Professional Documents
Culture Documents
Lab 5 Refrigeration-1
Lab 5 Refrigeration-1
Uploaded by
Ahmed MaqsoodCopyright:
Available Formats
You might also like
- ChillerDocument10 pagesChillerkhansartaj19995No ratings yet
- Refrigeration Unit Lab ReportDocument28 pagesRefrigeration Unit Lab ReportShinigdho Synthia79% (14)
- Lab Report 560751c42519eDocument16 pagesLab Report 560751c42519eNadiaNo ratings yet
- Refrigeration Lab CompleteDocument17 pagesRefrigeration Lab CompleteSyahirzabidiNo ratings yet
- (CARNOT CYCLE) Lab Report On Cooling and Heating Effects in An Air Conditioning SystemDocument12 pages(CARNOT CYCLE) Lab Report On Cooling and Heating Effects in An Air Conditioning SystemUmer Qureshi0% (1)
- Thermodynamics Lab ManualDocument9 pagesThermodynamics Lab ManualEr Shankar Singh Dhami100% (2)
- Chapter - 4-Simple Vapour Compression Refrigeration SystemDocument141 pagesChapter - 4-Simple Vapour Compression Refrigeration SystemMohamed Al-Odat0% (1)
- Experiment 1 RefrigerationDocument4 pagesExperiment 1 RefrigerationErlangga Sulaiman Razak100% (1)
- Refrigeration Lab Report: Ali Rida Bachir SID 8104461Document4 pagesRefrigeration Lab Report: Ali Rida Bachir SID 8104461NARE EDMUNDNo ratings yet
- Vapour Compression Refrigeration PDFDocument5 pagesVapour Compression Refrigeration PDFjose100% (1)
- Heat Pump and RefrigertorDocument10 pagesHeat Pump and RefrigertordohlalaNo ratings yet
- Performance and Efficiency Test of A Refrigeration Plant (Lecture)Document56 pagesPerformance and Efficiency Test of A Refrigeration Plant (Lecture)Anonymous xjV1llZS75% (4)
- BDA 37201 Engineering Lab V: Thermodynamics Air Conditioning SystemDocument18 pagesBDA 37201 Engineering Lab V: Thermodynamics Air Conditioning SystemMuhd I-dilNo ratings yet
- 4 Performance and Efficiency Test of A Refrigeration PlantDocument52 pages4 Performance and Efficiency Test of A Refrigeration PlantIvy Joy UbinaNo ratings yet
- Duyuru2018 Refrigeration CycleDocument4 pagesDuyuru2018 Refrigeration CycleGaurav KeshriNo ratings yet
- Rac Solution Set ADocument7 pagesRac Solution Set AMuhammad AkhtarNo ratings yet
- Refrigeration Laboratory Unit ExperimentDocument9 pagesRefrigeration Laboratory Unit Experimentsalim ekizNo ratings yet
- 1334337428180-Electr - QUESTION - BANK - TL - AC - AND - EM - Final PDFDocument116 pages1334337428180-Electr - QUESTION - BANK - TL - AC - AND - EM - Final PDFPankaj KumarNo ratings yet
- R and AC Presentation - 2Document73 pagesR and AC Presentation - 2teddiyfentawNo ratings yet
- Vapour Compression RefrigerationDocument38 pagesVapour Compression RefrigerationArvind75% (4)
- Me 171066Document6 pagesMe 171066Saad mubeenNo ratings yet
- Lab 3Document28 pagesLab 3Afiqah FaiqahNo ratings yet
- Exp 1 - Refrigeration UnitDocument33 pagesExp 1 - Refrigeration UnitastigeNo ratings yet
- PDF Report PhysicsDocument4 pagesPDF Report Physicsphysics a2No ratings yet
- Refregent and CryogenicsDocument7 pagesRefregent and CryogenicsViren ParwaniNo ratings yet
- Vapor Compression Refrigeration CycleDocument9 pagesVapor Compression Refrigeration CycleNisha KuttanNo ratings yet
- Refrigration and Air Conditioning MDTDocument10 pagesRefrigration and Air Conditioning MDTSudhir KoliNo ratings yet
- Performance of The Vapour Compression Cycle As A Refrigerator and As A Heat PumpDocument7 pagesPerformance of The Vapour Compression Cycle As A Refrigerator and As A Heat Pumptatoo1No ratings yet
- Rac Solution Set BDocument12 pagesRac Solution Set BxofigoNo ratings yet
- Refrigeration CycleDocument8 pagesRefrigeration CycleMohamed HassanainNo ratings yet
- Domestacic Refrigeration Test NEWDocument14 pagesDomestacic Refrigeration Test NEWmanoj kumarNo ratings yet
- Refrigeration and Air Conditioning: Lecture # 3Document38 pagesRefrigeration and Air Conditioning: Lecture # 3Owais AzharNo ratings yet
- Refrigeration - Test - Rig Lab ManualDocument6 pagesRefrigeration - Test - Rig Lab ManualSandeep SainiNo ratings yet
- Lesson 8 - Refrigeration CycleDocument17 pagesLesson 8 - Refrigeration CycleCameronNo ratings yet
