Professional Documents
Culture Documents
0 ratings0% found this document useful (0 votes)
0 viewsCBC Morphology
CBC Morphology
Uploaded by
Razaz AdilThe document discusses various topics related to CBC morphological assessment including red blood cell analysis, indices calculations, classification of anemia, and microscopic examination of blood films. Red blood cell analysis involves quantitative and qualitative characteristics to evaluate red blood cell concentration, size, and hemoglobin content. Anemia can be classified morphologically based on red blood cell size as macrocytic, microcytic, or normocytic, or etiologically based on causes related to reduced production, increased destruction, or blood loss.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You might also like
- ILMA - Haematology 2nd - Moore, KnightDocument687 pagesILMA - Haematology 2nd - Moore, Knightnivesiva24100% (1)
- Atlas of Canine and Feline Peripheral Blood SmearsDocument516 pagesAtlas of Canine and Feline Peripheral Blood SmearsLaura MewsNo ratings yet
- Atlas of Canine and Feline Peripheral Blood SmearsDocument516 pagesAtlas of Canine and Feline Peripheral Blood Smearserasmia100% (3)
- Blood InvestigationDocument70 pagesBlood InvestigationRohit RaiNo ratings yet
- Anemia BMLTDocument134 pagesAnemia BMLTRajkishor YadavNo ratings yet
- Anemias: DR Nargis FatimaDocument25 pagesAnemias: DR Nargis FatimaMuhammad Adil MemonNo ratings yet
- Anemia, Tic, DLC MbbsDocument50 pagesAnemia, Tic, DLC Mbbssharads221004No ratings yet
- Anemia and JaundiceDocument30 pagesAnemia and JaundiceRachit BirthdayNo ratings yet
- Approuch Anemia On Laboratory Based: Hematology 2011Document31 pagesApprouch Anemia On Laboratory Based: Hematology 2011VincentiusNo ratings yet
- ANEMIAS: Red Blood Cell Morphology: and Approach To DiagnosisDocument12 pagesANEMIAS: Red Blood Cell Morphology: and Approach To DiagnosisNoreen B. BañagadoNo ratings yet
- Tariq PresentationDocument24 pagesTariq PresentationHasnain AliNo ratings yet
- Anemia 1Document92 pagesAnemia 1Bikash PatgiriNo ratings yet
- Erythrocytic Indices and Their Significance Final-1Document22 pagesErythrocytic Indices and Their Significance Final-1Asad ullahNo ratings yet
- Hema, AnemiaDocument193 pagesHema, AnemiaTemechegnNo ratings yet
- All HematologyDocument484 pagesAll HematologyIrene NgesaNo ratings yet
- Hematologic Pathology p36-47Document12 pagesHematologic Pathology p36-47zeroun24No ratings yet
- Anemia and Its Types PresentationDocument21 pagesAnemia and Its Types PresentationAyesha ShabbirNo ratings yet
- RBC DisordersDocument49 pagesRBC DisordersGeraldine AgpesNo ratings yet
- AnemiaDocument32 pagesAnemiaSherina Christo100% (3)
- Approach To Anemia: Gayanirmalessvari.S. Final Year Mbbs Part - 2Document12 pagesApproach To Anemia: Gayanirmalessvari.S. Final Year Mbbs Part - 2K DineshNo ratings yet
- Rbcs Indices:: Mean Corpuscular Volume (MCV)Document2 pagesRbcs Indices:: Mean Corpuscular Volume (MCV)Jalal AlbadriNo ratings yet
- Heamatology Dr. Osama PDFDocument94 pagesHeamatology Dr. Osama PDFAnmar ZawahraNo ratings yet
- Anemia Clinical ConsiderationDocument39 pagesAnemia Clinical Considerationema dwi laksanaNo ratings yet
- Dr. Sailendra Nayak Assistant Professor MedicineDocument49 pagesDr. Sailendra Nayak Assistant Professor MedicineAishwarya JeeNo ratings yet
- AnaemiaDocument4 pagesAnaemiaSwayam AroraNo ratings yet
- INTERPRETATION OF CBC -FINALDocument57 pagesINTERPRETATION OF CBC -FINALmedschoolnotes95No ratings yet
- Anemia Its Laboratory DiagnosisDocument146 pagesAnemia Its Laboratory DiagnosisCh M MushahidNo ratings yet
- 1 Introduction To AnemiaDocument60 pages1 Introduction To AnemiaKhisha RangasNo ratings yet
- Hematology L5Document22 pagesHematology L5Haibat Sultan StationeryNo ratings yet
- Chapter Two Anemiarev - ATDocument153 pagesChapter Two Anemiarev - ATAemro TadeleNo ratings yet
- Hematological Investigation or Quantitative Evaluation of The Hematopoietic SystemDocument21 pagesHematological Investigation or Quantitative Evaluation of The Hematopoietic SystemMAMA LALANo ratings yet
- 7.1. Anemia 2023Document30 pages7.1. Anemia 2023MichellyTjoaNo ratings yet
- Hema II Chapter 3 - Anemiarev - ATDocument154 pagesHema II Chapter 3 - Anemiarev - AThannigadah7No ratings yet
- AnaemiaDocument21 pagesAnaemiaJohnson OlawaleNo ratings yet
- Approach To AnemiaDocument50 pagesApproach To AnemiaRishi ShresthaNo ratings yet
- 6 - AnemiaDocument32 pages6 - AnemiaSergio VasquezNo ratings yet
- HaematologyDocument28 pagesHaematologyfh fgNo ratings yet
- RBC, PCV, ESR, Blood IndicesDocument5 pagesRBC, PCV, ESR, Blood IndicesUjjwal Kumar MauryaNo ratings yet
- Curs Hematologie Ex Ob, An Generalit Engl Martie 2023 POSTATDocument55 pagesCurs Hematologie Ex Ob, An Generalit Engl Martie 2023 POSTATErland BordNo ratings yet
- COMPLETE BLOOD PICTURE Ok-1 PDFDocument78 pagesCOMPLETE BLOOD PICTURE Ok-1 PDFEmanuel100% (1)
- Strategi Diagnosis AnemiaDocument49 pagesStrategi Diagnosis Anemianiniek yusidaNo ratings yet
- 6-Un2-Ch5 (Corrected) PDFDocument21 pages6-Un2-Ch5 (Corrected) PDFlina hossamNo ratings yet
- Diagnostic Approach To Anemia: Archana M Agarwal, M.DDocument46 pagesDiagnostic Approach To Anemia: Archana M Agarwal, M.DHasan AbdulhalemNo ratings yet
- 1BMS326 1-16 Combined & CompressedDocument412 pages1BMS326 1-16 Combined & CompressedMitcheruNo ratings yet
- Interpretation of Laboratory ResultsDocument106 pagesInterpretation of Laboratory ResultsNgetwa Wiryboy stNo ratings yet
- Red and White Blood Cell DisordersDocument11 pagesRed and White Blood Cell DisordersVittorio Di PaoloNo ratings yet
- Red Blood Cell DisordersDocument106 pagesRed Blood Cell DisordersCharmaine Clarke-BlytheNo ratings yet
- They Are Important For The Classification of AnemiaDocument2 pagesThey Are Important For The Classification of AnemiathelordhaniNo ratings yet
- Hematology 1 (Laboratory) - Week 10-11 ModuleDocument8 pagesHematology 1 (Laboratory) - Week 10-11 ModuleJam RamosNo ratings yet
