Professional Documents
Culture Documents
0 ratings0% found this document useful (0 votes)
2 viewsApproach Blood Gases
Approach Blood Gases
Uploaded by
EmilyThis document provides guidance on interpreting venous and arterial blood gases. For venous blood gases, it instructs to check the pH, CO2, and HCO3 levels to determine if the patient has respiratory acidosis, metabolic acidosis, metabolic alkalosis with respiratory compensation, or respiratory alkalosis. It notes that for arterial blood gases, the oxygen levels should be checked first. It then defines the anion gap formula and lists common causes of a high anion gap, such as lactic acidosis, ketoacidosis, alcohols, toxins, and renal failure.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You might also like
- Introduction BCHN 222 2022Document39 pagesIntroduction BCHN 222 2022Francisca ManyisaNo ratings yet
- Protein Metabolism and Acids SamyA1Document170 pagesProtein Metabolism and Acids SamyA1Abby RahmanNo ratings yet
- Acid-Base Interpretation: APRIL 4, 2017Document9 pagesAcid-Base Interpretation: APRIL 4, 2017mohamedsmnNo ratings yet
- ATP Production AEROBIC Metabolism: ST STDocument11 pagesATP Production AEROBIC Metabolism: ST STSiir Pwnsalot100% (1)
- Bca Protein Metab 2Document69 pagesBca Protein Metab 2Genina MaylemNo ratings yet
- Electrolytes Water Balance PH Balance Trace MetalsDocument21 pagesElectrolytes Water Balance PH Balance Trace MetalsJohn Kevin Carl SaligumbaNo ratings yet
- A Concentration of Technology For High Quality Results: Clinical Chemistry SystemDocument2 pagesA Concentration of Technology For High Quality Results: Clinical Chemistry SystemHabibNo ratings yet
- Cholesterol SynthesisDocument28 pagesCholesterol SynthesisUbaid AhmedNo ratings yet
- Biochemistry Homework HelpDocument10 pagesBiochemistry Homework HelpNguyen Bao TranNo ratings yet
- Bản Sao Của Lesson3 - Lipid MetabolismDocument36 pagesBản Sao Của Lesson3 - Lipid Metabolismdnthanh.y2023No ratings yet
- Liver MetabolismDocument46 pagesLiver Metabolismescanorsama4000No ratings yet
- 8 ToxicologyDocument5 pages8 ToxicologyMd Sakil AminNo ratings yet
- SHS.109. 15 16 Amino Acid Catabolism and AnabolismDocument55 pagesSHS.109. 15 16 Amino Acid Catabolism and AnabolismFATIMA NAEEMNo ratings yet
- PDH Complex and TCA CycleDocument20 pagesPDH Complex and TCA CycleDarrion LouisNo ratings yet
- ELECTROLYTES (Na & K)Document3 pagesELECTROLYTES (Na & K)Alondra SagarioNo ratings yet
- Lecture 5 Protein Metabolism 1Document29 pagesLecture 5 Protein Metabolism 1Elizabeth LagunasNo ratings yet
- Causes of Metabolic AcidosisDocument1 pageCauses of Metabolic AcidosisfcarcamoNo ratings yet
- LD Atellica Solution Ous Menu Oct 2019-07128017 1800000007128017Document2 pagesLD Atellica Solution Ous Menu Oct 2019-07128017 1800000007128017Xương RồngNo ratings yet
- 3-Acetate Mevalonate PathwayDocument9 pages3-Acetate Mevalonate PathwayBhavana PalNo ratings yet
- ArpitDocument73 pagesArpitDurgesh PushkarNo ratings yet
- ABG Analysis and Acid Base BalanceDocument16 pagesABG Analysis and Acid Base BalancePrashin RocharamNo ratings yet
- Aldehydes Ketones and Carboxylic AcidDocument67 pagesAldehydes Ketones and Carboxylic AcidakshayisuniversalkingNo ratings yet
