Professional Documents
Culture Documents
178
178
Uploaded by
Joya BhatiagharuCopyright:
Available Formats
You might also like
- Reaction IntermediatesDocument12 pagesReaction IntermediatesJoya Bhatiagharu100% (3)
- P-S Cage and P-O Cage CompoundsDocument9 pagesP-S Cage and P-O Cage Compoundssatish100% (1)
- Unit 5 Answer KeyDocument2 pagesUnit 5 Answer Keyapi-273525891No ratings yet
- 2ca (Po) +6sio +10C 6casio +10Co+P 3nah Po +PH +3Naoh+3H O 4H Po +10O+6H O Red P White PDocument1 page2ca (Po) +6sio +10C 6casio +10Co+P 3nah Po +PH +3Naoh+3H O 4H Po +10O+6H O Red P White PJoya BhatiagharuNo ratings yet
- 10eng PDFDocument18 pages10eng PDFАхмед АбдуллаNo ratings yet
- Oxides of Phosphorus (P-O Cage Compounds) : Physical PropertiesDocument4 pagesOxides of Phosphorus (P-O Cage Compounds) : Physical Propertiessatish100% (1)
- Level 0 PhosphorousDocument10 pagesLevel 0 PhosphorousTimothy SaxenaNo ratings yet
- CBSE Class 12 Chemistry - The P Block Elements AssignmentDocument7 pagesCBSE Class 12 Chemistry - The P Block Elements AssignmentManickam Gnanashekaran0% (1)
- CHE 212 - Lecture 2Document20 pagesCHE 212 - Lecture 2Ifiok UsoroNo ratings yet
- Nitrogen Family QuesDocument2 pagesNitrogen Family QuesKamal KishoreNo ratings yet
- PhosphorousDocument8 pagesPhosphorousUJJWAL JHANo ratings yet
- EZ Series FSC-II Chemistry CH 4Document19 pagesEZ Series FSC-II Chemistry CH 4Furqan Zahid100% (1)
- 7 The P-Block Elements: SolutionsDocument24 pages7 The P-Block Elements: SolutionsMriganko Roy100% (1)
- P Block Elements Notes For Entrance ExaminationDocument13 pagesP Block Elements Notes For Entrance ExaminationSrijan ChakrabortyNo ratings yet
- Group 15 ElementsDocument1 pageGroup 15 ElementsVaibhavMittalNo ratings yet
- The P-Block Elements: White P: White Waxy/poisonous/ Soluble in CSDocument2 pagesThe P-Block Elements: White P: White Waxy/poisonous/ Soluble in CSSsNo ratings yet
- Chemistry of PhosphorusDocument7 pagesChemistry of PhosphorusChandra ReddyNo ratings yet
- Chemistry Himanshu Raj 1118163 PDFDocument12 pagesChemistry Himanshu Raj 1118163 PDFHimanshu rajNo ratings yet
- Part 2 Inorg. Struct.Document6 pagesPart 2 Inorg. Struct.Sahil RaundalNo ratings yet
- 15th Group Short NotesDocument3 pages15th Group Short NotessanjaysaikumarkNo ratings yet
- P Block Give Reasons (Without Answers)Document6 pagesP Block Give Reasons (Without Answers)zxcvb100% (1)
- Phosphorous: Dr. Rajeev SinghDocument12 pagesPhosphorous: Dr. Rajeev SinghVigyan PravahaNo ratings yet
- Lecture 14-Gr 15Document25 pagesLecture 14-Gr 15averagestudent838No ratings yet
- 12 - TPP PhenolsDocument7 pages12 - TPP PhenolsSaadia AsgharNo ratings yet
- CAREER PREP HANDOUTs CHAPTER # 4 & 5Document8 pagesCAREER PREP HANDOUTs CHAPTER # 4 & 5Muhammad Talha AsifNo ratings yet
- 6.P Block ElementsDocument24 pages6.P Block ElementsSSSSSSSSSSSSNo ratings yet
