2 affinity thus decreasing
the efficiency of Oxygen to be delivered to the ● Female 12 - 15 g/dL
tissues. ● Newborn 16.5 - 21.5 g/dL
● Caused by: oxidants (Nitrite, Primaquine,
dapsone, Benzocaine) or Methemoglobin
Reductase deficiency
Transcribed by: Don Filomeno Marfori B. Agus
Technical
● Pipetting errors
● Dirty and scratch cuvettes
● Deteriorated reagent
Biologic
● Lipemic Sample
● Leukocytosis
● Hgb S and Hgb C
● HCT or Packed cell volume is the volume of
packed RBC that occupies a given volume of
blood.
● Reported as % or L/L
● Use EDTA or Heparin as anticoagulant
● Seal with clay at least 4 mm long
● Centrifuge for 5 mins between speed of 10,000
x g-15,000 x g
● Two methods
○ Macrohematocrit (uses wintrobe tube)
○ Microhematocrit (uses capillary tube)
- Male 40-54% (0.40-0.54 L/L)
- Female 35-49% (0.35-0.49 L/L)
- Newborn 53-65% (0.53-0.65 L/L)
- Child 30-43% (0.30-0.43 L/L)
Falsely Decrease ↓
• Incomplete sealing of tubes.
• Hemolysis
• Over Anticoagulated Blood
Falsely Increased ↑
• Reading including the buffy coat
• Inadequate centrifugation
• Abnormal RBC Morphology: Macrocytic Anemia,
Spherocytosis, Thalassemia, Hypochromic Anemia,
Sickle Cell Anemia
You might also like
- Karen Case StudyDocument4 pagesKaren Case StudyEmily YipNo ratings yet
- r600 8 104Document247 pagesr600 8 104Mark Cheney100% (1)
- Central Venous Pressure InsertionDocument22 pagesCentral Venous Pressure Insertionjeizy17No ratings yet
- Irvine - Shadow ConversationsDocument29 pagesIrvine - Shadow ConversationsdugoutproNo ratings yet
- Phlebotomy Procedure 3rd PDFDocument4 pagesPhlebotomy Procedure 3rd PDFFranciska Gledy AmbaritaNo ratings yet
- PresDocument60 pagesPresebtissem Jbeli100% (1)
- Module 2.4 (PDF) (1) 1Document38 pagesModule 2.4 (PDF) (1) 1Miah TantanNo ratings yet
- Venous Blood Collection - PresentationDocument12 pagesVenous Blood Collection - PresentationMegbaruNo ratings yet
- CHECKLIST FOR Phlebotomy ProcedureDocument6 pagesCHECKLIST FOR Phlebotomy ProcedureDr.Nisha Prasad.No ratings yet
- Patient Care 2Document10 pagesPatient Care 2molaf17696No ratings yet
- Uf Health Pathology Laboratories Venipuncture ProcedureDocument6 pagesUf Health Pathology Laboratories Venipuncture ProcedureMikyla CorpuzNo ratings yet
- Cannula-Insertion NewDocument6 pagesCannula-Insertion Newلوريس أبو الفتوحNo ratings yet
- Module 8 - 9 - PMTP LabDocument5 pagesModule 8 - 9 - PMTP LabPeach Mango PieNo ratings yet
- (PH156) LAB 02 Principles of Blood Collection (LAB)Document4 pages(PH156) LAB 02 Principles of Blood Collection (LAB)Rosshlein Marian YangsonNo ratings yet
- Quick Overview On Drawing Blood: Selecting A SiteDocument6 pagesQuick Overview On Drawing Blood: Selecting A SiteVincent ReyesNo ratings yet
- Module 6-7 - PMTP LabDocument4 pagesModule 6-7 - PMTP LabPeach Mango PieNo ratings yet
- Lesson 4 (Laboratory) - Venipuncture Procedure (Needle and Syringe)Document2 pagesLesson 4 (Laboratory) - Venipuncture Procedure (Needle and Syringe)jechtnagase7No ratings yet
- VIII. Intravenous Cannulation TheoryDocument45 pagesVIII. Intravenous Cannulation TheoryNandhiniNo ratings yet
- Iv CannulationDocument1 pageIv Cannulationxofivig439No ratings yet
- Patient Care 3.Document11 pagesPatient Care 3.molaf17696No ratings yet
- Blood Culture & Sensitivity (2011734)Document11 pagesBlood Culture & Sensitivity (2011734)Najib AimanNo ratings yet
- 10-C Venous Blood CollectionDocument12 pages10-C Venous Blood Collectionasnake DagnewNo ratings yet
- Intravenous Fluid Insertion 2Document56 pagesIntravenous Fluid Insertion 2Genki Fay B. LequiganNo ratings yet