- Tutorial Anemia TerbaruDocument36 pagesTutorial Anemia TerbaruLuthfi AnshoriNo ratings yet
- Dr. Haryanto - Kuliah Approach Anemia 2011Document23 pagesDr. Haryanto - Kuliah Approach Anemia 2011YeniNo ratings yet
- Laboratory Diagnosis of Anemia: by Dr. Ankita Pal Department of PathologyDocument43 pagesLaboratory Diagnosis of Anemia: by Dr. Ankita Pal Department of PathologyHimanshiNo ratings yet
- Anemia and Its Oral MenifestationsDocument74 pagesAnemia and Its Oral MenifestationsBushra FaheemNo ratings yet
- تجميع اسئله الاخصائين 1Document36 pagesتجميع اسئله الاخصائين 1Osama BakheetNo ratings yet
- Diagnostic Laboratoric To Anemia: Prof. Dr. Adi Koesoema Aman SPPK (KH) - Dr. Malayana Nasution Mked. Clin - Path. SPPKDocument41 pagesDiagnostic Laboratoric To Anemia: Prof. Dr. Adi Koesoema Aman SPPK (KH) - Dr. Malayana Nasution Mked. Clin - Path. SPPKrubyniNo ratings yet
- Lecture On Anemias and Polycythemias by Dr. RoomiDocument30 pagesLecture On Anemias and Polycythemias by Dr. RoomiMudassar Roomi100% (1)
- 50 FullDocument5 pages50 Fullaulia haninNo ratings yet
- AnemiaDocument145 pagesAnemiaTegegne WorkNo ratings yet
- Second Practical - Hematology: 3 YearDocument44 pagesSecond Practical - Hematology: 3 YearAjigotto ubiq.No ratings yet
- Anemia OsamaDocument57 pagesAnemia Osamaosamafoud7710No ratings yet
- Lecture 2 - Clinical Pathology - AnemiaDocument56 pagesLecture 2 - Clinical Pathology - AnemiaSamuel GitongaNo ratings yet
- Anemia OsamaDocument57 pagesAnemia Osamaosamafoud7710No ratings yet
- Williams Manual of Hematology 10Th Edition Marshall A Lichtman Ebook Full ChapterDocument51 pagesWilliams Manual of Hematology 10Th Edition Marshall A Lichtman Ebook Full Chaptersteven.pinera406100% (9)
- MLS 123 MODULE 6 UNIT 4 Venipuncture Procedure Capillary PunctureDocument17 pagesMLS 123 MODULE 6 UNIT 4 Venipuncture Procedure Capillary PunctureVENUS LIRIA PANTINo ratings yet
- Blood Morphometry or Blood Film CommentDocument103 pagesBlood Morphometry or Blood Film CommentYangnuu TitusNo ratings yet
- Caso de EncefalitisDocument4 pagesCaso de EncefalitisJonathan CuevaNo ratings yet
- H560 BrochureDocument8 pagesH560 Brochuremicklemagdy50No ratings yet
- Malaria Parasite Detection Using Deep LearningDocument7 pagesMalaria Parasite Detection Using Deep LearningIJRASETPublicationsNo ratings yet
- Micropara LabDocument8 pagesMicropara LabFatima KateNo ratings yet
- Blood Cells Morphology and Parasites IdentificationDocument8 pagesBlood Cells Morphology and Parasites IdentificationAnge OuedraogoNo ratings yet
- Ajayi Deborah Siwes ReportDocument32 pagesAjayi Deborah Siwes ReportPAUL VICTORNo ratings yet
- Binda BIO3219 Lab5Document12 pagesBinda BIO3219 Lab5Ronaldo BindaNo ratings yet
- PERIPHERAL SMEAR - UG ClassDocument71 pagesPERIPHERAL SMEAR - UG Classaditya.3757No ratings yet
- Specification Accredited A Level Biology B Advancing Biology h422Document96 pagesSpecification Accredited A Level Biology B Advancing Biology h422Natasha PopliNo ratings yet
- Palmones Parasitology Lab TransesDocument19 pagesPalmones Parasitology Lab TransesJISOO KimNo ratings yet
- Lab Activity No. 5 - Slide PresentationDocument24 pagesLab Activity No. 5 - Slide PresentationChelsea Padilla Delos ReyesNo ratings yet
- The Importance of Trephine Biopsy and Aspirate in Bone Marrow AnalysisDocument5 pagesThe Importance of Trephine Biopsy and Aspirate in Bone Marrow Analysisoki harisandiNo ratings yet
- Standard Operating Procedure (Haematology) : R. K. Life Services Private LimitedDocument61 pagesStandard Operating Procedure (Haematology) : R. K. Life Services Private LimitedAniruddha ChatterjeeNo ratings yet
- Lab Manual ZOL536Document58 pagesLab Manual ZOL536RishavNo ratings yet
- C2 HEMA Lab L8 Reticulocyte Count Platelet Count and PBS 2Document6 pagesC2 HEMA Lab L8 Reticulocyte Count Platelet Count and PBS 2SkxNo ratings yet
- Morphology of HematologyDocument11 pagesMorphology of HematologyChabNo ratings yet
- Diagnosis of Parasitic InfectionsDocument22 pagesDiagnosis of Parasitic InfectionsInsatiable CleeNo ratings yet
- Bloodfilm Preparationandreporting 200512223320Document203 pagesBloodfilm Preparationandreporting 200512223320Alex StefanNo ratings yet
- Malaria Detection Using Image Processing and Machine LearningDocument11 pagesMalaria Detection Using Image Processing and Machine LearningdataprodcsNo ratings yet
- Atlas MalariaDocument4 pagesAtlas MalariaLaboratorium Pkm ManjaNo ratings yet
- Solution Manual For Human Anatomy Physiology Main Version 4th Edition Terry Martin Cynthia Prentice CraveDocument24 pagesSolution Manual For Human Anatomy Physiology Main Version 4th Edition Terry Martin Cynthia Prentice CraveNoahMcbridecekjg100% (44)
- MBBS Second Year Pathology Paper-I Important Question BankDocument11 pagesMBBS Second Year Pathology Paper-I Important Question BankAakansh Maheshwari 1No ratings yet
- PLT Interference-Malang-231125Document6 pagesPLT Interference-Malang-231125Beatrix RosellaNo ratings yet
- 3-Automated Screening of Sickle Cells Using A Smartphone-Based Microscope and Deep LearningDocument30 pages3-Automated Screening of Sickle Cells Using A Smartphone-Based Microscope and Deep LearningAli M. RiyathNo ratings yet
CBC Morphology
CBC Morphology
Uploaded by
Razaz Adil0 ratings0% found this document useful (0 votes)
0 views84 pagesThe document discusses various topics related to CBC morphological assessment including red blood cell analysis, indices calculations, classification of anemia, and microscopic examination of blood films. Red blood cell analysis involves quantitative and qualitative characteristics to evaluate red blood cell concentration, size, and hemoglobin content. Anemia can be classified morphologically based on red blood cell size as macrocytic, microcytic, or normocytic, or etiologically based on causes related to reduced production, increased destruction, or blood loss.