- Refrigeration and Air Conditioning: Lab ManualDocument48 pagesRefrigeration and Air Conditioning: Lab ManualcaxxvadgvadgfsNo ratings yet
- Exp. No. 02 Domestic Refrigerator Test Rig: Pimpri Chinchwad College of Engineering & Research, RavetDocument34 pagesExp. No. 02 Domestic Refrigerator Test Rig: Pimpri Chinchwad College of Engineering & Research, RavetAbcd EfgNo ratings yet
- Chapter 5-Compounded Vapor Compression Cyclee-ExamplesDocument65 pagesChapter 5-Compounded Vapor Compression Cyclee-Examplesm_alodat6144100% (1)
- Experiment 6 (Refrigerator) ) 1Document10 pagesExperiment 6 (Refrigerator) ) 1Meor Fitri SE100% (1)
- Vapor Compression Refrigeration Test Rig Lab Manual LatestDocument12 pagesVapor Compression Refrigeration Test Rig Lab Manual LatestAshish VermaNo ratings yet
- Carnot Refrigeration CycleDocument11 pagesCarnot Refrigeration CycleZaimNo ratings yet
- Operating Manual: Vapour Compression Cycle Test RigDocument11 pagesOperating Manual: Vapour Compression Cycle Test Rigp09me128No ratings yet
- 11 Refrigeration CyclesDocument18 pages11 Refrigeration CyclesHussamNo ratings yet
- Refrigerant Unit Lab ReportDocument19 pagesRefrigerant Unit Lab Reportakmal100% (2)
- Lab Heat PumpDocument9 pagesLab Heat PumpShahran IezzatNo ratings yet
- HVAC Cooling WaterDocument54 pagesHVAC Cooling WaterShiyamraj Thamodharan100% (1)
- Lab ReportDocument16 pagesLab ReportDaniel Razak0% (1)
- Vapor Compression Refrigeration Test Rig Computerised Lab ManualDocument12 pagesVapor Compression Refrigeration Test Rig Computerised Lab ManualAshish Verma50% (2)
- R & AC Lab ManualDocument30 pagesR & AC Lab ManualShashankNo ratings yet
- Exercice Coolpack - EnoncéDocument8 pagesExercice Coolpack - EnoncéTaha KarimeNo ratings yet
- Unit V (R&ac)Document139 pagesUnit V (R&ac)ragunath LakshmananNo ratings yet
- Experiment 4-Heat Pump July 2018Document8 pagesExperiment 4-Heat Pump July 2018Salihah AbdullahNo ratings yet
- Sunway Practical Lab Bicarbonate Decomposition 2012Document11 pagesSunway Practical Lab Bicarbonate Decomposition 2012venkieeNo ratings yet
- RAC Lab ManualDocument27 pagesRAC Lab ManualKewal SinghNo ratings yet
- Performance and Efficiency Test of Refrigeration Sysytem: (Mel Lab 3 Report)Document14 pagesPerformance and Efficiency Test of Refrigeration Sysytem: (Mel Lab 3 Report)Yhan SombilonNo ratings yet
- Vapor Jet RefrigeratorDocument11 pagesVapor Jet RefrigeratorAlyan YousafNo ratings yet
- Marvel Carbureter and Heat Control: As Used on Series 691 Nash Sixes Booklet SFrom EverandMarvel Carbureter and Heat Control: As Used on Series 691 Nash Sixes Booklet SNo ratings yet
- Mechanics of the Household: A Course of Study Devoted to Domestic Machinery and Household Mechanical AppliancesFrom EverandMechanics of the Household: A Course of Study Devoted to Domestic Machinery and Household Mechanical AppliancesNo ratings yet
- Oral and Practical Review: Reflections on the Part 147 CourseFrom EverandOral and Practical Review: Reflections on the Part 147 CourseNo ratings yet
- 709e101bf97d5b558234d6bfe44d50f5Document13 pages709e101bf97d5b558234d6bfe44d50f5Ahmed MaqsoodNo ratings yet
- Yellow Belt Capstone Project - Eric WilliamsonDocument12 pagesYellow Belt Capstone Project - Eric WilliamsonAhmed MaqsoodNo ratings yet
- Internal Combustion Engines and Gas TurbinesDocument71 pagesInternal Combustion Engines and Gas TurbinesAhmed MaqsoodNo ratings yet
- Multi Axis Course Challenge OGUNLADE SAHEEDDocument8 pagesMulti Axis Course Challenge OGUNLADE SAHEEDAhmed MaqsoodNo ratings yet
- NC Code TurningDocument8 pagesNC Code TurningAhmed MaqsoodNo ratings yet
- Water Chillers 93: Compressors and MotorsDocument2 pagesWater Chillers 93: Compressors and MotorsRohit ShresthaNo ratings yet
- Refrigeration & Liquefaction: J. G. Weisend IIDocument36 pagesRefrigeration & Liquefaction: J. G. Weisend IIsyafiqNo ratings yet
- Refrigeration and Air Conditiong Lab MannualDocument24 pagesRefrigeration and Air Conditiong Lab MannualCreative 360No ratings yet
- 1-Solar-Powered Absorption Chillers A Comprehensive and Critical ReviewDocument23 pages1-Solar-Powered Absorption Chillers A Comprehensive and Critical Reviewkhaledabdel1No ratings yet