- 3card Arterial WebDocument2 pages3card Arterial WebFaozan FikriNo ratings yet
- 9 - Vascular AccessDocument11 pages9 - Vascular Accessضبيان فرحانNo ratings yet
- Phlebotomy, ESR, CBCDocument6 pagesPhlebotomy, ESR, CBCAbi SulitNo ratings yet
- VENIPUNCTUREDocument10 pagesVENIPUNCTUREGreniyelNo ratings yet
- IV On Learning GuideDocument2 pagesIV On Learning Guidemzainal_18No ratings yet
- 6 VenipunctureDocument4 pages6 VenipunctureAldy JNo ratings yet
- IV Line PeriferDocument32 pagesIV Line PeriferAndikaChandraNo ratings yet
- Routine Venipuncture GuidelinesDocument6 pagesRoutine Venipuncture GuidelinesAndleeb AshrafNo ratings yet
- Station Exam - Pharm and MedsurgDocument9 pagesStation Exam - Pharm and MedsurgbriannareardonNo ratings yet
- Locating The Vein and Venipuncture ProceduresDocument33 pagesLocating The Vein and Venipuncture ProceduresAsha AsheNo ratings yet
- Practical Guidance On Venepuncture For Blood Donation PDFDocument2 pagesPractical Guidance On Venepuncture For Blood Donation PDFAshley Smith100% (1)
- Checklist For Iv Cannulation ProcedureDocument5 pagesChecklist For Iv Cannulation ProcedureMark Nel Nuñez100% (1)
- Inserting Iv Cannula: Procedure CD ID RemarksDocument1 pageInserting Iv Cannula: Procedure CD ID RemarksNicholas TagleNo ratings yet
- Intravenous Therapy Notes OnlineDocument5 pagesIntravenous Therapy Notes OnlineDexie James Ventenilla DizonNo ratings yet
- Technical ProcedureDocument142 pagesTechnical Procedureflavianamcl2012No ratings yet
- Intravenouss MedicationDocument37 pagesIntravenouss MedicationIRFAN CHANNANo ratings yet
- Lab Exercise 1 Blood Collection Techniques Part1Document8 pagesLab Exercise 1 Blood Collection Techniques Part1Janielle Medina FajardoNo ratings yet
- Checklist - Catheterization 1Document4 pagesChecklist - Catheterization 1Carlyn AguasNo ratings yet
- Central Venous LineDocument34 pagesCentral Venous Lineاسيرالاحزان100% (2)
- PMLS 2 Act.2Document3 pagesPMLS 2 Act.2jhanemil240No ratings yet
- Collection of Blood SamplesDocument12 pagesCollection of Blood Samplesmedicoprakash75% (4)
- (Bio 024) Lab Activity 12 - BloodDocument7 pages(Bio 024) Lab Activity 12 - BloodJelleane Paja TaoataoNo ratings yet
- Ivf NiceDocument22 pagesIvf NiceDianne LabisNo ratings yet
- Lab Activity Winged VeniDocument2 pagesLab Activity Winged VeniSkxNo ratings yet
- Nursing ProcedureDocument7 pagesNursing Proceduremilcah0% (1)
- Unit 4 ProcedureDocument11 pagesUnit 4 ProcedureRaxNo ratings yet
- MTS - Medical Injection Techniques & AdministrationDocument38 pagesMTS - Medical Injection Techniques & Administrationintansavira100% (1)
- Lesson 2. Basic Hematological Methods of ExaminationDocument9 pagesLesson 2. Basic Hematological Methods of ExaminationKhelly Joshua UyNo ratings yet
- Procedure in Blood Collection StudentsDocument25 pagesProcedure in Blood Collection Studentskimberly abianNo ratings yet
- IV Therapy: AdvantagesDocument9 pagesIV Therapy: AdvantagesKaren HutchinsonNo ratings yet
- PericardiocentesisDocument3 pagesPericardiocentesisThe Multilingual MedicNo ratings yet
- Iv Cannulation: Dr. Carla O. Pandrya, Span FK UphDocument21 pagesIv Cannulation: Dr. Carla O. Pandrya, Span FK UphCandice LavigneNo ratings yet
- Nasogastric Intubation Bladder CatheterizationDocument18 pagesNasogastric Intubation Bladder Catheterizationmolaf17696No ratings yet
- Intravenous Cannulation Jazeera University: Ismail Ahmed HersiDocument29 pagesIntravenous Cannulation Jazeera University: Ismail Ahmed HersiFaqay HersiNo ratings yet
- Final TracheostomyDocument6 pagesFinal TracheostomychandhomepcNo ratings yet