Original Description:
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document discusses various topics related to CBC morphological assessment including red blood cell analysis, indices calculations, classification of anemia, and microscopic examination of blood films. Red blood cell analysis involves quantitative and qualitative characteristics to evaluate red blood cell concentration, size, and hemoglobin content. Anemia can be classified morphologically based on red blood cell size as macrocytic, microcytic, or normocytic, or etiologically based on causes related to reduced production, increased destruction, or blood loss.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
0 views84 pagesCBC Morphology
CBC Morphology
Uploaded by
Razaz AdilThe document discusses various topics related to CBC morphological assessment including red blood cell analysis, indices calculations, classification of anemia, and microscopic examination of blood films. Red blood cell analysis involves quantitative and qualitative characteristics to evaluate red blood cell concentration, size, and hemoglobin content. Anemia can be classified morphologically based on red blood cell size as macrocytic, microcytic, or normocytic, or etiologically based on causes related to reduced production, increased destruction, or blood loss.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 84
Good CBC Morphological
Assessment
NAZAR AHMED MOHAMED ABD-ALLA
BSC - OMDURMAN AHLIA
HIGH DOPLOMA DGREE - ELZAEM EL-AZHARY
FORMER HEAD OF HEMATOLOGY & BLOOD BANK
MINISTRY OF HEALTH – LABORATORY ADMINISTRATION
KHARTOUM STATE
MARKETING MANAGER-LAB EQP –DIVISION
ALGAM COMPANY FOR DRUGS & CHEMICAL LTD
NAZAR AHMED SANGOOR 2008 1
Learning Objective
*1- TO KNOW Red Blood Cell Analysis .
*2- TO KNOW THE IMPORTANCE OF INDICES
CALCULATIONS IN BLOOD TESTING.
*3- TO Know THE Classification Of Anemia.
*4- TO KNOW How To Check Leishman Stain.
*5- TO KNOW The differential white cell
count.
NAZAR AHMED SANGOOR 2008 2
Learning Objective
*6- TO KNOW The Value of Good Morphological
Assessment.
*7- TO KNOW Microscopic Examination of Blood
Films.
*8- TO KNOW PROCEDURE FOR EXAMINATION OF
STAINED BLOOD SMEAR.
*9- TO KNOW Sources of error OF STAINED
BLOOD SMEAR.
NAZAR AHMED SANGOOR 2008 3
Red Blood Cell Analysis
*Red Blood Cell Are Define :
* 1-Three Quantative Value:
1- Volume of packed red cell (VPRC) or
hematocrite.
2- Hb concentration .
3- The red cell concentration per unite of
volume.
NAZAR AHMED SANGOOR 2008 4
Red Blood Cell Analysis
• *2- Three Qualative Characteristic Of The
Red Cell Population.
• 1- MCV (Mean Cell Volume)
• 2- MCH (Mean Cell Hemoglobin)
• 3-MCHC (Mean Cell Hemoglobin
Concentration)
NAZAR AHMED SANGOOR 2008 5
CONTENUE
• *1- volume of the red cell hematocrite :
• *It is the proportion of the volume of blood sample
that is occupied by red blood cell.
• *determine manually by centrifugation of blood at
agiving speed and time in standardized glass tube
with uniform bore.
• *The height of the column of red cell compared with
that of the total column of blood following
centrifugation yields the hematocrite .
NAZAR AHMED SANGOOR 2008 6
Source Of Inherent Error
• *Hematocrite measure the red cell
concentration not red cell mass there for:
• *1-Pateiont in shock or with volume
depletion may have normal or high
hematocrite measurement due to
hemoconcentration despite a decreased red
cell mass.
NAZAR AHMED SANGOOR 2008 7
Source Of Inherent Error
• *2- Trapping of plasma in the red cell column
• *The plasma may trapped in:
• *Sickle cell –microcytic cell- macrocytic cell-
spherocyte cell at a high level because of
increase cellular rigidity.
NAZAR AHMED SANGOOR 2008 8
Technical Error
• *1- poor sample mixing
• *2- Inappropriate anticoagulant
concentration
• *3-Insufficient centrifugation
NAZAR AHMED SANGOOR 2008 9
THE IMPORTANCE OF INDICES CALCULATIONS
IN BLOOD TESTING
• *The RBC indices is a mathematical calculation
to yield three clinical parameters useful in
diagnosing anemia.
• *1- Mean Corpuscular Volume (MCV):
• average RBC volume is calculated by dividing
the hematocrit value by the RBC count value.
• *MCV = Hematocrit (L/L) x 1000
Red cell count 10/L)
NAZAR AHMED SANGOOR 2008 10
* Normal values :are 76 to 96 femtoliters (fL).
*value is below 76 fL, then this indicates
microcytic anemia.
value greater than 96 would suggest
macrocytic anemia.
* falsely elevated:
*1-Agglutination of RBC as in cold agglutinin
disease .
*2- Sever hyperglassemia may cause somatic
swelling of RBC during analysis
NAZAR AHMED SANGOOR 2008 11
• *2-Mean Corpuscular Hemoglobin (MCH). This
describes the hemoglobin content of each RBC .
• *obtained by dividing the hemoglobin value by
the RBC count value.
• *The normal value ranges :from 27 to 32
picograms (pg).
• *Values below 27 pg are suggestive of
hypochromic anemia.
• * falsely elevated by hyperlibidemia or
leukocytosis.
NAZAR AHMED SANGOOR 2008 12
• *3-Mean Corpuscular Hemoglobin
Concentration (MCHC):
• *describing the hemoglobin concentration in
RBC’s.
• *MCHC := Hb (g/dl)/ hematocrit(L/L)
• *Normal values range : 31 to 36 g/dL.
• * Low value suggest hypochromic anemias.
• * MCHC affected by factor effect the PCV &Hb
g/dl
• *Note: Indices normal values may vary slightly
from region to region or lab to lab.
NAZAR AHMED SANGOOR 2008 13
Red Cell Distribution Width
• *Reflects the range of red cell sizes measured
within a sample.
• *RDW has been proposed to be useful in early
classification of anemia
• *1- become abnormal earlier in nutritional
deficiency anemia than any of the other red cell
parameter especially in case of IDA .
NAZAR AHMED SANGOOR 2008 14
Red Cell Distribution Width
• *2- useful in characterizing microcytic anemia
particularly distinguishing between IDA (high
RDW low MCV )and uncomplicated
heterozygous thalassemia (normal RDW low
MCV).
• *3-useful to identified the following (red cell
fragmentation –agglutination –dimorphic cell
populations).
• *4-Cold agglutinin disease increase MCV
&RDW volume .
NAZAR AHMED SANGOOR 2008 15
ANEMIAS MAY BE CLASSIFIED BY RDW AND
MCV
RDW = N MCV = N RDW = I MCV = N
Normocytic: acute hemorrhage sideroblastic
chronic liver disease early IDA
RDW = N MCV = D RDW = I MCV = D
Microcytic: heterozygous thalassemia iron deficiency anemia
anemia of chronic disease homozygous thalassemia
RDW = N MCV = I RDW = I MCV = I
Macrocytic: myelodysplastic syndromes B12 deficiency
aplastic anemia immune hemolytic with
marked reticulocytosis
NAZAR AHMED SANGOOR 2008 16
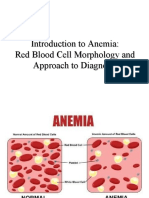
ANEMIA
• *Anemia is simply a reduction in the number
of circulating RBC’s and/or hemoglobin
concentration and\ or PCV below the normal
range according to age & sex.