- Danfoss Vex Tes5 Te 20 Sep2011Document19 pagesDanfoss Vex Tes5 Te 20 Sep2011ionut ciobanuNo ratings yet
- Alfawatercoolcondenser PDFDocument24 pagesAlfawatercoolcondenser PDFyeshig2000No ratings yet
- Sistema de Clima Volvo 1Document3 pagesSistema de Clima Volvo 1gustavoNo ratings yet
- Assignment 5Document2 pagesAssignment 5hossamkandil7No ratings yet
- Basic Refrigeration CycleDocument9 pagesBasic Refrigeration Cyclekhushal bhanderiNo ratings yet
- Lab Report 9Document5 pagesLab Report 9mamoona noreenNo ratings yet
- Cooling DubaiDocument1 pageCooling DubaiPaul BautistaNo ratings yet
- DOWNTIME: The Eight Types of Waste Air-Cooled Chiller Assembly Process Case StudyDocument4 pagesDOWNTIME: The Eight Types of Waste Air-Cooled Chiller Assembly Process Case Studynorthbride2008No ratings yet
- Mini Compressor & Cooling Units - Rigid CoolingDocument16 pagesMini Compressor & Cooling Units - Rigid CoolingronaldmarcelleNo ratings yet
- Waste Heat Recapture From Supermarket Refrigeration Systems: Final ReportDocument37 pagesWaste Heat Recapture From Supermarket Refrigeration Systems: Final ReportNevena AksićNo ratings yet
- Air Conditioning SystemDocument34 pagesAir Conditioning Systemshnslave100% (1)
- Condenser: Lesson 3 Lesson Title: Learning Outcomes: at The End of The Lesson, Students of BTLE Will Be Able ToDocument37 pagesCondenser: Lesson 3 Lesson Title: Learning Outcomes: at The End of The Lesson, Students of BTLE Will Be Able ToAliceNo ratings yet
- Tds - WC ChillerDocument21 pagesTds - WC Chillerjkhan_724384100% (2)
- Applied Thermodynamics - Refrigeration Cycle - Quiz 3Document88 pagesApplied Thermodynamics - Refrigeration Cycle - Quiz 3yashbhutada156No ratings yet
- Preliminary: Samsung Electronics Co., LTDDocument57 pagesPreliminary: Samsung Electronics Co., LTDManuel Figueroa PradoNo ratings yet
- Marks. A) TR Bell: RefrigerationDocument3 pagesMarks. A) TR Bell: RefrigerationKrishnaNo ratings yet
- Combined DataDocument5 pagesCombined DatasarikaNo ratings yet
- Air Conditioner (RHD Manual)Document13 pagesAir Conditioner (RHD Manual)Christian Chicana DiazNo ratings yet
- Effect of Capillary Tube Length On The Vcrs Performance: Experiment No. (1) Mechanical LabDocument15 pagesEffect of Capillary Tube Length On The Vcrs Performance: Experiment No. (1) Mechanical LabDilshad S FaisalNo ratings yet
- Assignment # 2: Thermo LabDocument4 pagesAssignment # 2: Thermo LabmjunaidNo ratings yet
- Cooling Tower CalculationsDocument4 pagesCooling Tower CalculationsDesiree MolinaNo ratings yet
- Chapter 5 Fundamentals of Refrigeration PDFDocument72 pagesChapter 5 Fundamentals of Refrigeration PDFmanipsgNo ratings yet
- 19XRV Catálogo ProdutoDocument92 pages19XRV Catálogo ProdutoMatheus Ribeiro Lemes100% (1)
- ME010 704: Refrigeration and Air ConditioningDocument2 pagesME010 704: Refrigeration and Air ConditioningAnonymous VDnLHNG7QQNo ratings yet
Lab 5 Refrigeration-1
Lab 5 Refrigeration-1
Uploaded by
Ahmed MaqsoodOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Lab 5 Refrigeration-1
Lab 5 Refrigeration-1
Uploaded by
Ahmed MaqsoodCopyright:
Available Formats
MENG 380 – Winter 2021
MENG 380 Thermodynamics Lab 5 - Refrigeration Lab
Introduction
In the previous lab, we examined the compressor of the refrigeration cycle. That experiment
observed the performance and calculated the efficiency. This allows us to observe the
refrigeration cycle as a complete system. We will use the Hampden Model H-6710 Refrigeration
cycle demonstrator for this experiment. For this lab, we will be finding the coefficient of
performance and coefficient of refrigeration.
Equipment Setup
The Hampden Model H-6710 Refrigeration demonstrator is shown in Figure 1. There are
thermocouples located on each side of the condenser to measure the temperature for both the
water and refrigerant. When the refrigerant passes through the condenser it is cooled by tap
water. The water is supplied from a faucet, and drained into a sink. There are pressure gauges
and thermocouples to measure the pressure and temperature of the refrigerant before and
after both the condenser and evaporator.