- Assisting Iv InfusionDocument3 pagesAssisting Iv InfusionDianne LabisNo ratings yet
- Catheter Written ReportDocument7 pagesCatheter Written ReportMARIANN JEAN ANDREA CULANAG MATALINESNo ratings yet
- Important Key NotesDocument5 pagesImportant Key NotesLUALHATI VILLASNo ratings yet
- Different in the therapy of pressure negtotheeva single-useFrom EverandDifferent in the therapy of pressure negtotheeva single-useNo ratings yet
- Muhammad Ramdhan Suwandi-N8d-360179141Document6 pagesMuhammad Ramdhan Suwandi-N8d-360179141Rija SunaNo ratings yet
- 43 Malayan Insurance Vs Regis BrokerageDocument2 pages43 Malayan Insurance Vs Regis BrokerageKlaire EsdenNo ratings yet
- InfoDocument3 pagesInfoIonuț MoNo ratings yet
- Bird But ActuallywadwaDocument57 pagesBird But ActuallywadwaSTEI - Hidayatullah Wildan Ghaly BucharyNo ratings yet
- Doctor Who Adventure Game Future Look PDF FreeDocument17 pagesDoctor Who Adventure Game Future Look PDF FreeDaran Pires TeixeiraNo ratings yet
- Box Speaker 15 Inch LapanganDocument3 pagesBox Speaker 15 Inch LapanganWilli Je WilliamNo ratings yet
- Edited LocalizationDocument23 pagesEdited LocalizationrodeliaNo ratings yet
- C.5 Doppler EffectDocument21 pagesC.5 Doppler Effectrandom rogersNo ratings yet
- TESLA AnalysisDocument19 pagesTESLA AnalysisMuhammad Rifki PutraNo ratings yet
- Nimbly Black Lives Matter GuideDocument16 pagesNimbly Black Lives Matter GuideDaniel WuNo ratings yet
- G R No 148560 Case Digest 1Document3 pagesG R No 148560 Case Digest 1Lea Rose ParafinaNo ratings yet
- Indian Standard: Guide For Testing Direct-Current (D C) MachinesDocument12 pagesIndian Standard: Guide For Testing Direct-Current (D C) MachinesRohith YerrabachalaNo ratings yet
- Cost Benefit Analysis For Peer To Peer Electricity MarketDocument17 pagesCost Benefit Analysis For Peer To Peer Electricity MarketDamar PranadiNo ratings yet
- Peppa Game Toy - Google SearchDocument1 pagePeppa Game Toy - Google SearchhienvvuNo ratings yet
- Perk Codes: WarningsDocument10 pagesPerk Codes: WarningsYash Raj100% (1)
- United States v. Adonte Young, 4th Cir. (2015)Document5 pagesUnited States v. Adonte Young, 4th Cir. (2015)Scribd Government DocsNo ratings yet
- Memoirs of A MartyrDocument138 pagesMemoirs of A MartyrManu SarvagyaNo ratings yet
- ClassifyingtheworldDocument4 pagesClassifyingtheworldapi-337646087No ratings yet
- Zhabert and GemmaDocument8 pagesZhabert and GemmaBautista Mark GironNo ratings yet
- S.No. District Name Zone School ID School NameDocument6 pagesS.No. District Name Zone School ID School NameAbhishek KumarNo ratings yet
- Coping With Employment Uncertainty: A Comparison of Employed and Unemployed WorkersDocument11 pagesCoping With Employment Uncertainty: A Comparison of Employed and Unemployed WorkersJAN STIVEN ASUNCIÓN FLORESNo ratings yet
- Daftar Stasiun Radio Di JakartaDocument1 pageDaftar Stasiun Radio Di Jakartadjarot2007No ratings yet
- Peck D, 1998Document9 pagesPeck D, 1998Rafael LeeNo ratings yet
- Urbanization and Rural-Urban Migration: Theory and Policy: Dyah Maya Nihayah, S.E., M.SiDocument26 pagesUrbanization and Rural-Urban Migration: Theory and Policy: Dyah Maya Nihayah, S.E., M.SivalkriyeNo ratings yet
- HondaLtd CaseDocument12 pagesHondaLtd CaseSiddharth VermaNo ratings yet
- Joker, The Laughing Christ of Postmodernity (Arslan Akhtar)Document5 pagesJoker, The Laughing Christ of Postmodernity (Arslan Akhtar)Raja Arslan AkhtarNo ratings yet
- The Nature and Aim of Theosophy: by J.D. BuckDocument18 pagesThe Nature and Aim of Theosophy: by J.D. BuckivahdamNo ratings yet