Professional Documents
Culture Documents
Step Up 2.1 Acid-Base Theories Problems Worksheet
Step Up 2.1 Acid-Base Theories Problems Worksheet
Uploaded by
HasatakiOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Step Up 2.1 Acid-Base Theories Problems Worksheet
Step Up 2.1 Acid-Base Theories Problems Worksheet
Uploaded by
HasatakiCopyright:
Available Formats
2.
1 Acid−Base Theories
Problems Worksheet
1. What is the difference between ionisation and dissociation?
2. Write an ionic equation for the following reactions:
a. Dilute nitric acid is added to a solution of potassium hydroxide
b. Dilute hydrochloric acid is poured over aluminium metal
c. Lithium carbonate is added to sulfuric acid
d. Gaseous hydrogen chloride is reacted with gaseous ammonia
Find the solutions online at stepupineducation.com © stepupineducation.com
3. What is the major limitation of the Arrhenius theory of acids and bases?
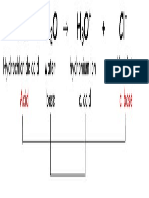
4. Explain how the following reaction could be identified as an acid−base reaction under the
Brønsted−Lowry theory but not under the Arrhenius theory:
HCl(g) + NH3(g) NH4Cl(s)
5. Identify the conjugate acid base pairs in the following reactions:
a. HNO3 + H2O NO3− + H3O+
b. H2S + NH3 HS− + NH4+
c. HSO3− + OH− SO32− + H2O
Find the solutions online at stepupineducation.com © stepupineducation.com
6. Write the conjugate base for each of the following acids:
a. HCl
b. CH3COOH
c. HSO4−
d. HCO3−
e. HF
Write the conjugate acid for each of the following bases:
f. NH3
g. HPO42−
h. ClO−
i. CO32−
j. S2−
Find the solutions online at stepupineducation.com © stepupineducation.com
7. Some species can behave as both acids or bases; they can accept a proton or donate a proton. They are
said to be amphoteric. H2PO4− is an example of an amphoteric ion.
a. Write an equation showing H2PO4− behaving as an acid:
b. Write an equation showing H2PO4− behaving as a base:
c. Give an example of another amphoteric ion.
8. Sulfuric acid is a polyprotic acid with two hydrogens capable of ionising.
a. Write equations showing the first and second reactions of H2SO4 with water.
b. Even though sulfuric acid contains two hydrogen atoms capable of ionisation, a solution does not
contain two times as many hydrogen ions as a solution of HCl with the same concentration.
Explain why not.
Find the solutions online at stepupineducation.com © stepupineducation.com
You might also like
- Chapter Test B: Chapter: Acids and BasesDocument7 pagesChapter Test B: Chapter: Acids and BasesWeng50% (2)
- 4.8 Introduction To Acid-Base Reactions StudentDocument3 pages4.8 Introduction To Acid-Base Reactions StudentSyed RazaNo ratings yet
- Test2 Ch17a Acid-Base Practice Problems PDFDocument12 pagesTest2 Ch17a Acid-Base Practice Problems PDFRaphael CastilloNo ratings yet
- Acid-Base Practice ProblemsDocument12 pagesAcid-Base Practice ProblemsDAKSH CHETAN HATHINo ratings yet
- Grade 10 Chemistry Chapter 14: Acids and Bases: Assignment 3 (Formal Task) 100 MarksDocument4 pagesGrade 10 Chemistry Chapter 14: Acids and Bases: Assignment 3 (Formal Task) 100 MarksPhantom BloodNo ratings yet
- General Organic and Biological Chemistry 6Th Edition Stoker Solutions Manual Full Chapter PDFDocument37 pagesGeneral Organic and Biological Chemistry 6Th Edition Stoker Solutions Manual Full Chapter PDFyhenryhnorc5100% (8)
- 7.1 Acids and Bases 22-23 PDFDocument143 pages7.1 Acids and Bases 22-23 PDFTomatoNo ratings yet
- CH 14 Study GuideDocument8 pagesCH 14 Study Guide4b00d1No ratings yet
- MC ch14 STPDocument28 pagesMC ch14 STPmashary aldawoodNo ratings yet
- Acid-Base Def & StrengthsDocument12 pagesAcid-Base Def & StrengthsCheu Hann JongNo ratings yet
- NS1Lec - Module 5 - NacionalesDocument5 pagesNS1Lec - Module 5 - NacionalesWindere Marie NacionalesNo ratings yet
- Activity 2.1 - Acids and Bases NMNMDocument3 pagesActivity 2.1 - Acids and Bases NMNMClarise CanANo ratings yet
- CH 14 Study GuideDocument4 pagesCH 14 Study Guide4b00d1No ratings yet