Expansion Refrigerant
Valve Flowmeter
Evaporator
(Inside)
Condenser
Water Input
Compressor
Condenser
Water
Condenser
Water Input BV-3
Flowmeter Condenser
Figure 1 Hampden Model H-6710 Refrigeration Demonstrator
Start Up/Shut Down Procedure
MENG 380 – Refrigeration Lab 1
The instructor will prepare the system for use by completing the following steps.
Connect the cooling water hoses and turn on the water
Open the valves to release the R134a refrigerant from its storage can into the system
You will complete the following steps to run the system
Closing the BV-3 valve and turning the flow meter valve fully open (counter-clockwise)
so that cooling water flow is temperature controlled
Setting the water heater set point as desired for the lab section
Starting the system and running it at 60 Hz for 1 hour to warm up
o Main AC
o Pump
o Heater
o Compressor
o Start the compressor by pressing the FWD button on the compressor motor
control panel and adjusting to 60 Hz using the arrow keys
The unit is shut down by reversing the start up procedure after returning the compressor
control frequency to 60 Hz. You must press the stop button on the compressor motor controller
and wait until the motor ramps down to a stop before starting to turn off the power. For long
term shut down the instructor will pump the R134a refrigerant into the storage can.
Condenser Theory
The condenser of the refrigeration cycle is a counter-flow, shell-and-tube heat exchanger. If the
heat exchanger is completely insulated, we can assume that there is no heat loss in the pipes,
and we can assume that the water absorbs heat lost by the refrigerant so it can later expand in
the evaporator, gaining heat. The goal of the condenser in the refrigeration cycle is to cool the
hot refrigerant with water. In order to calculate the heat gained by the water we must use the
following equation
Q= ṁc ∆ T Eqn 1
Where Q is the heat gained from the refrigerant per unit time, ṁ is the mass flow rate (in
lbm/s), c is the specific heat (in Btu/lbm°F) and ΔT is the change in temperature (in °F). The
value of the specific heat can vary by temperature, but for water the change is minimal for the
range of temperatures you will observe. For this lab the specific heat value will be 1 Btu/lbm °F.
To calculate the heat loss of the refrigerant, one must understand what is occurring in the
condenser. The refrigerant is going through a phase change. When the refrigerant leaves the
compressor, it leaves at a high pressure, high temperature gas. When the refrigerant passes
though the condenser it changes from a gas to a liquid. The refrigerant will leave as a low
temperature, high pressure liquid. Since the refrigerant is going through the phase change and
a drop in temperature, the specific heat cannot be used. Therefore, equation 1 will be invalid
for finding the heat loss of the refrigerant. In order to find the heat loss of the refrigerant you
must find the change in enthalpy. The equation is as follows:
MENG 380 – Refrigeration Lab 2
Q=H 2−H 3 Eqn2
where H 2 is the enthalpy (measured in Btu) of the refrigerant entering the condenser and H 3 is
the enthalpy of the refrigerant exiting the condenser. The change of enthalpy can be also
obtained by using specific enthalpy or enthalpy per unit mass. The unit of specific enthalpy is
Btu/lbm. The equation will be:
q=h 2−h3 Eqn 3
Where h2 and h3 are the specific enthalpy of the refrigerant entering and exiting the condenser
and q is the heat gained per unit mass.
Since the refrigerant is entering the condenser as a gas, the enthalpy values are obtained in the
superheat tables. The refrigerant exits the condenser as a liquid, and the enthalpy are found in
the saturation chart. Keep in mind that the refrigerant exiting the condenser is liquid and not
vapor. Make sure that the proper value of enthalpy is selected.
The refrigerant mass flow rate must be also taken into account. The refrigerant flow meter is
located on the Hampden Model H-6710 Refrigeration demonstrator. The refrigerant flowmeter
has its own scale and the values must be looked up in Table A-1 excerpted from the Hampden
Model H-6710 Refrigeration demonstrator user manual. When reading the flowmeter, you
must read your value from the middle of the sphere located within the flowmeter.
The effectiveness can be calculated once the data has been obtained. Effectiveness is defined
as:
ε = Actual Heat Loss ¿ Hot Fluid ¿
Maximum Possible Heat Loss By Hot Fluid Eqn 4
The actual heat loss is relatively easy to find, but finding the maximum heat loss can be harder
to find. First, what is the maximum heat loss? The maximum heat loss is the maximum amount
of heat loss by the hot fluid and transferred into the cold fluid. The exit temperature of the hot
fluid can only be as cool as the cold fluid entering the condenser.
For equation 4 to be valid, the enthalpy of the refrigerant at the inlet temperature of the water
must be found which is the lowest enthalpy value that the refrigerant can reach. The maximum
heat loss for the refrigerant will be:
Q=H 2−H max Eqn 5
where Hmax is the enthalpy of the refrigerant at the water inlet temperature.
The final effectiveness equation will be:
H 2 −H 3
ε= Eqn 6
H 2−H max
Full Cycle Theory
MENG 380 – Refrigeration Lab 3
The refrigeration cycle can be viewed as a reversed heat engine. The difference between the
two is that in the refrigeration cycle work is used to pump heat out of the system where in a
heat engine heat instead is used to produce work. Depending on the flow direction of the
refrigerant the system can act as a refrigerator or a heater. With a reversing valve as shown in
the figure below it can provide cooling or heating. In either the cooling or heating mode this is
also called a heat pump since heat is pumped into and out of the system.