• *This means that the hemoglobin value will
drop below 13 g/dL in the adult male and
below 11 g/dL in the female.
NAZAR AHMED SANGOOR 2008 17
NAZAR AHMED SANGOOR 2008 18
Classification Of Anemia
• *A e ia’s may be classified in more than one
way.
• *1- morphologically in which there are three
general types of anemia:
*a. macrocytic
*b. microcytic
*c. normocytic
.
NAZAR AHMED SANGOOR 2008 19
Classification Of Anemia
• *2- pathophysiological or etiological classification
*two general groups of anemias appear.
* A-Those anemias that are caused by a reduction
in the number of RBC’s due to a maturation defect.
*B-The other group of anemias are those due to
increased RBC destruction
NAZAR AHMED SANGOOR 2008 20
Classification Of Anemia
• *Morphological Classification Of Anemia
• *Groups anemia by red blood cell size.
• *1- Macrocytic Normochromic Red Blood Cell
• *A- vitamin B12 deficiency. Folic acid deficiency:
• 1- pernicious anemia 2- sprue
• 3- following gastrectomy 4- dietary
• 5- abnormal intrinsic factor 6- tape worm
infection
• *B- disease of the liver:
NAZAR AHMED SANGOOR 2008 21
Classification Of Anemia
• *2- Normocytic Normochromic Red Blood Cell:
• *A- defective formation of the blood cell or the
presence of tumor cell in the bone marrow
• 1- a plastic anemia . 2- leukemia .
• 3- Hodgkin's disease . 4- multiple myeloma.
NAZAR AHMED SANGOOR 2008 22
Classification Of Anemia
• 5-leukoerythroblastosis. 6- metstatic cancer
• 7- anemia associated with renal and endocrine
disease.
• 8- anemia associated with inflammatory disease
(chronic disorder).
NAZAR AHMED SANGOOR 2008 23
CONTENUE
• *B- Abnormal hemoglobin increased
destruction of the red blood cell
• 1-certain acquired hemolytic anemia.
• 2- paroxysmal nocturnal hemoglobinuria.
• 3- sickle cell anemia.
• 4- hemolytic disease of the new born.
• 5- anemia of chronic renal insufficiency.
NAZAR AHMED SANGOOR 2008 24
*3-Microcytic Anemia
• A-iron deficiency anemia
• B-thalassemia
• C- sidroblastic anemia
• D- chronic blood loss
NAZAR AHMED SANGOOR 2008 25
*Classification Of Anemia According To
Causes (kinetic )
• *Involves evaluating production, destruction and loss
• *1- decrease or impair production of red blood
cell:
• A- bone marrow damage, Infiltration, Atrophy.
• 1- leukemia . 2- leukoerythroblastosis.
• 3-aplastic anemia. 4- lymphoma .
• 5- multiple myLoma. 6- myelofibrosis.
• 7- pure red cell aplasia.
NAZAR AHMED SANGOOR 2008 26
*B- Decrease Erythropoietin
• 1- inflammatory process.
• 2 – anemia of chronic disorder.
• 3- chronic renal disease .
• 4- hypothyroidism.
• *C- Vitamin And Mineral Deficiencies
• 1-iron deficiency anemia.
• 2- vitamin B12 and folic acid deficiency.
• *D- Defect In Globin Synthesis
• 1- ά & β thalassemia. 2- μ thalassemia trait.
NAZAR AHMED SANGOOR 2008 27
• * E- iron over load
1- sidroblastic anemia.
2-hemochromatosis.
*F- ineffective erythropoiesis :
• 1- congenital dyserythropoietic anemia.
• 2- increased RBC destruction.
NAZAR AHMED SANGOOR 2008 28
*2-Destruction And Loss
1-Intrinsic Defect With In RBC:
• 1- Hereditary Membrane Defect:
• A- spherocytosis
• B- Elleptocytosis
• C- Abetalipoproteinemia
• D- stomatocytosis
• E- Rh null disease
NAZAR AHMED SANGOOR 2008 29
*1-Intrinsic Defect With In RBC:
• *2- Hereditary Enzyme Defect:
• A- Glucose -6- phosphate dehydrogenase.
• B- Pyruvate Kinas.
• *3- Hereditary Hemoglobin Defect
(Hemoglobinopathy):
• 1- Sickled cell (disease & trait ) .
• 2- HbC.
• 3- HbE .
• 4- HbD.
NAZAR AHMED SANGOOR 2008 30
• *4- Hereditary Defective Globin Synthesis
• A- α or β Thalassemia (Major or minor).
• B- Sickle – β thalassemia.
• C- ε β thalassemia.
• D- HbH Disease.
NAZAR AHMED SANGOOR 2008 31
*2- Extra Corpuscular Causes:
• * A-Non Acquired Hemolytic Anemia
• 1- chemical, toxin, venom .
• 2- physical trauma disorder causing fragmentation
• a- Burns. b- Cardiac Replacement Valve.
• c- Microangiopathic Hemolytic Anemia.
• d- Hemolytic Uremic Syndrome.
• 3- Infection ( parasitic).
NAZAR AHMED SANGOOR 2008 32
*2-Extra Corpuscular Causes: (immune
hemolytic anemia)
*B- Acquired Hemolytic Anemia
• 1- Isoimmune Anemia.
• a- Incompatible Blood Transfusion
• b- HDN
• 2- Autoimmune Antibodies.
• a- warm
• b- cold (CHAD)
• 3- Drugs.
NAZAR AHMED SANGOOR 2008 33
• 4-Miscellaneous.
1- Anemia Of Liver Disease
• 2- Sulfhemoglobinemia
• 3- Porphyries
• 4- Methemoglobinemia
• 5- Acute Blood Loss (e.g. car accident)
• 6-Proximal Nocturnal Hemoglobinurea
NAZAR AHMED SANGOOR 2008 34
*How To Check Leishman Stain
• *Check the nuclose of wbc & the cytoplasm
• *Check if there is eosinophil to see the pinkish
color of granules.
• *Check the reddish brown color of RBC.
• *To obtain good staining film avoid the air
which well evaporate the glycerol.
• *Buffer the stain by use buffered DW
• PH 7.2-7.4.
NAZAR AHMED SANGOOR 2008 35
Staining characteristics of a correctly stained
normal film
Nuclei Purple
Cytoplasm
Erythrocytes Deep pink
Reticulocytes Grey-blue
Neutrophils Orange-pink
Lymphocytes Blue ; some small
lymphocytes deep blue
Monocytes Grey-blue
NAZAR AHMED SANGOOR 2008
36
*Staining characteristics of a correctly
stained normal film
Basophils Blue
Granules
Neutrophils Fine purple
Eosinophils Red-orange
Basophils Purple-black
Monocytes Fine reddish (azurophil)
Platelets Purple
NAZAR AHMED SANGOOR 2008 37
Staining faults
Too blue Smear too thick
Inadequate time in buffer
Buffer pH too high
Staining time too long
Diluted stain overused,
requires replenishment
Stock MGG solution
incorrectly made or made
from impure dye
Stock solution left
exposed to bright daylight
NAZAR AHMED SANGOOR 2008 38
Staining faults
Too pink Excessive time in buffer
Buffer pH too low
Stock MGG solution
incorrectly made or made
from impure dye
Cover slip mounted before
film is completely dry
Too faint Staining time too short
Excessive washing after
staining
NAZAR AHMED SANGOOR 2008 39
Staining faults
Stain deposit Stain solution left in
uncovered jar or tray
Stain solution not filtered
Dirty slides
Blue background Inadequate fixation
Prolonged storage before
fixation
Blood
anticoagulated with
heparin
NAZAR AHMED SANGOOR 2008 40
*Making a blood film
*A blood film may be made from non-
anticoagulated (native) blood, obtained either
from a vein or capillary.
