Professional Documents
Culture Documents
Accreditation Process Flowchart
Accreditation Process Flowchart
Uploaded by
Myo ThazinOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Accreditation Process Flowchart
Accreditation Process Flowchart
Uploaded by
Myo ThazinCopyright:
Available Formats
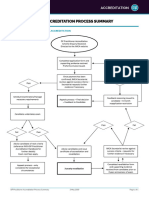
ACCREDITATION PROCESS FOR
NEW AND RENEWING FACILITIES
Facility submits an application
and fee for a New Facility
A P P L I C AT I O N
Facility submits
renewal ACR accepts application
application/fee (45-day window for testing begins)
within 60 days
ACR notifies the FDA/
CMS (if applicable)
Facility submits FDA sends the new
Testing Package mammo facility a
6-month provisional
certificate
ACR reviews the Testing Package
and images sent to reviewers
Clinical Phantom Image
Image Review
Review (if applicable)
REVIEW
ACR issues Final Report
Facility
reviews Final Report DEFICIENCY Option to repeat,
noting areas for potential appeal or withdraw
improvement
APPROVAL
3-year certificate & accreditation
toolkit sent to facility.
A P P R O VA L
Facility added to ACR Accredited
Facility list on ACR website
ACR notifies the FDA/CMS
(if applicable)
Renewal notice
sent 8 months Facility submits post-
prior to certificate accreditation survey
expiration date
accreditationsupport.acr.org | 1-800-227-6440 (Breast) 1-800-770-0145 (Non-Breast)
You might also like
- HVAC System Qualification Protocol (Validation) - Pharmaceutical GuidelinesDocument18 pagesHVAC System Qualification Protocol (Validation) - Pharmaceutical GuidelinesFabiola Olivares100% (2)
- Compex Certification ProcessDocument1 pageCompex Certification ProcessMarkus_17No ratings yet
- Fluoroscopy QC Guidance May 10 2019Document8 pagesFluoroscopy QC Guidance May 10 2019Candra GunawanNo ratings yet
- Itp For Site Preparation & Earth WorksDocument17 pagesItp For Site Preparation & Earth WorksDaniel Martinez50% (2)
- Terms of Calibration Service (Rev. 7, May 2014)Document1 pageTerms of Calibration Service (Rev. 7, May 2014)John Paul RosNo ratings yet
- Department Circular No.4 Series of 2017Document16 pagesDepartment Circular No.4 Series of 2017M.C RocaNo ratings yet
- IAA Part66 FeesDocument2 pagesIAA Part66 FeesDhananjaya Nirmal PereraNo ratings yet
- Medical Physicist'S Mammography QC Test Summary Full-Field Digital - PlanmedDocument5 pagesMedical Physicist'S Mammography QC Test Summary Full-Field Digital - Planmedmaysen mhamdiNo ratings yet
- 09 Feb 2021 FDA Citizen - S Charter CSL 02 February 2021Document71 pages09 Feb 2021 FDA Citizen - S Charter CSL 02 February 2021Raeanne Sabado BangitNo ratings yet
- Circular 59-00-9-1Document17 pagesCircular 59-00-9-1genk74No ratings yet
- ALXN-QD-0012543 Appendix 3 'Changes' For ALXN-QD-0012421Document2 pagesALXN-QD-0012543 Appendix 3 'Changes' For ALXN-QD-0012421Rupesh PawarNo ratings yet
- CAPA ProcedureDocument15 pagesCAPA Proceduresudar1477No ratings yet
- ACR Quality Control - MRI - Breast MRI (Revised 10-21-2020) - Accreditation SupportDocument5 pagesACR Quality Control - MRI - Breast MRI (Revised 10-21-2020) - Accreditation SupportJulio MartiniNo ratings yet
- Design Changes STC Per Advisory Circular AC21-8Document28 pagesDesign Changes STC Per Advisory Circular AC21-8kinqpinzNo ratings yet
- Ashghal Test List January-2015Document111 pagesAshghal Test List January-2015tuski24No ratings yet
- Flow ChartsDocument11 pagesFlow ChartsDarshan ChaudhariNo ratings yet
- Qa&Qmc Unit 1 Part Vi Nabl AccreditationDocument7 pagesQa&Qmc Unit 1 Part Vi Nabl AccreditationAbdul WashiqueNo ratings yet
- Ma App 25Document2 pagesMa App 25ahmedNo ratings yet
- Supplementary Training Modules On Good Manufacturing PracticeDocument27 pagesSupplementary Training Modules On Good Manufacturing PracticeRavindra ShilimkarNo ratings yet
- PBU PPVC MAS Flowchart New Application RenewalDocument1 pagePBU PPVC MAS Flowchart New Application RenewalNicky LimNo ratings yet
- Installation of Static EquipmentsDocument5 pagesInstallation of Static Equipmentsmansih457No ratings yet
- Samm Policy 8 (SP8) - Requirements For The Accreditation of Site Testing and Calibration LaboratoriesDocument8 pagesSamm Policy 8 (SP8) - Requirements For The Accreditation of Site Testing and Calibration LaboratoriesTauke SengNo ratings yet
- IMCA Download 17554 DP Practitioner Accreditation Scheme Process 1Document1 pageIMCA Download 17554 DP Practitioner Accreditation Scheme Process 1Talisma Consultoria e TreinamentosNo ratings yet