According to the second law of thermodynamics, heat cannot be transferred from a medium at
a lower temperature to one at a higher temperature without providing mechanical work. The
goal of the heat engine is to absorb thermal energy from a high temperature region and
convert it into work, while releasing heat to a lower temperature region. The heat engine must
obey the second law of thermodynamics. However, the refrigeration cycle is slightly more
complex. The goal of the refrigeration cycle is to remove thermal energy from a lower
temperature region and transfer it to a higher temperature region. The compressor does this
work to make the refrigeration cycle possible.
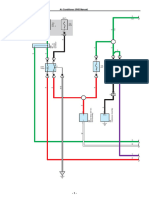
The refrigeration cycle has four main components: the evaporator, compressor, condenser, and
expansion valve. The schematic of a simple refrigeration cycle is shown in Figure 2.
MENG 380 – Refrigeration Lab 4
Figure 2 Schematic diagram of the refrigeration system
The purpose of the evaporator is to transfer heat from a higher temperature environment to
the refrigerant. The evaporator will also cause the low-pressure refrigerant to vaporize as heat
is transferred from the evaporator to the refrigerant. From the evaporator the low-pressure
refrigerant gas then goes to the compressor. The compressor will compress the refrigerant
from a low-pressure gas to a high pressure, high temperature gas. Work is done on the
compressor by electrical means in order to achieve pressurization. After the compressor, the
refrigerant then goes through a condenser. The condenser will remove any heat from the
refrigerant, which will cause the gas to liquefy but remain at high pressure. Finally, the
refrigerant will pass through an expander, which will cause the refrigerant to go from being
high pressure to low pressure. This process causes the cooling effect and heat is transferred.
The cycle will then repeat itself.
The effectiveness equation for the evaporator in terms of Figure 2 will be:
h4 −h1
ε= Eqn 7
h4 −hmax
Where h4 is the enthalpy of the refrigerant entering the evaporator, h1 is the enthalpy of the
refrigerant leaving the evaporator, and hmax is the enthalpy of refrigerant at the temperature of
the water in the evaporator.
MENG 380 – Refrigeration Lab 5
In order to find the coefficient of performance or the coefficient of refrigeration you will need
to find the work done on the cycle, the heat added to the refrigerant, and the heat rejected by
the refrigerant. To accomplish this you will need to find enthalpy values at certain locations on
the cycle. By assuming a steady-flow system to Figure 2, we will obtain the following equations:
1. For the evaporator,
h1 −h4 =q add Eqn 8
2. For the Compressor,
h2 −h1=wk comp Eqn 9
3. For the condenser,
h2 −h3=qrej Eqn 10
4. For the Expansion Valve,
h3 −h4 =0 Eqn 11
By finding work of the compressor, heat added, and heat rejected, you can easily calculate the
coefficient of performance, and the coefficient of refrigeration. The coefficient of performance
for a heat pump can be defined as:
Qrej
COPH ¿ Eqn 12
Wk cycle
In addition, the coefficient of performance for refrigeration is defined as:
Q add
COPR ¿ Eqn 13
Wk cycle
In terms of enthalpy the coefficient of performance will be:
h2−h 3
COPH ¿ Eqn 14
h2−h 1
In addition, the coefficient of refrigeration will be:
h1−h 4
COPR ¿ Eqn 15
h2−h1
MENG 380 – Refrigeration Lab 6
Performing the lab
1. Start the system and allow it to run for 20 minutes with the BV-3 valve closed and the
flow meter fully open so the thermostat controls the water flow rate through the
condenser.
2. Use this time to make sure you understand the system and determine what data you
need to collect to perform the calculations.
3. Collect the data for the thermostatically controlled system.
4. Open the BV-3 valve and adjust the flow meter to a flow rate of 2 gpm (gallons per
minute). Allow the system to stabilize for 5 minutes and collect the data under this
condition.
5. Adjust the flow meter to a flow rate of 1 gpm, allow the system to stabilize for 5 minutes
and collect the data under this condition.
6. Adjust the flow meter to a flow rate of 0.5 gpm, allow the system to stabilize for 5
minutes and collect the data under this condition.
7. Shut down the system as described earlier. Fully open the flow meter and shut off the
water making sure you do not shut off the water for other experiments.
Report
This will be a formal lab report by your entire laboratory group and should include the following
items in addition to the standard text describing what you did and what you learned.
A nicely drawn block diagram of the refrigerant path showing where all of the
measurements were taken.
A plot of the COPR and COPH vs. water flow rate.