- Chapter 5 - PhenolsDocument29 pagesChapter 5 - Phenolsdr.3nonaNo ratings yet
- N P As SB Bi Small Size High I.E, High E.N Absence of D-Orbital NCL Does Not Exist PCL Does Exist (Empty D-Orbital) N (N N)Document7 pagesN P As SB Bi Small Size High I.E, High E.N Absence of D-Orbital NCL Does Not Exist PCL Does Exist (Empty D-Orbital) N (N N)Eswara ReddyNo ratings yet
- Worksheet Chapter 4.I ANSWERSDocument2 pagesWorksheet Chapter 4.I ANSWERSkhalid badranNo ratings yet
- Answers F3 ChemsitryDocument19 pagesAnswers F3 Chemsitryabdifatahomarali.soNo ratings yet
- Sintesis Asam SalisilatDocument5 pagesSintesis Asam SalisilatRohaniNo ratings yet
- Previous Year Board Exam QuestionsDocument19 pagesPrevious Year Board Exam QuestionsRishabh AgarwalNo ratings yet
- Null 12Document10 pagesNull 12Swastik DasNo ratings yet
- Phenols: Bio-Organic Chemistry-I BBT-202 Lecture-13Document37 pagesPhenols: Bio-Organic Chemistry-I BBT-202 Lecture-13Sate AhmadNo ratings yet
- Praktikum Anorganik Nitrogen Dan AmmoniaDocument30 pagesPraktikum Anorganik Nitrogen Dan Ammoniaqurrota ainynNo ratings yet
- Phenol 203Document47 pagesPhenol 203ajibolaakorede20No ratings yet
- GRP 15 - PBlock CHEMHACKDocument10 pagesGRP 15 - PBlock CHEMHACKSATYA PRAKASH SINGHNo ratings yet
- Unit - 3 P-Block Elements-Ii: WWW - Nammakalvi.inDocument5 pagesUnit - 3 P-Block Elements-Ii: WWW - Nammakalvi.inAakaash C.K.100% (1)
- Namma Kalvi 12th Chemistry Unit 3 Study Material em 215020Document5 pagesNamma Kalvi 12th Chemistry Unit 3 Study Material em 215020Aakaash C.K.No ratings yet
- Phosphorus - Allotropic Forms: Presentation By: Aakriti Roy (XII Science)Document7 pagesPhosphorus - Allotropic Forms: Presentation By: Aakriti Roy (XII Science)Aakriti RoyNo ratings yet
- Halides of PhosphorusDocument9 pagesHalides of PhosphorusSavita ChemistryNo ratings yet
- CHAPTER 4 Lecture (Compatibility Mode)Document26 pagesCHAPTER 4 Lecture (Compatibility Mode)Shakeel AhmedNo ratings yet
- FenolDocument43 pagesFenolRedyNo ratings yet
- Qualitative Testfor Elementsin Organic CompoundDocument8 pagesQualitative Testfor Elementsin Organic Compoundidon'tgiveachogiwaNo ratings yet
- 15th GroupDocument37 pages15th GroupSai Sasivardhan GampaNo ratings yet
- Unit 11. Alcohols, Phenols and Ethers One Mark Questions: Ans: EthanolDocument10 pagesUnit 11. Alcohols, Phenols and Ethers One Mark Questions: Ans: EthanolDeva RajNo ratings yet
- Reasoning Questions in P Block ElementsDocument15 pagesReasoning Questions in P Block ElementsAbhi WaliaNo ratings yet
- Inorganic ChemistryDocument13 pagesInorganic Chemistrybhargavthegreat123No ratings yet
- Praktikum Anorganik Nitrogen Dan AmmoniaDocument24 pagesPraktikum Anorganik Nitrogen Dan Ammoniaqurrota ainynNo ratings yet