- Class: Interlaboratory Comparisons/ Proficiency Testing Document Number 11Document6 pagesClass: Interlaboratory Comparisons/ Proficiency Testing Document Number 11Francesca PorcelliNo ratings yet
- CT Accreditation Program Clinical Image Quality GuideDocument47 pagesCT Accreditation Program Clinical Image Quality GuideAndreasNo ratings yet
- 2-Lab Activity Flow ChartDocument1 page2-Lab Activity Flow ChartgouseyaNo ratings yet
- WRAP Audit Procedure - (06.09.2022)Document1 pageWRAP Audit Procedure - (06.09.2022)Abdur Rahman SohagNo ratings yet
- 007 - RCS Survey Instruction and Approval - MV FRIO CONFINDANCEDocument2 pages007 - RCS Survey Instruction and Approval - MV FRIO CONFINDANCEfernando pradoNo ratings yet
- DO - 126 - s2016 - Star RatingDocument4 pagesDO - 126 - s2016 - Star RatingBenedict PadillaNo ratings yet
- BPV GUI 05 Issue 1 ASME PRD Guide For ReviewsDocument10 pagesBPV GUI 05 Issue 1 ASME PRD Guide For Reviewstuandongnguyen1111No ratings yet
- 2383 01Document3 pages2383 01OSCAR YOBANY VEGA HERNANDEZNo ratings yet
- Medical Physicist'S Mammography QC Test Summary Full-Field Digital - SiemensDocument4 pagesMedical Physicist'S Mammography QC Test Summary Full-Field Digital - SiemensChoi Min YoungNo ratings yet
- Nabh Accreditation / Certification TimelineDocument4 pagesNabh Accreditation / Certification TimelineCHANDU LAL JAISWALNo ratings yet
- System Audit Types Master GlossaryDocument9 pagesSystem Audit Types Master GlossaryBaljeet SinghNo ratings yet
- IqpqoqDocument5 pagesIqpqoqShiva KrishnaNo ratings yet
- AccreditationProcess ClinicalTrialDocument2 pagesAccreditationProcess ClinicalTrialshahidashraftNo ratings yet
- GDP Qualification Summary Report - Mind MapDocument1 pageGDP Qualification Summary Report - Mind MapManjunath ChikkamaniNo ratings yet
- Qualification and Validation-34163308Document31 pagesQualification and Validation-34163308atif saadaoui100% (1)
- Change Control Matrix For Facility ChangeDocument2 pagesChange Control Matrix For Facility ChangePrem Goel100% (1)
- Nsa Rev14 (1) EnglishDocument15 pagesNsa Rev14 (1) EnglishOtavio CastroNo ratings yet
- NABL CronologyDocument3 pagesNABL CronologyvermadevanjNo ratings yet
- ACR ClinicalTestingInstructionsDocument8 pagesACR ClinicalTestingInstructionsbrianNo ratings yet
- NABLDocument2 pagesNABLvermadevanjNo ratings yet
- SubrahmanyamDocument5 pagesSubrahmanyamSiva SubrahmanyamNo ratings yet
- MGN 537 ACS Jun - 15Document7 pagesMGN 537 ACS Jun - 15Arjun VohraNo ratings yet
- Flowchart - CPE Provider-ADocument1 pageFlowchart - CPE Provider-AKharinne SisonNo ratings yet
- Control Plan - Tie Rod 1.375'-12-2A UNFX12.78, 84B515663ADP1.Document4 pagesControl Plan - Tie Rod 1.375'-12-2A UNFX12.78, 84B515663ADP1.Himanshu MishraNo ratings yet
- Kredensialing Oleh Himpunan Seminat Farmasi Rumah Sakit Indonesia 2012Document54 pagesKredensialing Oleh Himpunan Seminat Farmasi Rumah Sakit Indonesia 2012spiderNo ratings yet
- DC 04 4th DraftDocument14 pagesDC 04 4th DraftChristan John AbrenicaNo ratings yet
- 1 - Accreditation International Equivalence and BenefitsDocument29 pages1 - Accreditation International Equivalence and BenefitsRajesh PatelNo ratings yet
- Qualification of HVACDocument37 pagesQualification of HVACSf BztprkNo ratings yet
- 4.ASME Certification Program OveriewDocument26 pages4.ASME Certification Program OveriewudomNo ratings yet
- FoSCoS - FSSAIDocument2 pagesFoSCoS - FSSAIshashilandtNo ratings yet
- Inmetro - RBLE Catalog ConsultationDocument2 pagesInmetro - RBLE Catalog ConsultationRapha MarxNo ratings yet
- Pravin MaliDocument60 pagesPravin MalikuttiNo ratings yet
- APIC Guideline SupplierQualification Appendix 3 Checklist 2009Document16 pagesAPIC Guideline SupplierQualification Appendix 3 Checklist 2009dinu344No ratings yet
- Sec 8 D 3 App 4Document2 pagesSec 8 D 3 App 4Emilse GonzalezNo ratings yet
- 498Document11 pages498khushnaaz855No ratings yet
- Airman Certification Standards: Instrument Rating - Airplane (2024): FAA-S-ACS-8CFrom EverandAirman Certification Standards: Instrument Rating - Airplane (2024): FAA-S-ACS-8CNo ratings yet
- Airman Certification Standards: Remote Pilot - Small Unmanned Aircraft Systems (2024): FAA-S-ACS-10BFrom EverandAirman Certification Standards: Remote Pilot - Small Unmanned Aircraft Systems (2024): FAA-S-ACS-10BNo ratings yet