A plot of the heat flows vs. flow rate
MENG 380 – Refrigeration Lab 7
Rosemont/Brooks Flowmeter Calibration Data
Freon F-134a
Scale Flow Flow Scale Flow Flow Scale Flow Flow
Reading (lbm/min) (kg/min) Reading (lbm/min) (kg/min) Reading (lbm/min) (kg/min)
100 1.91 0.87 68 1.26 0.57 36 0.59 0.27
98 1.87 0.85 66 1.22 0.55 34 0.56 0.25
96 1.84 0.83 64 1.18 0.54 32 0.52 0.24
94 1.80 0.82 62 1.14 0.52 30 0.48 0.22
92 1.76 0.80 60 1.09 0.49 28 0.44 0.20
90 1.72 0.78 58 1.05 0.48 26 0.40 0.18
88 1.68 0.76 56 1.01 0.46 24 0.36 0.16
86 1.64 0.74 54 0.97 0.44 22 0.33 0.15
84 1.60 0.73 52 0.92 0.42 20 0.29 0.13
82 1.55 0.70 50 0.88 0.40 18 0.25 0.11
80 1.51 0.68 48 0.84 0.38 16 0.22 0.10
78 1.47 0.67 46 0.80 0.36 14 0.18 0.082
76 1.43 0.65 44 0.76 0.34 12 0.14 0.064
74 1.39 0.63 42 0.72 0.33 10 0.11 0.050
72 1.35 0.61 40 0.68 0.31 8 0.07 0.032
70 1.31 0.59 38 0.63 0.29 6 0.03 0.014
MENG 380 – Refrigeration Lab 8
Pre-Lab Name: _______________________
1. What is a vapor compression cycle?
2. How is the performance of a vapor compression refrigerator measured?
3. Can a vapor compression refrigeration system operate both as a refrigerator and a heater?
4. Why is water used in this lab?
MENG 380 – Refrigeration Lab 9
You might also like
- ChillerDocument10 pagesChillerkhansartaj19995No ratings yet
- Refrigeration Unit Lab ReportDocument28 pagesRefrigeration Unit Lab ReportShinigdho Synthia79% (14)
- Lab Report 560751c42519eDocument16 pagesLab Report 560751c42519eNadiaNo ratings yet
- Refrigeration Lab CompleteDocument17 pagesRefrigeration Lab CompleteSyahirzabidiNo ratings yet
- (CARNOT CYCLE) Lab Report On Cooling and Heating Effects in An Air Conditioning SystemDocument12 pages(CARNOT CYCLE) Lab Report On Cooling and Heating Effects in An Air Conditioning SystemUmer Qureshi0% (1)
- Thermodynamics Lab ManualDocument9 pagesThermodynamics Lab ManualEr Shankar Singh Dhami100% (2)
- Chapter - 4-Simple Vapour Compression Refrigeration SystemDocument141 pagesChapter - 4-Simple Vapour Compression Refrigeration SystemMohamed Al-Odat0% (1)
- Experiment 1 RefrigerationDocument4 pagesExperiment 1 RefrigerationErlangga Sulaiman Razak100% (1)
- Refrigeration Lab Report: Ali Rida Bachir SID 8104461Document4 pagesRefrigeration Lab Report: Ali Rida Bachir SID 8104461NARE EDMUNDNo ratings yet
- Vapour Compression Refrigeration PDFDocument5 pagesVapour Compression Refrigeration PDFjose100% (1)
- Heat Pump and RefrigertorDocument10 pagesHeat Pump and RefrigertordohlalaNo ratings yet
- Performance and Efficiency Test of A Refrigeration Plant (Lecture)Document56 pagesPerformance and Efficiency Test of A Refrigeration Plant (Lecture)Anonymous xjV1llZS75% (4)
- BDA 37201 Engineering Lab V: Thermodynamics Air Conditioning SystemDocument18 pagesBDA 37201 Engineering Lab V: Thermodynamics Air Conditioning SystemMuhd I-dilNo ratings yet
- 4 Performance and Efficiency Test of A Refrigeration PlantDocument52 pages4 Performance and Efficiency Test of A Refrigeration PlantIvy Joy UbinaNo ratings yet
- Duyuru2018 Refrigeration CycleDocument4 pagesDuyuru2018 Refrigeration CycleGaurav KeshriNo ratings yet
- Rac Solution Set ADocument7 pagesRac Solution Set AMuhammad AkhtarNo ratings yet
- Refrigeration Laboratory Unit ExperimentDocument9 pagesRefrigeration Laboratory Unit Experimentsalim ekizNo ratings yet
- 1334337428180-Electr - QUESTION - BANK - TL - AC - AND - EM - Final PDFDocument116 pages1334337428180-Electr - QUESTION - BANK - TL - AC - AND - EM - Final PDFPankaj KumarNo ratings yet
- R and AC Presentation - 2Document73 pagesR and AC Presentation - 2teddiyfentawNo ratings yet
- Vapour Compression RefrigerationDocument38 pagesVapour Compression RefrigerationArvind75% (4)
- Me 171066Document6 pagesMe 171066Saad mubeenNo ratings yet
- Lab 3Document28 pagesLab 3Afiqah FaiqahNo ratings yet
- Exp 1 - Refrigeration UnitDocument33 pagesExp 1 - Refrigeration UnitastigeNo ratings yet
- PDF Report PhysicsDocument4 pagesPDF Report Physicsphysics a2No ratings yet
- Refregent and CryogenicsDocument7 pagesRefregent and CryogenicsViren ParwaniNo ratings yet