* from EDTA-anticoagulated blood Chelation of
calcium by EDTA hinders platelets aggregation so
that platelets are evenly spread and their numbers
can be assessed more easily.
NAZAR AHMED SANGOOR 2008 41
*Films prepared from capillary blood usually show
prominent platelet aggregation .
*films from native venous blood often show small
Aggregates .
*Films prepared from native venous or capillary
blood are free of artifacts due to storage or the
effects of the anticoagulant.
NAZAR AHMED SANGOOR 2008 42
*Good laboratory practice includes:
*recording the date and time the specimen is
received in the laboratory.
*making a film shortly after receipt of the specimen.
*In this way the length of any delay in transit is
known and attribution of morphological changes to
prolonged storage of EDTA-anticoagulated blood.
NAZAR AHMED SANGOOR 2008 43
*A drop of blood (either native or
anticoagulated) is placed near one end of the
slide.
*Anticoagulated blood from screw-top
containers can be applied to the slide using a
capillary tube, which is then discarded.
NAZAR AHMED SANGOOR 2008 44
*A drop of blood from specimen containers with
penetrable lids can be applied to the slide by means
of a special device that perforates the lid.
*The spreader is applied at an angle of 25–30°, in
front of the drop of blood, and is drawn back into it.
*Once the blood has run along its back edge, the
spreader is advanced with a smooth steady motion
so that a thin film of blood is spread over the slide.
NAZAR AHMED SANGOOR 2008 45
*If the angle of the spreader is too obtuse or the
speed of spreading is too fast, the film
will be too short.
*An experienced operator learns to recognize
blood with a higher than normal haematocrit
(Hct), which is more viscous and requires a more
acute angle to make a satisfactory film and,
conversely, blood with a lower than normal Hct,
which requires a more obtuse angle.
NAZAR AHMED SANGOOR 2008 46
*The differential white cell count
*A differential white cell count is the assigning of
leucocytes to their individual categories, this
categorization being expressed as a percentage or,
when the WBC is available, as an absolute count.
*The ICSH recommends that the differential
leucocyte count be expressed in absolute
numbers.
NAZAR AHMED SANGOOR 2008 47
*Cells that are normally present in the peripheral
blood can be assigned to five or six categories,
depending on whether non-segmented or band
forms of neutrophils are separated from segmented
neutrophils or are counted with them.
*The differential count also includes any abnormal
cells that may be present.
*NRBC can be included as separate category in the
differential count or, alternatively,their number can
be expressed per 100 white cells.
NAZAR AHMED SANGOOR 2008 48
The Value of Good Morphological
Assessment
*1. Many blood diseases can confidently be
diagnosed, or at least strongly suspected, on the
morphology.
*This can save a great deal of time, money, and even
discomfort for the patient .
*2. There are some diseases where the FBC can be
completely normal and only the morphology will
show abnormalities, such as lead poisoning.
NAZAR AHMED SANGOOR 2008 49
*3. It has been pointed out that the absolute amount
of plasma water affects the counts and many of the
measurements in the FBC, which may produce
spuriously abnormal results.
* Plasma water does not affect the morphology, and
this may be of assistance in such cases.
*4. The presence in a report of an obviously careful
morphological assessment indicates that the FBC as a
whole was looked at by a professional.
NAZAR AHMED SANGOOR 2008 50
*Blood films is essential in cases of
1. Leukopenia. 2. Leukocytosis.
3. Thrombocytosis. 4. Thrombocytopenia.
5. Anemia of unknown etiology.
6. A moderately to severely raised RDW even in an
otherwise normal count.
7. A moderate to severe left shift with a normal WCC.
8. The presence of nucleated RBCs.
NAZAR AHMED SANGOOR 2008 51
*Microscopic Examination of Blood Films
*1-Survey the film at x10 magnification to get a general
impression of its quality.
*2- Find an area where the red cells are evenly
distributed, just touching but not overlapping, and
study their morphology at x 40.
*3-Scan the film to get an impression whether the
leucocytes are increased or decreased.
NAZAR AHMED SANGOOR 2008 52
*Microscopic Examination of Blood Films
*4- Identify any unusual or abnormal
cells.
*5-Estimate the relative proportion of
platelets and note the presence of
abnormally large platelets.
*6-Use the x100 lens for studying the
fine details of the cell morphology.
NAZAR AHMED SANGOOR 2008 53
*PROCEDURE FOR EXAMINATION OF STAINED BLOOD
SMEAR
**1- Check the blood smear to ensure that it is well
made .
*The tail of the film should be smooth
**2- Examine the blood smear using the low power
(10x) objective.
*A- the red cell and white blood cell and platelets
must be correctly stained
NAZAR AHMED SANGOOR 2008 54
The Smear
NAZAR AHMED SANGOOR 2008 55
PROCEDURE FOR EXAMINATION OF STAINED
BLOOD SMEAR
• *B- check that there is even distribution of the
WBC on the smear.
• *C- Estimate the WBC count by noting the number
of WBC / field and the number of WBC in relation
to the RBC count it should agree with the test
result obtained
NAZAR AHMED SANGOOR 2008 56
CONTENUE
• *D- when scanning the blood smear it is
important to note any thing unusual or irregular
such as large abnormal looking cell or rouleaux
formation of the RBC.
• *E- choose the area of the blood smear where
the differential count is to begin place a drop of
oil on the slid and carefully change the oil
immersion to objective (100x ).
• **3- perform the differential cell count and at the
same time examine the morphology of the WBC.
NAZAR AHMED SANGOOR 2008 57
* type of examination can be choose
*1- using the cross – sectional or crenellation
technique .
*The wbc are counted in consecutive fields as
the blood film moved from side to side .
*Counting should begin in the thin area of the
smear where the red blood cell are slightly
over lapping and procedure into the thicker
area.
NAZAR AHMED SANGOOR 2008 58
*2-longitudinal method
• *The wbc are counted in consecutive field from tail
toward the head of the smear.
• *This is the ideal method if the smear is thin enough
so that the wbc may be identified all the way to the
beginning of the smear.
NAZAR AHMED SANGOOR 2008 59
*3- The battlement field
*uses a pattern of consecutive field beginning near
the tail on a horizontal edge count three
consecutive horizontal edge field ( moving away
from the tail count two fields toward the center of
the smear count this fields horizontal moving away
from the tail count two fields vertically to the edge.
NAZAR AHMED SANGOOR 2008 60
Recommended Method For Differential
NAZAR AHMED SANGOOR 2008 61
CONTENUE
• **4- Identify each wbc seen and record on
differential cell counted until 100 wbc have been
counted.