- Metabolic Acidosis - Endocrine and Metabolic Disorders - Merck Manuals Professional EditionDocument2 pagesMetabolic Acidosis - Endocrine and Metabolic Disorders - Merck Manuals Professional Editionmaulidanabilah5No ratings yet
- Amino Acid CatabolismDocument31 pagesAmino Acid CatabolismLidya YudithNo ratings yet
- Biochemistry High Yield PointsDocument18 pagesBiochemistry High Yield PointsAnonymous S0H8cqgnfiNo ratings yet
- 11 - Carbohydrate MetabolismDocument68 pages11 - Carbohydrate MetabolismcheckmateNo ratings yet
- USMLE Preparatory Online Resource_ Effective Biochemistry and Genetics Teaching Relatively Short Time_Dr Kumar Ponnusamy Urea Cycle & Nitrogen Metabolism_ST Matthew's University School of Medicine 2010Document185 pagesUSMLE Preparatory Online Resource_ Effective Biochemistry and Genetics Teaching Relatively Short Time_Dr Kumar Ponnusamy Urea Cycle & Nitrogen Metabolism_ST Matthew's University School of Medicine 2010Dr Kumar Ponnusamy100% (1)
- Water Soluble VitaminsDocument55 pagesWater Soluble VitaminsDr. M. Prasad Naidu100% (1)
- Types of AntidotesDocument1 pageTypes of AntidotesAprilVivienCuNo ratings yet
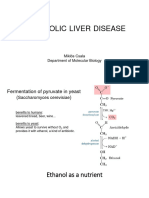
- PBC 01 Csala Alcohol 2023Document31 pagesPBC 01 Csala Alcohol 2023mir.wakilmsNo ratings yet
- N-Metabolism AA II 2018 HandoutDocument19 pagesN-Metabolism AA II 2018 HandoutlinNo ratings yet
- Phenyl AcetoneDocument2 pagesPhenyl Acetoneunderworldinc100% (1)
- Neonatal HyperammonaemiaDocument12 pagesNeonatal HyperammonaemiaGawri AbeyNo ratings yet
- GC Toxicology Case Studies Apr 28 2013Document36 pagesGC Toxicology Case Studies Apr 28 2013Grace Anastasia Ginting SinusingaNo ratings yet
- Manajemen Asidosis MetabolikDocument22 pagesManajemen Asidosis MetabolikriyanhstNo ratings yet
- 1.Beta-Oxidation 2.minor Fatty Acid Oxidation Alpha Oxidation Omega Oxidation Peroxisomal Beta OxidationDocument31 pages1.Beta-Oxidation 2.minor Fatty Acid Oxidation Alpha Oxidation Omega Oxidation Peroxisomal Beta OxidationNityantiniNo ratings yet
- Engineering of Biological Processes Metabolic Pathways: Pertemuan 2Document26 pagesEngineering of Biological Processes Metabolic Pathways: Pertemuan 2Fauzan RahmanNo ratings yet
- Porg Lab-PrelimDocument46 pagesPorg Lab-PrelimVincent BustamanteNo ratings yet
- CHEM 180: Christian MANAHAN Anna Esperanza LEGASPIDocument19 pagesCHEM 180: Christian MANAHAN Anna Esperanza LEGASPIAnna LegaspiNo ratings yet
- Pharmacognosy NotesDocument7 pagesPharmacognosy NotesGeniNo ratings yet
- IEMs Kul Nutrisi DR LanangDocument29 pagesIEMs Kul Nutrisi DR LanangChaliza AdnanNo ratings yet
- Mls 218 Protein-MetDocument45 pagesMls 218 Protein-MetZainabNo ratings yet
- K (Anion Gap 12) (Anion Gap 12) Acute Asthma Hypovolemia: - Vomit - Pyloric StenosisDocument4 pagesK (Anion Gap 12) (Anion Gap 12) Acute Asthma Hypovolemia: - Vomit - Pyloric StenosisAhmad Asyraf AzmanNo ratings yet
- Common Drugs AntidotesDocument3 pagesCommon Drugs AntidotesJhix JadraqueNo ratings yet
- Toxic AlcoholsDocument3 pagesToxic Alcoholsمحمد العمريNo ratings yet
- Engineering of Biological Processes Lecture 1: Metabolic PathwaysDocument27 pagesEngineering of Biological Processes Lecture 1: Metabolic Pathwaysagnarindra01_8550147No ratings yet
- General Protein Metabolism NUB Full 2022Document11 pagesGeneral Protein Metabolism NUB Full 2022PIH SHTNo ratings yet
- Blood Gas InterpretationDocument67 pagesBlood Gas Interpretationmajdy2006No ratings yet