- 9.1.conjugate Acid-Base PairsDocument11 pages9.1.conjugate Acid-Base PairsFelicia GunawanNo ratings yet
- CH 10 and 11 Acid-Base QuestionsDocument8 pagesCH 10 and 11 Acid-Base QuestionsNap DoNo ratings yet
- 8 2 Bronsted-Lowry Acids and BasesDocument13 pages8 2 Bronsted-Lowry Acids and Basesribots2002No ratings yet
- 8 2 Bronsted-Lowry Acids and BasesDocument27 pages8 2 Bronsted-Lowry Acids and BasesLyka BugarinNo ratings yet
- Acid Base Part1Document3 pagesAcid Base Part1Alex IoannouNo ratings yet
- Ejercicios PH 2Document4 pagesEjercicios PH 2Mario PeñaNo ratings yet
- Acid and Base WorksheetDocument4 pagesAcid and Base Worksheetapi-270967967No ratings yet
- v2 Physical Science 12 2 A Guide To Acids and BasesDocument10 pagesv2 Physical Science 12 2 A Guide To Acids and BasesOnalenna LegodiNo ratings yet
- Inorganic Cha 4Document21 pagesInorganic Cha 4Adugnaw BiksNo ratings yet
- Chapter 7Document259 pagesChapter 7Hafizszul Feyzul100% (1)
- UNIT 2.docx Grade 12 Chemistry Note and WSDocument27 pagesUNIT 2.docx Grade 12 Chemistry Note and WSmesfin yonasNo ratings yet
- Acid Base Theory WorksheetDocument3 pagesAcid Base Theory WorksheetKepala SMA Kusuma BangsaNo ratings yet
- Conjugate Acid and Base PairsDocument1 pageConjugate Acid and Base Pairsfelisita wisangNo ratings yet
- 4.3 Acid-Base ReactionsDocument15 pages4.3 Acid-Base Reactionshala madridNo ratings yet
- Modern Chemistry Chapter Test :ADocument6 pagesModern Chemistry Chapter Test :ARayan AltamimiNo ratings yet
- WORK SHEET - Ionic & Acid-Base ReactionsDocument5 pagesWORK SHEET - Ionic & Acid-Base ReactionsAndrej ZafirovikjNo ratings yet
- Bronsted Lowry+WorksheetDocument4 pagesBronsted Lowry+WorksheetHo Hsiao JiunNo ratings yet
- Acid and Base Worksheet: © 2004 Cavalcade Publishing, All Rights ReservedDocument4 pagesAcid and Base Worksheet: © 2004 Cavalcade Publishing, All Rights ReservedMiasco Joy AnnNo ratings yet
- Acid Base WorksheetDocument4 pagesAcid Base WorksheetKirsten TroupeNo ratings yet
- Acid Base WorksheetDocument4 pagesAcid Base WorksheetIyanaNo ratings yet
- Acid-Base WorksheetDocument4 pagesAcid-Base WorksheetMay LanieNo ratings yet
- Acid-Base WorksheetDocument4 pagesAcid-Base WorksheetJoseph ZhangNo ratings yet
- Chem Lec. Module 5Document8 pagesChem Lec. Module 5Aivan NovillaNo ratings yet
- Acid Base Review Honors ChemDocument6 pagesAcid Base Review Honors Chemhdlee888No ratings yet
- Skoog Fac 10e Sag Ch07Document20 pagesSkoog Fac 10e Sag Ch07u112021140No ratings yet
- Chapter 4 Reactions in SolutionsDocument29 pagesChapter 4 Reactions in SolutionsMohamed AlQallafNo ratings yet
- Acid-Base Chemistry. Extra Practice Problems General Types/Groups of ProblemsDocument13 pagesAcid-Base Chemistry. Extra Practice Problems General Types/Groups of ProblemsYeabisraNo ratings yet
- Ib Chem Answers 8Document2 pagesIb Chem Answers 8baguette baguettoNo ratings yet
- Study Guide HonorsDocument3 pagesStudy Guide Honorsapi-294237871No ratings yet
- Ionic EquilibriumDocument23 pagesIonic EquilibriumjamzeethsNo ratings yet
- 7.0 Ionic EquilibriaDocument124 pages7.0 Ionic EquilibriaTasya KassimNo ratings yet
- H PO (Aq) + CN (Aq) HCN (Aq) + HPO (Aq) CN HPO H PO HPO H PODocument10 pagesH PO (Aq) + CN (Aq) HCN (Aq) + HPO (Aq) CN HPO H PO HPO H POPisosNo ratings yet
- WS4. Lewis Bronsted-Lowry Acids Worksheet (HL)Document4 pagesWS4. Lewis Bronsted-Lowry Acids Worksheet (HL)Yuvraj GuptaNo ratings yet
- Ionic Equilibrium (4 Marks)Document7 pagesIonic Equilibrium (4 Marks)Nagesh NangiNo ratings yet
- 09 Acids and BasesDocument87 pages09 Acids and BasesvincentNo ratings yet
- New QB Acid and Base 1Document24 pagesNew QB Acid and Base 1Irmak CoşkunNo ratings yet
- Acids Bases Practice Theories EquationsDocument5 pagesAcids Bases Practice Theories EquationsJhiean IruguinNo ratings yet
- Acids and Bases Review Hon-18Document2 pagesAcids and Bases Review Hon-18api-368121935No ratings yet
- Acids and Bases-QuestionsDocument4 pagesAcids and Bases-Questionsreem ahmedNo ratings yet