- Vapor Compression Refrigeration CycleDocument9 pagesVapor Compression Refrigeration CycleNisha KuttanNo ratings yet
- Refrigration and Air Conditioning MDTDocument10 pagesRefrigration and Air Conditioning MDTSudhir KoliNo ratings yet
- Performance of The Vapour Compression Cycle As A Refrigerator and As A Heat PumpDocument7 pagesPerformance of The Vapour Compression Cycle As A Refrigerator and As A Heat Pumptatoo1No ratings yet
- Rac Solution Set BDocument12 pagesRac Solution Set BxofigoNo ratings yet
- Refrigeration CycleDocument8 pagesRefrigeration CycleMohamed HassanainNo ratings yet
- Domestacic Refrigeration Test NEWDocument14 pagesDomestacic Refrigeration Test NEWmanoj kumarNo ratings yet
- Refrigeration and Air Conditioning: Lecture # 3Document38 pagesRefrigeration and Air Conditioning: Lecture # 3Owais AzharNo ratings yet
- Refrigeration - Test - Rig Lab ManualDocument6 pagesRefrigeration - Test - Rig Lab ManualSandeep SainiNo ratings yet
- Lesson 8 - Refrigeration CycleDocument17 pagesLesson 8 - Refrigeration CycleCameronNo ratings yet
- Refrigeration and Air Conditioning: Lab ManualDocument48 pagesRefrigeration and Air Conditioning: Lab ManualcaxxvadgvadgfsNo ratings yet
- Exp. No. 02 Domestic Refrigerator Test Rig: Pimpri Chinchwad College of Engineering & Research, RavetDocument34 pagesExp. No. 02 Domestic Refrigerator Test Rig: Pimpri Chinchwad College of Engineering & Research, RavetAbcd EfgNo ratings yet
- Chapter 5-Compounded Vapor Compression Cyclee-ExamplesDocument65 pagesChapter 5-Compounded Vapor Compression Cyclee-Examplesm_alodat6144100% (1)
- Experiment 6 (Refrigerator) ) 1Document10 pagesExperiment 6 (Refrigerator) ) 1Meor Fitri SE100% (1)
- Vapor Compression Refrigeration Test Rig Lab Manual LatestDocument12 pagesVapor Compression Refrigeration Test Rig Lab Manual LatestAshish VermaNo ratings yet
- Carnot Refrigeration CycleDocument11 pagesCarnot Refrigeration CycleZaimNo ratings yet
- Operating Manual: Vapour Compression Cycle Test RigDocument11 pagesOperating Manual: Vapour Compression Cycle Test Rigp09me128No ratings yet
- 11 Refrigeration CyclesDocument18 pages11 Refrigeration CyclesHussamNo ratings yet
- Refrigerant Unit Lab ReportDocument19 pagesRefrigerant Unit Lab Reportakmal100% (2)
- Lab Heat PumpDocument9 pagesLab Heat PumpShahran IezzatNo ratings yet
- HVAC Cooling WaterDocument54 pagesHVAC Cooling WaterShiyamraj Thamodharan100% (1)
- Lab ReportDocument16 pagesLab ReportDaniel Razak0% (1)
- Vapor Compression Refrigeration Test Rig Computerised Lab ManualDocument12 pagesVapor Compression Refrigeration Test Rig Computerised Lab ManualAshish Verma50% (2)
- R & AC Lab ManualDocument30 pagesR & AC Lab ManualShashankNo ratings yet
- Exercice Coolpack - EnoncéDocument8 pagesExercice Coolpack - EnoncéTaha KarimeNo ratings yet
- Unit V (R&ac)Document139 pagesUnit V (R&ac)ragunath LakshmananNo ratings yet
- Experiment 4-Heat Pump July 2018Document8 pagesExperiment 4-Heat Pump July 2018Salihah AbdullahNo ratings yet
- Sunway Practical Lab Bicarbonate Decomposition 2012Document11 pagesSunway Practical Lab Bicarbonate Decomposition 2012venkieeNo ratings yet
- RAC Lab ManualDocument27 pagesRAC Lab ManualKewal SinghNo ratings yet
- Performance and Efficiency Test of Refrigeration Sysytem: (Mel Lab 3 Report)Document14 pagesPerformance and Efficiency Test of Refrigeration Sysytem: (Mel Lab 3 Report)Yhan SombilonNo ratings yet
- Vapor Jet RefrigeratorDocument11 pagesVapor Jet RefrigeratorAlyan YousafNo ratings yet
- Marvel Carbureter and Heat Control: As Used on Series 691 Nash Sixes Booklet SFrom EverandMarvel Carbureter and Heat Control: As Used on Series 691 Nash Sixes Booklet SNo ratings yet
- Mechanics of the Household: A Course of Study Devoted to Domestic Machinery and Household Mechanical AppliancesFrom EverandMechanics of the Household: A Course of Study Devoted to Domestic Machinery and Household Mechanical AppliancesNo ratings yet
- Oral and Practical Review: Reflections on the Part 147 CourseFrom EverandOral and Practical Review: Reflections on the Part 147 CourseNo ratings yet