- © Dept. of Medical and Clinical Biochemistry Upjš in Košice, Medical Faculty Eva Ďurovcová, MD, PHDDocument51 pages© Dept. of Medical and Clinical Biochemistry Upjš in Košice, Medical Faculty Eva Ďurovcová, MD, PHDPaulina PaskeviciuteNo ratings yet
- Alcohol Metabolism LectureDocument52 pagesAlcohol Metabolism LectureHassan HarunaNo ratings yet
- General Tox ApproachDocument15 pagesGeneral Tox ApproachAnonymous 4txA8N8etNo ratings yet
- 228 Carbohydrate MetabolismDocument43 pages228 Carbohydrate MetabolismAmanullahNo ratings yet
- KodsDocument1 pageKodsJeremiah GatchalianNo ratings yet
- H Berle 2012Document30 pagesH Berle 2012Stoica AlexandruNo ratings yet
- Ch9 Tricardoxylic Acid Cycle LectureDocument16 pagesCh9 Tricardoxylic Acid Cycle Lecturearyanchaudhary77798No ratings yet
- Oilfield Services: Anti-Foams Antioxidants / Iron Control EthyleneaminesDocument2 pagesOilfield Services: Anti-Foams Antioxidants / Iron Control EthyleneaminesDarmawanSaputraNo ratings yet
- Mbbs II Catabolism of AasDocument42 pagesMbbs II Catabolism of AasamareaneneNo ratings yet
- Amino Acid Metabolism: Inborn ErrorsDocument18 pagesAmino Acid Metabolism: Inborn ErrorsAdedoyin BankoleNo ratings yet
- Sodium and PotassiumDocument4 pagesSodium and PotassiumLUALHATI VILLASNo ratings yet
Approach Blood Gases
Approach Blood Gases
Uploaded by
Emily0 ratings0% found this document useful (0 votes)
2 views1 pageThis document provides guidance on interpreting venous and arterial blood gases. For venous blood gases, it instructs to check the pH, CO2, and HCO3 levels to determine if the patient has respiratory acidosis, metabolic acidosis, metabolic alkalosis with respiratory compensation, or respiratory alkalosis. It notes that for arterial blood gases, the oxygen levels should be checked first. It then defines the anion gap formula and lists common causes of a high anion gap, such as lactic acidosis, ketoacidosis, alcohols, toxins, and renal failure.
Original Description:
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document provides guidance on interpreting venous and arterial blood gases. For venous blood gases, it instructs to check the pH, CO2, and HCO3 levels to determine if the patient has respiratory acidosis, metabolic acidosis, metabolic alkalosis with respiratory compensation, or respiratory alkalosis. It notes that for arterial blood gases, the oxygen levels should be checked first. It then defines the anion gap formula and lists common causes of a high anion gap, such as lactic acidosis, ketoacidosis, alcohols, toxins, and renal failure.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
0 ratings0% found this document useful (0 votes)
2 views1 pageApproach Blood Gases
Approach Blood Gases
Uploaded by
EmilyThis document provides guidance on interpreting venous and arterial blood gases. For venous blood gases, it instructs to check the pH, CO2, and HCO3 levels to determine if the patient has respiratory acidosis, metabolic acidosis, metabolic alkalosis with respiratory compensation, or respiratory alkalosis. It notes that for arterial blood gases, the oxygen levels should be checked first. It then defines the anion gap formula and lists common causes of a high anion gap, such as lactic acidosis, ketoacidosis, alcohols, toxins, and renal failure.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