- 13 Ionic Equilibria Notes PDFDocument37 pages13 Ionic Equilibria Notes PDFUchiha YogesNo ratings yet
- Acids and BasesDocument7 pagesAcids and Basessmdali14No ratings yet
- Ch14 Study QuestionsDocument3 pagesCh14 Study QuestionsКанат ТютеновNo ratings yet
- 5031 Acid Base WorksheetDocument5 pages5031 Acid Base WorksheetSaima Usman/TCHR/MGBNo ratings yet
- Testbank3 Syror Baser+svar PDFDocument10 pagesTestbank3 Syror Baser+svar PDFSandileVilaneNo ratings yet
- Practice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersFrom EverandPractice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersNo ratings yet
- Inorganic Cha 4Document21 pagesInorganic Cha 4Adugnaw BiksNo ratings yet
- Kromatogram Mimyak JintanDocument2 pagesKromatogram Mimyak JintanPutri ElafifahNo ratings yet
- MCAT Mnemonics Juan CarlosDocument3 pagesMCAT Mnemonics Juan Carlosjuan carlosNo ratings yet
- Quant Chem AnalDocument4 pagesQuant Chem AnalMisheelt TsolmonbaatarNo ratings yet
- Certificate of Analysis of Black Cumin Seed OilDocument1 pageCertificate of Analysis of Black Cumin Seed OilAbhishek SoniNo ratings yet
- Neptune Fish Concentrate Technical Data SheetDocument1 pageNeptune Fish Concentrate Technical Data SheetKatherine Leyla Anchayhua TorresNo ratings yet
- Test Bank For Organic Chemistry 4 Edition Janice SmithDocument24 pagesTest Bank For Organic Chemistry 4 Edition Janice SmithStevenMcclainpwdt100% (55)
- Chapter 16auDocument97 pagesChapter 16auShekaina Faith Cuizon LozadaNo ratings yet
- ACID Base Equil P Test MCDocument5 pagesACID Base Equil P Test MCctyre34No ratings yet
- Denumire Substanta Formula Chimica Clorura de Vinil CH2 CH-CLDocument21 pagesDenumire Substanta Formula Chimica Clorura de Vinil CH2 CH-CLLayla NicoNo ratings yet
- Acid Base Chemistry and Buffers Concept Test: Problem 1Document4 pagesAcid Base Chemistry and Buffers Concept Test: Problem 1Carlos A. Villanueva HilaroNo ratings yet
- Acid-Base RXNS PDFDocument4 pagesAcid-Base RXNS PDFJenny SabinoNo ratings yet
- Total Amino Acids AOAC 2018.06 Pre PrintDocument17 pagesTotal Amino Acids AOAC 2018.06 Pre PrintDuy Tien NguyenNo ratings yet
- Biosynthesis of Nonessential Amino AcidsDocument18 pagesBiosynthesis of Nonessential Amino Acidsاسيل الحسينNo ratings yet
- Week 2Document15 pagesWeek 2ALPHEAUS-MBA AwajibenejiNo ratings yet
- Answers of Organic Chemistry DPP For GOC (Conceptual Improvement of GOC)Document5 pagesAnswers of Organic Chemistry DPP For GOC (Conceptual Improvement of GOC)Krishna SinglaNo ratings yet
- Asam Amino Kuda LautDocument14 pagesAsam Amino Kuda LautIvan HeriansyahNo ratings yet
- Hydrochloric Acid Corrosion Resistant AlloysDocument2 pagesHydrochloric Acid Corrosion Resistant AlloysZlatko PartličNo ratings yet
- 2021 SEM 4 CC 9 OrganicDocument3 pages2021 SEM 4 CC 9 OrganicGaurav KumarNo ratings yet
- Class XII Chemistry Practical Record Vol2Document22 pagesClass XII Chemistry Practical Record Vol2yashrevasya2006No ratings yet
- 4 - Amino Acids and PeptidesDocument27 pages4 - Amino Acids and PeptidesJehssa Jee SarmientoNo ratings yet
- Lab Relative WK Acid Strengths Inq Student HandoutDocument3 pagesLab Relative WK Acid Strengths Inq Student HandoutMuhammad HamidNo ratings yet
- Biochem MidtermsDocument3 pagesBiochem MidtermsJhayneNo ratings yet
- Buffers Complete Handout 2020 With Answer KeyDocument14 pagesBuffers Complete Handout 2020 With Answer KeyRadhika RaniNo ratings yet
- EZ Nin Reagent DatasheetDocument2 pagesEZ Nin Reagent DatasheetJoshadow59No ratings yet
- 18 AbequilDocument29 pages18 AbequilSam H. SalehNo ratings yet
- Chapter Three Amino Acids and Peptides: Mary K. Campbell Shawn O. FarrellDocument29 pagesChapter Three Amino Acids and Peptides: Mary K. Campbell Shawn O. Farrellnora buanNo ratings yet
- Sulfuric Acid - AbsorberDocument18 pagesSulfuric Acid - AbsorberskskskskNo ratings yet
- Acid Base Equilibria Tutorial - With AnswersDocument10 pagesAcid Base Equilibria Tutorial - With AnswersNguYen QuE AnhNo ratings yet