- 709e101bf97d5b558234d6bfe44d50f5Document13 pages709e101bf97d5b558234d6bfe44d50f5Ahmed MaqsoodNo ratings yet
- Yellow Belt Capstone Project - Eric WilliamsonDocument12 pagesYellow Belt Capstone Project - Eric WilliamsonAhmed MaqsoodNo ratings yet
- Internal Combustion Engines and Gas TurbinesDocument71 pagesInternal Combustion Engines and Gas TurbinesAhmed MaqsoodNo ratings yet
- Multi Axis Course Challenge OGUNLADE SAHEEDDocument8 pagesMulti Axis Course Challenge OGUNLADE SAHEEDAhmed MaqsoodNo ratings yet
- NC Code TurningDocument8 pagesNC Code TurningAhmed MaqsoodNo ratings yet
- Water Chillers 93: Compressors and MotorsDocument2 pagesWater Chillers 93: Compressors and MotorsRohit ShresthaNo ratings yet
- Refrigeration & Liquefaction: J. G. Weisend IIDocument36 pagesRefrigeration & Liquefaction: J. G. Weisend IIsyafiqNo ratings yet
- Refrigeration and Air Conditiong Lab MannualDocument24 pagesRefrigeration and Air Conditiong Lab MannualCreative 360No ratings yet
- 1-Solar-Powered Absorption Chillers A Comprehensive and Critical ReviewDocument23 pages1-Solar-Powered Absorption Chillers A Comprehensive and Critical Reviewkhaledabdel1No ratings yet
- Danfoss Vex Tes5 Te 20 Sep2011Document19 pagesDanfoss Vex Tes5 Te 20 Sep2011ionut ciobanuNo ratings yet
- Alfawatercoolcondenser PDFDocument24 pagesAlfawatercoolcondenser PDFyeshig2000No ratings yet
- Sistema de Clima Volvo 1Document3 pagesSistema de Clima Volvo 1gustavoNo ratings yet
- Assignment 5Document2 pagesAssignment 5hossamkandil7No ratings yet
- Basic Refrigeration CycleDocument9 pagesBasic Refrigeration Cyclekhushal bhanderiNo ratings yet
- Lab Report 9Document5 pagesLab Report 9mamoona noreenNo ratings yet
- Cooling DubaiDocument1 pageCooling DubaiPaul BautistaNo ratings yet
- DOWNTIME: The Eight Types of Waste Air-Cooled Chiller Assembly Process Case StudyDocument4 pagesDOWNTIME: The Eight Types of Waste Air-Cooled Chiller Assembly Process Case Studynorthbride2008No ratings yet
- Mini Compressor & Cooling Units - Rigid CoolingDocument16 pagesMini Compressor & Cooling Units - Rigid CoolingronaldmarcelleNo ratings yet
- Waste Heat Recapture From Supermarket Refrigeration Systems: Final ReportDocument37 pagesWaste Heat Recapture From Supermarket Refrigeration Systems: Final ReportNevena AksićNo ratings yet
- Air Conditioning SystemDocument34 pagesAir Conditioning Systemshnslave100% (1)
- Condenser: Lesson 3 Lesson Title: Learning Outcomes: at The End of The Lesson, Students of BTLE Will Be Able ToDocument37 pagesCondenser: Lesson 3 Lesson Title: Learning Outcomes: at The End of The Lesson, Students of BTLE Will Be Able ToAliceNo ratings yet
- Tds - WC ChillerDocument21 pagesTds - WC Chillerjkhan_724384100% (2)
- Applied Thermodynamics - Refrigeration Cycle - Quiz 3Document88 pagesApplied Thermodynamics - Refrigeration Cycle - Quiz 3yashbhutada156No ratings yet
- Preliminary: Samsung Electronics Co., LTDDocument57 pagesPreliminary: Samsung Electronics Co., LTDManuel Figueroa PradoNo ratings yet
- Marks. A) TR Bell: RefrigerationDocument3 pagesMarks. A) TR Bell: RefrigerationKrishnaNo ratings yet
- Combined DataDocument5 pagesCombined DatasarikaNo ratings yet
- Air Conditioner (RHD Manual)Document13 pagesAir Conditioner (RHD Manual)Christian Chicana DiazNo ratings yet
- Effect of Capillary Tube Length On The Vcrs Performance: Experiment No. (1) Mechanical LabDocument15 pagesEffect of Capillary Tube Length On The Vcrs Performance: Experiment No. (1) Mechanical LabDilshad S FaisalNo ratings yet
- Assignment # 2: Thermo LabDocument4 pagesAssignment # 2: Thermo LabmjunaidNo ratings yet
- Cooling Tower CalculationsDocument4 pagesCooling Tower CalculationsDesiree MolinaNo ratings yet
- Chapter 5 Fundamentals of Refrigeration PDFDocument72 pagesChapter 5 Fundamentals of Refrigeration PDFmanipsgNo ratings yet
- 19XRV Catálogo ProdutoDocument92 pages19XRV Catálogo ProdutoMatheus Ribeiro Lemes100% (1)
- ME010 704: Refrigeration and Air ConditioningDocument2 pagesME010 704: Refrigeration and Air ConditioningAnonymous VDnLHNG7QQNo ratings yet