- 07 P Block ElementsDocument1 page07 P Block Elementsshamsul aminNo ratings yet
- Leep507 PDFDocument15 pagesLeep507 PDFUdit ChaudharyNo ratings yet
- Chemlab - PM2 AssignmentDocument2 pagesChemlab - PM2 AssignmentJei y’allNo ratings yet
- Answer P BlockDocument26 pagesAnswer P BlockvikiasNo ratings yet
- I Am Sharing PHOSPHORUS EDITED With YouDocument3 pagesI Am Sharing PHOSPHORUS EDITED With YouJjjj HsjsjsNo ratings yet
- Synthesis of Furan and ThiopheneDocument10 pagesSynthesis of Furan and ThiopheneNorman FerdinalNo ratings yet
- Tugas Anor - Kelompok5 - PKUDocument20 pagesTugas Anor - Kelompok5 - PKUAndriati RahayuNo ratings yet
- Conjugate Acid Base Pairs: Name - Chem Worksheet 19-2Document1 pageConjugate Acid Base Pairs: Name - Chem Worksheet 19-2Bindu Mohanan PillaiNo ratings yet
- Inorganic Chemistry 2 Semester: Nitrogen Group Oxygen Group Halogens Noble Gases Acids and Bases SolventsDocument200 pagesInorganic Chemistry 2 Semester: Nitrogen Group Oxygen Group Halogens Noble Gases Acids and Bases Solventshbhv240No ratings yet
- ذرةDocument15 pagesذرةzahrakerim21No ratings yet
- 175Document2 pages175Joya BhatiagharuNo ratings yet
- 418Document2 pages418Joya BhatiagharuNo ratings yet
- Atomic StructureDocument23 pagesAtomic StructureJoya BhatiagharuNo ratings yet
178
178
Uploaded by
Joya BhatiagharuOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
178
178
Uploaded by
Joya BhatiagharuCopyright:
Available Formats
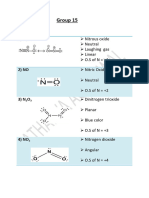
̥̊Topic
̥̊̊ Chemistry of Phosphorus and Its Compounds
Question Serial No 178
Question Type Single correct answer
Subtopic Allotropic forms of Phosphorus- Preparation, Properties, Uses
Instructions Choose the correct option.
Question Text A tetratomic molecule, which is Y, reacts with X to form P 4O10 along with a colourless
inert gas, nitrogen. It is also known that X is a sweet-scented gas, which is commonly
called laughing gas. The product of the reaction of Y with concentrated nitric acid is:
Options A Phosphoric acid
B Phosphonic acid
C Phosphorus acid
D Metaphosphoric acid
Answer A
Solution Laughing gas is the common name for dinitrogen oxide. This indicates that the
sweet-smelling gas (X) is N2O. The reaction of N2O with Y produces an oxide of
phosphorus P4O10 along with nitrogen gas. This infers that the tetratomic molecule is
P4.
The reaction can be written as follows:
P 4 +N2 O→ P4 O10+ N2
Consequently, X is N2O and Y is P4.
Concentrated nitric acid is a strong oxidising agent that oxidises non–metals and
their compounds. I is oxidised to iodic acid, C to carbon dioxide, S to sulphuric acid
and P4 to phosphoric acid. The reaction of concentrated nitric acid with phosphorus
(P4) is given below:
[ O]
P 4 +20HNO3 → 4 H3 PO 4 +20 NO 2+ 4 H 2 O
Thus, the final product is phosphoric acid, that is H 3PO4.
Hence, the correct answer is option A.