• *If nucleated red cell have been counted enumerate
them on a separate counter these cell are not to be
included in the 100 cell differential count but are
reported as # of NRBC /100 WBC
NAZAR AHMED SANGOOR 2008 62
CONTENUE
• *If any megakaryocytic cell or fragments
smudge cell or epithelial cell are seen these
cell should also be enumerated in the same
manner as the NRBC and reported as the
• # /100 wbc
NAZAR AHMED SANGOOR 2008 63
CONTENUE
*2- Examine the RBC morphology in a thin area
of the slide where only a few of the RBC slight
over lap not any variation from normal and
classify these irregularities as slight, moderate,
marked ,or sever
NAZAR AHMED SANGOOR 2008 64
ERYTHROCYTE MORPHOLOGY EVALUATION
CRITERIA
Morphology \ Normal SL MO MAR SEV
Characteristics Limits
Acanthocyte none 1-5 6-10 11-20 >20
Anisocytosis 0-2 3-10 11-20 21-50 >50
Basophilic Stippling 0-1 1-5 6-10 11-20 >20
Burr 0-2 3-10 11-20 21-50 >50
Elliptocytes 0-2 3-10 11-20 21-50 >50
Helmet (Bite) none 1-5 6-10 11-20 >20
Howell-Jolly none 1-2 3-5 6-10 >10
Hypochromia 0-2 3-10 11-20 21-50 >50
Macrocytes 0-5 6-10 11-20 21-50 >50
Microcytes 0-5 6-10 11-20 21-50 >50
Pappenheimer bodies None 1-2 3-5 6-10 >10
NAZAR AHMED SANGOOR 2008 65
ERYTHROCYTE MORPHOLOGY EVALUATION
CRITERIA
Morphology Normal SL MO MAR SEV
Characteristics Limits
Poikilocytosis 0-2 3-10 11-20 21-50 >50
Polychromatophilia (adult) 0-1 2-5 6-10 11-20 >50
Polychromatophilia (infant) 1-6 7-15 16-20 21-50 >50
Schistocytes None 1-5 6-10 11-20 >20
Sickle Cells None (if present in any number, report as positive)
Spherocytes 0-2 3-10 11-20 21-50 >50
Stomatocytes 0-2 3-10 11-20 21-50 >50
Target Cells (Codocytes) 0-2 3-10 11-20 21-50 >50
Tear Drop Cells 0-2 2-5 6-10 11-20 >20
Rouleaux Formation
Aggregates of 3 to 4 RBC’s = 1+
Aggregates of 5 to 10 RBC’s = 2+
Aggregates of >10 RBC’s = 3+ only a few free RBC’s noted
NAZAR AHMED SANGOOR 2008 66
CONTENUE
*3- Examine the platelets on the smear for
morphology and number present.
* Determine the approximate number of platelets
per field a normal blood smear (normal RBC &
normal platelets count )should show approximately
8-20 platelets per field .
One method for reporting platelets estimates is to
determine the average number of platelets per
field 5 to 10 different field and multiply this result
by 20.000 .
NAZAR AHMED SANGOOR 2008 67
Platelet Estimation Report Platelet
Estimation
• 0-49.000 /ml (marked decrease)
• 50.000-99.000/ml (moderate decrease)
• 100.000-149.000/ml (slight decrease)
• 150.000-199.000/ml (low normal )
• 200.000-400.000/ml (normal )
• 401.000-599.000 /ml (slight increase)
• 600.000-800.000/ml (moderate increase)
• Above 800.000/ml (marked increase )
NAZAR AHMED SANGOOR 2008 68
DISCUSSION
*1- when reporting manual differential Wbcc the result are
reported as a percentage of each cell type present.
*The most accurate and also the most preferable method of
reporting is the absolute count because the result expressed
in percent is relative number and can be misleading to the
clinician.
*The absolute count is calculated as:
Absolute# of cell/L=% of cell type in deferential X Wbc/L
NAZAR AHMED SANGOOR 2008 69
• *2- when counting a deferential the
following outline may be followed
• *A- WBC :
• *- check for even distribution and estimate the
number present also look for any gross
abnormalities present on the smear
• *-perform the differential count
• *-examine for morphology abnormality
NAZAR AHMED SANGOOR 2008 70
*Relative And Absolute Normal Adult Values
Normal Range: WBC Count = 5000 µL to 10,000/µL
Relative Values Absolute Values Bands = 0 to 6% Bands = 0 to
600/µL
Neutrophils = 55 to 68% Neutrophils = 2,000 to 6800/µL
Lymphocytes = 20 to 40% Lymphocytes = 1000 to 4000/µ
Monocytes = 2 to 8% Monocytes = 200 to 800/µL
Eosinophils = 0 to 3% Eosinophils = 0 to 300/µL
Basophils = 0 to 1% Basophils = 0 to 100/µL
NAZAR AHMED SANGOOR 2008 71
CONTENUE
• *B- RBC : Examine for
• -size and shape
• -Relative Hb content
• -polychromatophilia & NRBC
• -inclusion
• -rouleaux formation and agglutination
• -parasite.
NAZAR AHMED SANGOOR 2008 72
• *C- Platelets
• - Estimate number present
• -Examine for morphologic abnormalities
• 3-When studding stain smear do not examine
in the thick area always look for the cell where
it well distributed
NAZAR AHMED SANGOOR 2008 73
*4- when the white count below 1.000 it may be
difficult to find many wbc on stained smear so
differential may be perform by counting 50 wbc
annotation on the report must be made that only
50 wbc were counted (alternatively Buffy coat
smear may be perform
NAZAR AHMED SANGOOR 2008 74
*5- when the differential show an abnormal
distribution of cell type as
a- over 10%eosinophils b- over 2%
basophile
• c- over 11%monocytes d- more lymphocyte
than neutrophil (except for children)
• *A 200 cell differential may performed then
the result are average (divided by 2)and
notation is giving that 200 cell where counted
NAZAR AHMED SANGOOR 2008 75
• *6- before reporting the platelets is decrease
• *Scan the film for clump on low power especially
the feathered edge also recheck the tube for clot
• *7- if the differential count show the present of
immature granulocyte cell this is termed a shift to
left (leukemia, bacterial infection)
• A shift to right refer to an increase number of
hyper segmented neutrophils.
NAZAR AHMED SANGOOR 2008 76
*Sources of error
• *Irregular spread with ridges and long tail:
Edge of spreader dirty or chipped; dusty slide
• *Holes in film: Slide contaminated with fat or
grease
• *Irregular leucocyte and platelet distribution,
especially in tail: poor film-making technique
• *Film too short and too thick: spreader held at
incorrect angle
NAZAR AHMED SANGOOR 2008 77
*Sources of error
• *Film extending to end of slide: blood drop
too large
• *Short thin film: blood drop too small
• *Film extends to edge of slide: spreader too
wide or not positioned correctly
• *Cellular degenerative changes: delay in
fixing, inadequate fixing time or methanol
contaminated with water
NAZAR AHMED SANGOOR 2008 78
ANY QUESTION ?
NAZAR AHMED SANGOOR 2008 79
Learning Outcome
*1- KNOWING Red Blood Cell Analysis .
