Professional Documents
Culture Documents
CHEM Midterm Notes
CHEM Midterm Notes
Uploaded by
Kharylle Ann Iglesias0 ratings0% found this document useful (0 votes)
26 views7 pagesThe document describes various pieces of laboratory equipment and their functions, including:
1) Acid buret, distilling flask, dropper, erlenmeyer flask, evaporating dish, and florence flask which are used to store, measure, mix, heat, or separate chemicals.
2) Clamps, stands, rings and shields which are used to support or insulate heated equipment.
3) Condensers, funnels, pipettes and aspirators which transport or dispense liquids.
4) Test tubes, racks, thermometers and watch glasses which hold samples or measure properties.
5) Bunsen burners, alcohol lamps and wire gauzes which provide controlled heat sources.
Original Description:
Notes
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document describes various pieces of laboratory equipment and their functions, including:
1) Acid buret, distilling flask, dropper, erlenmeyer flask, evaporating dish, and florence flask which are used to store, measure, mix, heat, or separate chemicals.
2) Clamps, stands, rings and shields which are used to support or insulate heated equipment.
3) Condensers, funnels, pipettes and aspirators which transport or dispense liquids.
4) Test tubes, racks, thermometers and watch glasses which hold samples or measure properties.
5) Bunsen burners, alcohol lamps and wire gauzes which provide controlled heat sources.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
0 ratings0% found this document useful (0 votes)
26 views7 pagesCHEM Midterm Notes
CHEM Midterm Notes
Uploaded by
Kharylle Ann IglesiasThe document describes various pieces of laboratory equipment and their functions, including:
1) Acid buret, distilling flask, dropper, erlenmeyer flask, evaporating dish, and florence flask which are used to store, measure, mix, heat, or separate chemicals.
2) Clamps, stands, rings and shields which are used to support or insulate heated equipment.
3) Condensers, funnels, pipettes and aspirators which transport or dispense liquids.
4) Test tubes, racks, thermometers and watch glasses which hold samples or measure properties.
5) Bunsen burners, alcohol lamps and wire gauzes which provide controlled heat sources.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
You are on page 1of 7
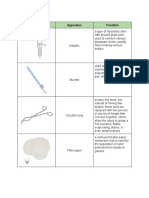
Acid buret – is a glass vial with graduation Distilling Flask – used to separate
marks. It is used in the laboratory for accurately mixtures of two liquids with
measuring and dispensing liquids. different boiling points.
Dropper – used to transfer
small quantities of liquids.
Adapter – used to connect pieces of ground
glass joint glassware to each other, to a vacuum
source, or to a water aspirator. It is also used to Erlenmeyer Flask – used for storing
lead liquids from a condenser to a receiving flask and mixing of chemicals in a
during distillation. laboratory setting.
Evaporating Dish – used to
evaporate excess solvent to
produce a concentrated solution
Alkali/Base Buret – are used for base titrants. or a solid precipitate of the
Basically, this is used in titrations where the dissolved substance.
analyte is an acid.
Florence Flask – is designed for
uniform heating, boiling, distillation
and ease of swirling.
Beaker – A cylindrical borosilicate Funnel – used to channel liquid or
glass container used as a receptable fine-grained substances into
for solid and liquid substances. containers with a small opening.
Glass rod/Stirring rod – used to mix chemicals
Buret clamp – a clamp and liquids for laboratory purposes.
which is used to secure
a buret on a stand.
Graduated Cylinder – used for measuring
Volumes (amounts) of liquids accurately.
Clay shield – used to insulate Iron clamp/Test tube clamp
the Bunsen burner flame from – used to hold test tubes in
the surroundings. It is also used place during heating
to support materials when operations and/or reactions.
heating.
Iron Ring – used to hold or
Clay triangle – used in conjunction Support beakers during
with the clay shield to create a stable experiments while connected
framework in which to place a to the iron stand.
substance while it is heated to
a high temperature. Iron stand – supports the iron ring
when heating substances or mixtures
Condenser – used in the laboratory to condense in a flask or beakers.
hot vapors into liquids during distillation.
Pipette – used in chemistry to
transport a measured volume of liquid,
often as a media dispenser.
Crucible Tong – It is used to safely handle hot
materials at a very high temperature.
Pipettor/ Aspirator- used to
draw liquids in pipettes.
Test tube – are containers for heating Alcohol lamp –
small amounts of liquids or solids with
a Bunsen burner or alcohol burner.