Exams CBSE , CUET
Taxonomy Applying
Difficulty Level Difficult
You might also like
- Reaction IntermediatesDocument12 pagesReaction IntermediatesJoya Bhatiagharu100% (3)
- P-S Cage and P-O Cage CompoundsDocument9 pagesP-S Cage and P-O Cage Compoundssatish100% (1)
- Unit 5 Answer KeyDocument2 pagesUnit 5 Answer Keyapi-273525891No ratings yet
- 2ca (Po) +6sio +10C 6casio +10Co+P 3nah Po +PH +3Naoh+3H O 4H Po +10O+6H O Red P White PDocument1 page2ca (Po) +6sio +10C 6casio +10Co+P 3nah Po +PH +3Naoh+3H O 4H Po +10O+6H O Red P White PJoya BhatiagharuNo ratings yet
- 10eng PDFDocument18 pages10eng PDFАхмед АбдуллаNo ratings yet
- Oxides of Phosphorus (P-O Cage Compounds) : Physical PropertiesDocument4 pagesOxides of Phosphorus (P-O Cage Compounds) : Physical Propertiessatish100% (1)
- Level 0 PhosphorousDocument10 pagesLevel 0 PhosphorousTimothy SaxenaNo ratings yet
- CBSE Class 12 Chemistry - The P Block Elements AssignmentDocument7 pagesCBSE Class 12 Chemistry - The P Block Elements AssignmentManickam Gnanashekaran0% (1)
- CHE 212 - Lecture 2Document20 pagesCHE 212 - Lecture 2Ifiok UsoroNo ratings yet
- Nitrogen Family QuesDocument2 pagesNitrogen Family QuesKamal KishoreNo ratings yet
- PhosphorousDocument8 pagesPhosphorousUJJWAL JHANo ratings yet
- EZ Series FSC-II Chemistry CH 4Document19 pagesEZ Series FSC-II Chemistry CH 4Furqan Zahid100% (1)
- 7 The P-Block Elements: SolutionsDocument24 pages7 The P-Block Elements: SolutionsMriganko Roy100% (1)
- P Block Elements Notes For Entrance ExaminationDocument13 pagesP Block Elements Notes For Entrance ExaminationSrijan ChakrabortyNo ratings yet
- Group 15 ElementsDocument1 pageGroup 15 ElementsVaibhavMittalNo ratings yet
- The P-Block Elements: White P: White Waxy/poisonous/ Soluble in CSDocument2 pagesThe P-Block Elements: White P: White Waxy/poisonous/ Soluble in CSSsNo ratings yet
- Chemistry of PhosphorusDocument7 pagesChemistry of PhosphorusChandra ReddyNo ratings yet
- Chemistry Himanshu Raj 1118163 PDFDocument12 pagesChemistry Himanshu Raj 1118163 PDFHimanshu rajNo ratings yet
- Part 2 Inorg. Struct.Document6 pagesPart 2 Inorg. Struct.Sahil RaundalNo ratings yet
- 15th Group Short NotesDocument3 pages15th Group Short NotessanjaysaikumarkNo ratings yet
- P Block Give Reasons (Without Answers)Document6 pagesP Block Give Reasons (Without Answers)zxcvb100% (1)
- Phosphorous: Dr. Rajeev SinghDocument12 pagesPhosphorous: Dr. Rajeev SinghVigyan PravahaNo ratings yet
- Lecture 14-Gr 15Document25 pagesLecture 14-Gr 15averagestudent838No ratings yet
- 12 - TPP PhenolsDocument7 pages12 - TPP PhenolsSaadia AsgharNo ratings yet
- CAREER PREP HANDOUTs CHAPTER # 4 & 5Document8 pagesCAREER PREP HANDOUTs CHAPTER # 4 & 5Muhammad Talha AsifNo ratings yet
- 6.P Block ElementsDocument24 pages6.P Block ElementsSSSSSSSSSSSSNo ratings yet
- Chapter 5 - PhenolsDocument29 pagesChapter 5 - Phenolsdr.3nonaNo ratings yet
- N P As SB Bi Small Size High I.E, High E.N Absence of D-Orbital NCL Does Not Exist PCL Does Exist (Empty D-Orbital) N (N N)Document7 pagesN P As SB Bi Small Size High I.E, High E.N Absence of D-Orbital NCL Does Not Exist PCL Does Exist (Empty D-Orbital) N (N N)Eswara ReddyNo ratings yet
- Worksheet Chapter 4.I ANSWERSDocument2 pagesWorksheet Chapter 4.I ANSWERSkhalid badranNo ratings yet
- Answers F3 ChemsitryDocument19 pagesAnswers F3 Chemsitryabdifatahomarali.soNo ratings yet
- Sintesis Asam SalisilatDocument5 pagesSintesis Asam SalisilatRohaniNo ratings yet