You are on page 1of 1
Approach Blood Gases
Venous Blood Gases
1. Check patient ID and date/time of the gas
2. Check the pH – are they acidotic or alkalotic?
3. Check the CO2 – CO2 is acidic
a. Low pH + high CO2 = respiratory acidosis
b. Low pH + low CO2 = metabolic acidosis
c. High pH + high CO2 = metabolic alkalosis + resp compensation
d. High pH + low CO2 = respiratory alkalosis
4. Check the HCO3 – HCO3 is alkalotic
a. If HCO3 follows the pH (both are high) = metabolic alkalosis
b. If HCO3 is opposite to the pH (e.g. low HCO3, high pH) = respiratory process with metabolic
compensation
When approaching an arterial blood gas, check the oxygen levels first
Anion Gap
Anion Gap = Na – (HCO3 + Cl)
Normal range 4-12
Causes of High Anion Gap
Lactate
Left Total Knee Toxins – ethanol, methanol, aspirin (salicylates)
Replacement ★ Ketones
Renal – uraemia, renal tubular acidosis
Cyanide, carbon monoxide
Alcoholic ketoacidosis
Toluene
Methanol, metformin
Uraemia
Diabetic ketoacidosis
CAT MUDPILES
Phenformin, pyroglutamic acid, paraldehyde, propylene glycol,
paracetamol
Iron, isoniazid
Lactate (numerous causes)
Ethanol, ethylene glycol
Salicylates (aspirin metabolism)
Glycols (ethylene glycol and propylene glycol)
Oxoproline (from panadol metabolism)
L-Lactate
D-Lactate
GOAL MARK
Methanol
Aspirin
Renal Failure
Ketoacidosis
You might also like
- Introduction BCHN 222 2022Document39 pagesIntroduction BCHN 222 2022Francisca ManyisaNo ratings yet
- Protein Metabolism and Acids SamyA1Document170 pagesProtein Metabolism and Acids SamyA1Abby RahmanNo ratings yet
- Acid-Base Interpretation: APRIL 4, 2017Document9 pagesAcid-Base Interpretation: APRIL 4, 2017mohamedsmnNo ratings yet
- ATP Production AEROBIC Metabolism: ST STDocument11 pagesATP Production AEROBIC Metabolism: ST STSiir Pwnsalot100% (1)
- Bca Protein Metab 2Document69 pagesBca Protein Metab 2Genina MaylemNo ratings yet
- Electrolytes Water Balance PH Balance Trace MetalsDocument21 pagesElectrolytes Water Balance PH Balance Trace MetalsJohn Kevin Carl SaligumbaNo ratings yet
- A Concentration of Technology For High Quality Results: Clinical Chemistry SystemDocument2 pagesA Concentration of Technology For High Quality Results: Clinical Chemistry SystemHabibNo ratings yet
- Cholesterol SynthesisDocument28 pagesCholesterol SynthesisUbaid AhmedNo ratings yet
- Biochemistry Homework HelpDocument10 pagesBiochemistry Homework HelpNguyen Bao TranNo ratings yet
- Bản Sao Của Lesson3 - Lipid MetabolismDocument36 pagesBản Sao Của Lesson3 - Lipid Metabolismdnthanh.y2023No ratings yet
- Liver MetabolismDocument46 pagesLiver Metabolismescanorsama4000No ratings yet
- 8 ToxicologyDocument5 pages8 ToxicologyMd Sakil AminNo ratings yet
- SHS.109. 15 16 Amino Acid Catabolism and AnabolismDocument55 pagesSHS.109. 15 16 Amino Acid Catabolism and AnabolismFATIMA NAEEMNo ratings yet