Test tube brush – used for cleaning test tubes
and narrow-mouthed laboratory glassware, such
as beakers and flask.
Test tube holder - used for holding a test tube in
place when the tube is hot or should not be
touched.
Test tube rack – used to hold upright multiple
test tubes at the same time.
Commonly used when
various different solutions
are needed to work
simultaneously.
Thermometer – used to measure temperatures
or temperature changes with a high degree of
precision.
Water bath – used to incubate samples in water
at a constant temperature over a long period of
time. Also used to enable certain chemical
reactions to occur.
Watch glass – is used to evaporate a liquid, to
hold solids while being weighed, for heating a
small amount of substance and as a cover for a One of the common operations in the laboratory
beaker. is the use of a Bunsen burner. It produces a
single open flame which is used for heating
and combustion. Combustion is commonly
called burning. The substance that burns is
usually referred to as fuel. Sufficient air or
Wire gauze – is placed on the support ring that is oxygen is needed for the complete combustion of
attached to the retort stand between the Bunsen a fuel.
burner and the beakers or other glassware or
flasks during heating.
1. Bunsen Burner
Note the gas inlet and the nozzle or gas spud
Complete combustion of a fuel yields at the base of the burner.
carbon dioxide and water vapor. This
reaction typically gives off heat and a
non-luminous flame. The general Before lighting the Bunsen burner, make
equation for a complete combustion sure that the gas regulator is closed and
reaction is the airholes are open.
Open the gas regulator slightly.
fuel + O2(g) → CO2(g) + H2O(g) + heat Light a matchstick and hold it just slightly
above the mouth of the burner.
Incomplete combustion occurs when Slowly open the gas cock until you have a
the supply of air or oxygen is poor. It flame of about ten cm high.
yields carbon monoxide and pure Open and close the air holes and note the
carbon aside from carbon dioxide and difference in the color of the flame.
water vapor. This pure carbon is called
soot. The flame produced is a luminous Put out the flame by turning off the gas
one. cock and close the gas regulator. NEVER
BLOW OUT THE GAS FLAME.
fuel + O2(g) → CO2(g) + H2O(g)
+ CO(g) + C(s) + heat
2. Measurement of volume of liquids.
Parts of a bunsen burner:
In reading the volume, place the graduated
cylinder on a flat surface and keep the eye at
level with the lower meniscus of the liquid
Record the volumes of liquids in approximately
two decimal places.
NOTE: For reading the volume of colored liquids,
keep the eye level with the upper
meniscus.
3. Transferring Liquids
- Hold the beaker with water with one hand
and a glass rod with the other.
- Hold the glass rod against the lip of the
beaker containing the water and put the
free end of the rod into an empty 250 mL
beaker.
- Carefully pour the water and let it glide
down the glass rod into the empty
Parts of a Bunsen Burner and Their Functions:
beaker
Barrel - where the fuel and air mixes
Air hole – entrance of the air 4. Heating liquids in test tubes
Mouth – place of ignition
Gas inlet – entrance of the fuel gas - Hold the test tube with a test tube holder
Gas regulator – valve that controls the and position it at a 45˚ angle.
fuel gas coming into the gas spud - Heat the liquid by moving the test tube
Gas spud – regulates/controls the amount slowly back and forth through the flame
of fuel gas to be combusted in such a manner that the top of the flame
Collar – movable part that is near the top of the liquid but does
regulates/controls the amount of air not touch the empty part of the test
coming in and to close and open the air tube.
holes
Base – supports the Bunsen burner 5. Investigating odors
Rubber tubing – attached to the gas inlet
and connected to the gas source - Many substances have characteristic
odors. Some have agreeable odors
while others have disagreeable or Heat energy is transferred from a hot metal to
irritating odors. water until the metal and the water have
- Be cautious in investigating odors. Some reached the same temperature. This transfer is
vapors, when inhaled, can be lethal. done in an insulated container to minimize heat
- Never take a direct sniff of the vapor at losses to the surroundings. It is safe to assume
the mouth of the container where the that all the heat lost by the metal (Qx) is absorbed
concentration of the vapor is high. by the water and is equal to the heat gained by
- When it is necessary to smell chemicals in the water, (Qw).
the laboratory, cup your hand above the
container and WAFT OR FAN THE This relationship can be used to calculate specific
VAPOR towards your face. heat of a metal because both the mass of the
- Try not to breathe in through your nose metal and its temperature change can be
but bring in just enough to detect the measured.
smell.