*2- KNOWING THE IMPORTANCE OF INDICES
CALCULATIONS IN BLOOD TESTING.
*3- KNOWING THE Classification Of Anemia.
*4- KNOWING How To Check Leishman Stain.
*5- KNOWING The differential white cell
count.
NAZAR AHMED SANGOOR 2008 80
Learning Outcome
*6- KNOWING The Value of Good
Morphological Assessment.
*7- KNOWING Microscopic Examination of
Blood Films.
*8- KNOWING PROCEDURE FOR
EXAMINATION OF STAINED BLOOD SMEAR.
*9- KNOWING Sources of error OF STAINED
BLOOD SMEAR.
NAZAR AHMED SANGOOR 2008 81
Summary
*1- CBC morphological assessment is very
important diagnostic tool need to be done
professionally by expert person who
followed good laboratory practices and get
enough knowledge to examine the smear.
NAZAR AHMED SANGOOR 2008 82
Summary
*2- person who examine the smear
should have background knowledge
about normal and abnormal blood cell
morphology.
*3- interpretation of result obtain is very
important.
NAZAR AHMED SANGOOR 2008 83
THANK YOU FOR GOOD ATTENTION
NAZAR AHMED SANGOOR 2008 84
You might also like
- ILMA - Haematology 2nd - Moore, KnightDocument687 pagesILMA - Haematology 2nd - Moore, Knightnivesiva24100% (1)
- Atlas of Canine and Feline Peripheral Blood SmearsDocument516 pagesAtlas of Canine and Feline Peripheral Blood SmearsLaura MewsNo ratings yet
- Atlas of Canine and Feline Peripheral Blood SmearsDocument516 pagesAtlas of Canine and Feline Peripheral Blood Smearserasmia100% (3)
- Blood InvestigationDocument70 pagesBlood InvestigationRohit RaiNo ratings yet
- Anemia BMLTDocument134 pagesAnemia BMLTRajkishor YadavNo ratings yet
- Anemias: DR Nargis FatimaDocument25 pagesAnemias: DR Nargis FatimaMuhammad Adil MemonNo ratings yet
- Anemia, Tic, DLC MbbsDocument50 pagesAnemia, Tic, DLC Mbbssharads221004No ratings yet
- Anemia and JaundiceDocument30 pagesAnemia and JaundiceRachit BirthdayNo ratings yet
- Approuch Anemia On Laboratory Based: Hematology 2011Document31 pagesApprouch Anemia On Laboratory Based: Hematology 2011VincentiusNo ratings yet
- ANEMIAS: Red Blood Cell Morphology: and Approach To DiagnosisDocument12 pagesANEMIAS: Red Blood Cell Morphology: and Approach To DiagnosisNoreen B. BañagadoNo ratings yet
- Tariq PresentationDocument24 pagesTariq PresentationHasnain AliNo ratings yet
- Anemia 1Document92 pagesAnemia 1Bikash PatgiriNo ratings yet
- Erythrocytic Indices and Their Significance Final-1Document22 pagesErythrocytic Indices and Their Significance Final-1Asad ullahNo ratings yet
- Hema, AnemiaDocument193 pagesHema, AnemiaTemechegnNo ratings yet
- All HematologyDocument484 pagesAll HematologyIrene NgesaNo ratings yet
- Hematologic Pathology p36-47Document12 pagesHematologic Pathology p36-47zeroun24No ratings yet
- Anemia and Its Types PresentationDocument21 pagesAnemia and Its Types PresentationAyesha ShabbirNo ratings yet
- RBC DisordersDocument49 pagesRBC DisordersGeraldine AgpesNo ratings yet
- AnemiaDocument32 pagesAnemiaSherina Christo100% (3)
- Approach To Anemia: Gayanirmalessvari.S. Final Year Mbbs Part - 2Document12 pagesApproach To Anemia: Gayanirmalessvari.S. Final Year Mbbs Part - 2K DineshNo ratings yet
- Rbcs Indices:: Mean Corpuscular Volume (MCV)Document2 pagesRbcs Indices:: Mean Corpuscular Volume (MCV)Jalal AlbadriNo ratings yet
- Heamatology Dr. Osama PDFDocument94 pagesHeamatology Dr. Osama PDFAnmar ZawahraNo ratings yet
- Anemia Clinical ConsiderationDocument39 pagesAnemia Clinical Considerationema dwi laksanaNo ratings yet
- Dr. Sailendra Nayak Assistant Professor MedicineDocument49 pagesDr. Sailendra Nayak Assistant Professor MedicineAishwarya JeeNo ratings yet
- AnaemiaDocument4 pagesAnaemiaSwayam AroraNo ratings yet
- INTERPRETATION OF CBC -FINALDocument57 pagesINTERPRETATION OF CBC -FINALmedschoolnotes95No ratings yet
- Anemia Its Laboratory DiagnosisDocument146 pagesAnemia Its Laboratory DiagnosisCh M MushahidNo ratings yet
- 1 Introduction To AnemiaDocument60 pages1 Introduction To AnemiaKhisha RangasNo ratings yet
- Hematology L5Document22 pagesHematology L5Haibat Sultan StationeryNo ratings yet
- Chapter Two Anemiarev - ATDocument153 pagesChapter Two Anemiarev - ATAemro TadeleNo ratings yet
- Hematological Investigation or Quantitative Evaluation of The Hematopoietic SystemDocument21 pagesHematological Investigation or Quantitative Evaluation of The Hematopoietic SystemMAMA LALANo ratings yet
- 7.1. Anemia 2023Document30 pages7.1. Anemia 2023MichellyTjoaNo ratings yet
- Hema II Chapter 3 - Anemiarev - ATDocument154 pagesHema II Chapter 3 - Anemiarev - AThannigadah7No ratings yet
- AnaemiaDocument21 pagesAnaemiaJohnson OlawaleNo ratings yet
- Approach To AnemiaDocument50 pagesApproach To AnemiaRishi ShresthaNo ratings yet
- 6 - AnemiaDocument32 pages6 - AnemiaSergio VasquezNo ratings yet
- HaematologyDocument28 pagesHaematologyfh fgNo ratings yet
- RBC, PCV, ESR, Blood IndicesDocument5 pagesRBC, PCV, ESR, Blood IndicesUjjwal Kumar MauryaNo ratings yet
- Curs Hematologie Ex Ob, An Generalit Engl Martie 2023 POSTATDocument55 pagesCurs Hematologie Ex Ob, An Generalit Engl Martie 2023 POSTATErland BordNo ratings yet
- COMPLETE BLOOD PICTURE Ok-1 PDFDocument78 pagesCOMPLETE BLOOD PICTURE Ok-1 PDFEmanuel100% (1)
- Strategi Diagnosis AnemiaDocument49 pagesStrategi Diagnosis Anemianiniek yusidaNo ratings yet
- 6-Un2-Ch5 (Corrected) PDFDocument21 pages6-Un2-Ch5 (Corrected) PDFlina hossamNo ratings yet
- Diagnostic Approach To Anemia: Archana M Agarwal, M.DDocument46 pagesDiagnostic Approach To Anemia: Archana M Agarwal, M.DHasan AbdulhalemNo ratings yet
- 1BMS326 1-16 Combined & CompressedDocument412 pages1BMS326 1-16 Combined & CompressedMitcheruNo ratings yet
- Interpretation of Laboratory ResultsDocument106 pagesInterpretation of Laboratory ResultsNgetwa Wiryboy stNo ratings yet