- PDH Complex and TCA CycleDocument20 pagesPDH Complex and TCA CycleDarrion LouisNo ratings yet
- ELECTROLYTES (Na & K)Document3 pagesELECTROLYTES (Na & K)Alondra SagarioNo ratings yet
- Lecture 5 Protein Metabolism 1Document29 pagesLecture 5 Protein Metabolism 1Elizabeth LagunasNo ratings yet
- Causes of Metabolic AcidosisDocument1 pageCauses of Metabolic AcidosisfcarcamoNo ratings yet
- LD Atellica Solution Ous Menu Oct 2019-07128017 1800000007128017Document2 pagesLD Atellica Solution Ous Menu Oct 2019-07128017 1800000007128017Xương RồngNo ratings yet
- 3-Acetate Mevalonate PathwayDocument9 pages3-Acetate Mevalonate PathwayBhavana PalNo ratings yet
- ArpitDocument73 pagesArpitDurgesh PushkarNo ratings yet
- ABG Analysis and Acid Base BalanceDocument16 pagesABG Analysis and Acid Base BalancePrashin RocharamNo ratings yet
- Aldehydes Ketones and Carboxylic AcidDocument67 pagesAldehydes Ketones and Carboxylic AcidakshayisuniversalkingNo ratings yet
- Metabolic Acidosis - Endocrine and Metabolic Disorders - Merck Manuals Professional EditionDocument2 pagesMetabolic Acidosis - Endocrine and Metabolic Disorders - Merck Manuals Professional Editionmaulidanabilah5No ratings yet
- Amino Acid CatabolismDocument31 pagesAmino Acid CatabolismLidya YudithNo ratings yet
- Biochemistry High Yield PointsDocument18 pagesBiochemistry High Yield PointsAnonymous S0H8cqgnfiNo ratings yet
- 11 - Carbohydrate MetabolismDocument68 pages11 - Carbohydrate MetabolismcheckmateNo ratings yet
- USMLE Preparatory Online Resource_ Effective Biochemistry and Genetics Teaching Relatively Short Time_Dr Kumar Ponnusamy Urea Cycle & Nitrogen Metabolism_ST Matthew's University School of Medicine 2010Document185 pagesUSMLE Preparatory Online Resource_ Effective Biochemistry and Genetics Teaching Relatively Short Time_Dr Kumar Ponnusamy Urea Cycle & Nitrogen Metabolism_ST Matthew's University School of Medicine 2010Dr Kumar Ponnusamy100% (1)
- Water Soluble VitaminsDocument55 pagesWater Soluble VitaminsDr. M. Prasad Naidu100% (1)
- Types of AntidotesDocument1 pageTypes of AntidotesAprilVivienCuNo ratings yet
- PBC 01 Csala Alcohol 2023Document31 pagesPBC 01 Csala Alcohol 2023mir.wakilmsNo ratings yet
- N-Metabolism AA II 2018 HandoutDocument19 pagesN-Metabolism AA II 2018 HandoutlinNo ratings yet
- Phenyl AcetoneDocument2 pagesPhenyl Acetoneunderworldinc100% (1)
- Neonatal HyperammonaemiaDocument12 pagesNeonatal HyperammonaemiaGawri AbeyNo ratings yet
- GC Toxicology Case Studies Apr 28 2013Document36 pagesGC Toxicology Case Studies Apr 28 2013Grace Anastasia Ginting SinusingaNo ratings yet
- Manajemen Asidosis MetabolikDocument22 pagesManajemen Asidosis MetabolikriyanhstNo ratings yet