- Previous Year Board Exam QuestionsDocument19 pagesPrevious Year Board Exam QuestionsRishabh AgarwalNo ratings yet
- Null 12Document10 pagesNull 12Swastik DasNo ratings yet
- Phenols: Bio-Organic Chemistry-I BBT-202 Lecture-13Document37 pagesPhenols: Bio-Organic Chemistry-I BBT-202 Lecture-13Sate AhmadNo ratings yet
- Praktikum Anorganik Nitrogen Dan AmmoniaDocument30 pagesPraktikum Anorganik Nitrogen Dan Ammoniaqurrota ainynNo ratings yet
- Phenol 203Document47 pagesPhenol 203ajibolaakorede20No ratings yet
- GRP 15 - PBlock CHEMHACKDocument10 pagesGRP 15 - PBlock CHEMHACKSATYA PRAKASH SINGHNo ratings yet
- Unit - 3 P-Block Elements-Ii: WWW - Nammakalvi.inDocument5 pagesUnit - 3 P-Block Elements-Ii: WWW - Nammakalvi.inAakaash C.K.100% (1)
- Namma Kalvi 12th Chemistry Unit 3 Study Material em 215020Document5 pagesNamma Kalvi 12th Chemistry Unit 3 Study Material em 215020Aakaash C.K.No ratings yet
- Phosphorus - Allotropic Forms: Presentation By: Aakriti Roy (XII Science)Document7 pagesPhosphorus - Allotropic Forms: Presentation By: Aakriti Roy (XII Science)Aakriti RoyNo ratings yet
- Halides of PhosphorusDocument9 pagesHalides of PhosphorusSavita ChemistryNo ratings yet
- CHAPTER 4 Lecture (Compatibility Mode)Document26 pagesCHAPTER 4 Lecture (Compatibility Mode)Shakeel AhmedNo ratings yet
- FenolDocument43 pagesFenolRedyNo ratings yet
- Qualitative Testfor Elementsin Organic CompoundDocument8 pagesQualitative Testfor Elementsin Organic Compoundidon'tgiveachogiwaNo ratings yet
- 15th GroupDocument37 pages15th GroupSai Sasivardhan GampaNo ratings yet
- Unit 11. Alcohols, Phenols and Ethers One Mark Questions: Ans: EthanolDocument10 pagesUnit 11. Alcohols, Phenols and Ethers One Mark Questions: Ans: EthanolDeva RajNo ratings yet
- Reasoning Questions in P Block ElementsDocument15 pagesReasoning Questions in P Block ElementsAbhi WaliaNo ratings yet
- Inorganic ChemistryDocument13 pagesInorganic Chemistrybhargavthegreat123No ratings yet
- Praktikum Anorganik Nitrogen Dan AmmoniaDocument24 pagesPraktikum Anorganik Nitrogen Dan Ammoniaqurrota ainynNo ratings yet
- 07 P Block ElementsDocument1 page07 P Block Elementsshamsul aminNo ratings yet
- Leep507 PDFDocument15 pagesLeep507 PDFUdit ChaudharyNo ratings yet
- Chemlab - PM2 AssignmentDocument2 pagesChemlab - PM2 AssignmentJei y’allNo ratings yet
- Answer P BlockDocument26 pagesAnswer P BlockvikiasNo ratings yet
- I Am Sharing PHOSPHORUS EDITED With YouDocument3 pagesI Am Sharing PHOSPHORUS EDITED With YouJjjj HsjsjsNo ratings yet
- Synthesis of Furan and ThiopheneDocument10 pagesSynthesis of Furan and ThiopheneNorman FerdinalNo ratings yet
- Tugas Anor - Kelompok5 - PKUDocument20 pagesTugas Anor - Kelompok5 - PKUAndriati RahayuNo ratings yet
- Conjugate Acid Base Pairs: Name - Chem Worksheet 19-2Document1 pageConjugate Acid Base Pairs: Name - Chem Worksheet 19-2Bindu Mohanan PillaiNo ratings yet
- Inorganic Chemistry 2 Semester: Nitrogen Group Oxygen Group Halogens Noble Gases Acids and Bases SolventsDocument200 pagesInorganic Chemistry 2 Semester: Nitrogen Group Oxygen Group Halogens Noble Gases Acids and Bases Solventshbhv240No ratings yet
- ذرةDocument15 pagesذرةzahrakerim21No ratings yet
- 175Document2 pages175Joya BhatiagharuNo ratings yet
- 418Document2 pages418Joya BhatiagharuNo ratings yet
- Atomic StructureDocument23 pagesAtomic StructureJoya BhatiagharuNo ratings yet