- Red and White Blood Cell DisordersDocument11 pagesRed and White Blood Cell DisordersVittorio Di PaoloNo ratings yet
- Red Blood Cell DisordersDocument106 pagesRed Blood Cell DisordersCharmaine Clarke-BlytheNo ratings yet
- They Are Important For The Classification of AnemiaDocument2 pagesThey Are Important For The Classification of AnemiathelordhaniNo ratings yet
- Hematology 1 (Laboratory) - Week 10-11 ModuleDocument8 pagesHematology 1 (Laboratory) - Week 10-11 ModuleJam RamosNo ratings yet
- Tutorial Anemia TerbaruDocument36 pagesTutorial Anemia TerbaruLuthfi AnshoriNo ratings yet
- Dr. Haryanto - Kuliah Approach Anemia 2011Document23 pagesDr. Haryanto - Kuliah Approach Anemia 2011YeniNo ratings yet
- Laboratory Diagnosis of Anemia: by Dr. Ankita Pal Department of PathologyDocument43 pagesLaboratory Diagnosis of Anemia: by Dr. Ankita Pal Department of PathologyHimanshiNo ratings yet
- Anemia and Its Oral MenifestationsDocument74 pagesAnemia and Its Oral MenifestationsBushra FaheemNo ratings yet
- تجميع اسئله الاخصائين 1Document36 pagesتجميع اسئله الاخصائين 1Osama BakheetNo ratings yet
- Diagnostic Laboratoric To Anemia: Prof. Dr. Adi Koesoema Aman SPPK (KH) - Dr. Malayana Nasution Mked. Clin - Path. SPPKDocument41 pagesDiagnostic Laboratoric To Anemia: Prof. Dr. Adi Koesoema Aman SPPK (KH) - Dr. Malayana Nasution Mked. Clin - Path. SPPKrubyniNo ratings yet
- Lecture On Anemias and Polycythemias by Dr. RoomiDocument30 pagesLecture On Anemias and Polycythemias by Dr. RoomiMudassar Roomi100% (1)
- 50 FullDocument5 pages50 Fullaulia haninNo ratings yet
- AnemiaDocument145 pagesAnemiaTegegne WorkNo ratings yet
- Second Practical - Hematology: 3 YearDocument44 pagesSecond Practical - Hematology: 3 YearAjigotto ubiq.No ratings yet
- Anemia OsamaDocument57 pagesAnemia Osamaosamafoud7710No ratings yet
- Lecture 2 - Clinical Pathology - AnemiaDocument56 pagesLecture 2 - Clinical Pathology - AnemiaSamuel GitongaNo ratings yet
- Anemia OsamaDocument57 pagesAnemia Osamaosamafoud7710No ratings yet
- Williams Manual of Hematology 10Th Edition Marshall A Lichtman Ebook Full ChapterDocument51 pagesWilliams Manual of Hematology 10Th Edition Marshall A Lichtman Ebook Full Chaptersteven.pinera406100% (9)
- MLS 123 MODULE 6 UNIT 4 Venipuncture Procedure Capillary PunctureDocument17 pagesMLS 123 MODULE 6 UNIT 4 Venipuncture Procedure Capillary PunctureVENUS LIRIA PANTINo ratings yet
- Blood Morphometry or Blood Film CommentDocument103 pagesBlood Morphometry or Blood Film CommentYangnuu TitusNo ratings yet
- Caso de EncefalitisDocument4 pagesCaso de EncefalitisJonathan CuevaNo ratings yet
- H560 BrochureDocument8 pagesH560 Brochuremicklemagdy50No ratings yet
- Malaria Parasite Detection Using Deep LearningDocument7 pagesMalaria Parasite Detection Using Deep LearningIJRASETPublicationsNo ratings yet
- Micropara LabDocument8 pagesMicropara LabFatima KateNo ratings yet
- Blood Cells Morphology and Parasites IdentificationDocument8 pagesBlood Cells Morphology and Parasites IdentificationAnge OuedraogoNo ratings yet
- Ajayi Deborah Siwes ReportDocument32 pagesAjayi Deborah Siwes ReportPAUL VICTORNo ratings yet
- Binda BIO3219 Lab5Document12 pagesBinda BIO3219 Lab5Ronaldo BindaNo ratings yet
- PERIPHERAL SMEAR - UG ClassDocument71 pagesPERIPHERAL SMEAR - UG Classaditya.3757No ratings yet
- Specification Accredited A Level Biology B Advancing Biology h422Document96 pagesSpecification Accredited A Level Biology B Advancing Biology h422Natasha PopliNo ratings yet
- Palmones Parasitology Lab TransesDocument19 pagesPalmones Parasitology Lab TransesJISOO KimNo ratings yet
- Lab Activity No. 5 - Slide PresentationDocument24 pagesLab Activity No. 5 - Slide PresentationChelsea Padilla Delos ReyesNo ratings yet
- The Importance of Trephine Biopsy and Aspirate in Bone Marrow AnalysisDocument5 pagesThe Importance of Trephine Biopsy and Aspirate in Bone Marrow Analysisoki harisandiNo ratings yet
- Standard Operating Procedure (Haematology) : R. K. Life Services Private LimitedDocument61 pagesStandard Operating Procedure (Haematology) : R. K. Life Services Private LimitedAniruddha ChatterjeeNo ratings yet
- Lab Manual ZOL536Document58 pagesLab Manual ZOL536RishavNo ratings yet
- C2 HEMA Lab L8 Reticulocyte Count Platelet Count and PBS 2Document6 pagesC2 HEMA Lab L8 Reticulocyte Count Platelet Count and PBS 2SkxNo ratings yet
- Morphology of HematologyDocument11 pagesMorphology of HematologyChabNo ratings yet
- Diagnosis of Parasitic InfectionsDocument22 pagesDiagnosis of Parasitic InfectionsInsatiable CleeNo ratings yet
- Bloodfilm Preparationandreporting 200512223320Document203 pagesBloodfilm Preparationandreporting 200512223320Alex StefanNo ratings yet
- Malaria Detection Using Image Processing and Machine LearningDocument11 pagesMalaria Detection Using Image Processing and Machine LearningdataprodcsNo ratings yet
- Atlas MalariaDocument4 pagesAtlas MalariaLaboratorium Pkm ManjaNo ratings yet
- Solution Manual For Human Anatomy Physiology Main Version 4th Edition Terry Martin Cynthia Prentice CraveDocument24 pagesSolution Manual For Human Anatomy Physiology Main Version 4th Edition Terry Martin Cynthia Prentice CraveNoahMcbridecekjg100% (44)
- MBBS Second Year Pathology Paper-I Important Question BankDocument11 pagesMBBS Second Year Pathology Paper-I Important Question BankAakansh Maheshwari 1No ratings yet
- PLT Interference-Malang-231125Document6 pagesPLT Interference-Malang-231125Beatrix RosellaNo ratings yet
- 3-Automated Screening of Sickle Cells Using A Smartphone-Based Microscope and Deep LearningDocument30 pages3-Automated Screening of Sickle Cells Using A Smartphone-Based Microscope and Deep LearningAli M. RiyathNo ratings yet