- 1.Beta-Oxidation 2.minor Fatty Acid Oxidation Alpha Oxidation Omega Oxidation Peroxisomal Beta OxidationDocument31 pages1.Beta-Oxidation 2.minor Fatty Acid Oxidation Alpha Oxidation Omega Oxidation Peroxisomal Beta OxidationNityantiniNo ratings yet
- Engineering of Biological Processes Metabolic Pathways: Pertemuan 2Document26 pagesEngineering of Biological Processes Metabolic Pathways: Pertemuan 2Fauzan RahmanNo ratings yet
- Porg Lab-PrelimDocument46 pagesPorg Lab-PrelimVincent BustamanteNo ratings yet
- CHEM 180: Christian MANAHAN Anna Esperanza LEGASPIDocument19 pagesCHEM 180: Christian MANAHAN Anna Esperanza LEGASPIAnna LegaspiNo ratings yet
- Pharmacognosy NotesDocument7 pagesPharmacognosy NotesGeniNo ratings yet
- IEMs Kul Nutrisi DR LanangDocument29 pagesIEMs Kul Nutrisi DR LanangChaliza AdnanNo ratings yet
- Mls 218 Protein-MetDocument45 pagesMls 218 Protein-MetZainabNo ratings yet
- K (Anion Gap 12) (Anion Gap 12) Acute Asthma Hypovolemia: - Vomit - Pyloric StenosisDocument4 pagesK (Anion Gap 12) (Anion Gap 12) Acute Asthma Hypovolemia: - Vomit - Pyloric StenosisAhmad Asyraf AzmanNo ratings yet
- Common Drugs AntidotesDocument3 pagesCommon Drugs AntidotesJhix JadraqueNo ratings yet
- Toxic AlcoholsDocument3 pagesToxic Alcoholsمحمد العمريNo ratings yet
- Engineering of Biological Processes Lecture 1: Metabolic PathwaysDocument27 pagesEngineering of Biological Processes Lecture 1: Metabolic Pathwaysagnarindra01_8550147No ratings yet
- General Protein Metabolism NUB Full 2022Document11 pagesGeneral Protein Metabolism NUB Full 2022PIH SHTNo ratings yet
- Blood Gas InterpretationDocument67 pagesBlood Gas Interpretationmajdy2006No ratings yet
- © Dept. of Medical and Clinical Biochemistry Upjš in Košice, Medical Faculty Eva Ďurovcová, MD, PHDDocument51 pages© Dept. of Medical and Clinical Biochemistry Upjš in Košice, Medical Faculty Eva Ďurovcová, MD, PHDPaulina PaskeviciuteNo ratings yet
- Alcohol Metabolism LectureDocument52 pagesAlcohol Metabolism LectureHassan HarunaNo ratings yet
- General Tox ApproachDocument15 pagesGeneral Tox ApproachAnonymous 4txA8N8etNo ratings yet
- 228 Carbohydrate MetabolismDocument43 pages228 Carbohydrate MetabolismAmanullahNo ratings yet
- KodsDocument1 pageKodsJeremiah GatchalianNo ratings yet
- H Berle 2012Document30 pagesH Berle 2012Stoica AlexandruNo ratings yet
- Ch9 Tricardoxylic Acid Cycle LectureDocument16 pagesCh9 Tricardoxylic Acid Cycle Lecturearyanchaudhary77798No ratings yet
- Oilfield Services: Anti-Foams Antioxidants / Iron Control EthyleneaminesDocument2 pagesOilfield Services: Anti-Foams Antioxidants / Iron Control EthyleneaminesDarmawanSaputraNo ratings yet
- Mbbs II Catabolism of AasDocument42 pagesMbbs II Catabolism of AasamareaneneNo ratings yet
- Amino Acid Metabolism: Inborn ErrorsDocument18 pagesAmino Acid Metabolism: Inborn ErrorsAdedoyin BankoleNo ratings yet
- Sodium and PotassiumDocument4 pagesSodium and PotassiumLUALHATI VILLASNo ratings yet