The bottom of the test tube should be at least one
CALORIMETRY – half inch above the
bottom of the beaker.
Calorimetry is the science of measuring a
quantity of heat. Fill the beaker with tap water so that the height of
Heat is a form of energy associated with the water in the beaker is about two
the motion of atoms or molecules of a inches higher than the top of the metal sample.
substance. There should be no water inside the test
Heat, Q, is measured in energy units such tube.
as joules (J) or calories (cal).
Temperature, T, is measured in degrees The nested cups with the cardboard cover and
Celsius,°C. the thermometer are referred to as a calorimeter.
Temperature and heat are related to
each other by the specific heat, cp, of a It is important that the transfer takes place
substance, defined as the quantity of heat quickly and carefully to minimize heat loss to
needed to raise the temperature one gram the surroundings and to avoid splashing.
of a substance by one degree Celsius
(J/g-°C). the specific heat of the metal versus the atomic
The relationship between quantity of heat mass of the scorresponding metal. THE
(Q), specific heat (cp), mass (m) and SPECIFIC HEAT IS INVERSELY
temperature change (∆T) is PROPORTIONAL TO THE ATOMIC MASS OF
mathematically expressed by the THE METAL.
equation:
Q = mcp∆ T or Joules = (g)(J/g -°C)(°C)
The amount of heat needed to raise the
temperature of 1 g of water by 1 degree Celsius is
the basis of the calorie.
Thus, the specific heat of water is exactly 1.00
cal/g∙0C. The SI unit of energy is the joule and it
is related to the calorie by: 1 calorie = 4.184 J.
Thus, the specific heat of water is also 4.184
J/g∙0C. The specific heat of a substance
relates to its capacity to absorb heat energy.
The higher the specific heat of a substance
the more energy required to change its
temperature.
In this experiment, calorimetry is used to
determine the specific heat of a metal.
HEAT OF COMBUSTION
The combustion of organic compounds
like alcohols produces large quantities
of energy.
Ethanol is a commonly used fuel in motor
cars and its usage is increasing because it
is a form of renewable energy.
A good fuel is any substance which gives
out large amounts of energy when it is
burnt. In most cases, fuels are burnt in
oxygen (air), i.e., they are oxidized.
This experiment aims to investigate the
relationship between the number of carbon atoms
in an alcohol chain and its standard enthalpy
change of combustion. what relationship can be drawn between the
number of carbon atoms and their standard
The heat of combustion (standard enthalpy enthalpies of combustion? – They are directly
change of combustion) is the enthalpy change proportional to each other.
when one mole of the compound undergoes
complete combustion in excess oxygen under 5 types of combustion and define each type.
standard conditions. 1. Complete - ccurs in an unlimited supply of
It is given the symbol ΔH˚comb and standard air, oxygen in particular. Also, complete
conditions simply refer to room conditions with a combustion is also known as clean
temperature of 298K and a pressure of 1 atm. combustion.
2. Incomplete - takes place when the air is
The combustion of alcohol is an exothermic in limited supply. And as opposed to
process. It releases heat to the surrounding complete combustion it is otherwise
resulting to a negative value. known as dirty combustion. Due to lack of
oxygen, the fuel will not react completely.
3. Rapid - Rapid energy needs external heat
NOTE: Alcohols are organic substances that are energy for the reaction to occur. The
flammable and easily catch fire when combustion produces a large amount of
exposed to naked flames. It is a fire hazard. Care heat and light energy and does so rapidly.
must be taken to ensure that any The combustion will carry on as long as
spills are being cleaned up immediately. Safety the fuel is available.
goggles must be worn while 4. Spontaneous - it requires no external
performing this experiment. A fire extinguisher energy for the combustion to start. It
should also be kept close by in case happens due to self-heating. A substance
of emergencies. with low-ignition temperatures gets heated
and this heat is unable to escape.
5. Explosive - when the reaction occurs very
rapidly. The reaction occurs when
something ignites to produce heat, light
and sound energy, The simple way to
describe is it to call it an explosion.
Examples of combustion in everyday life
- Burning of Wood or Coal to heat your
home
- Burning of Petrol or Diesel to run your Car
- Combustion of Natural Gas or LPG to
cook for on your stovetop
- For the production of energy in thermal LABORATORY SAFETY & HAZARDS
power plantt
- Fireworks Do (What to do in a Laboratory….)
Ethanol (C2H6O) Wear appropriate eye protection, and
Propanol (C3H8O) inform teacher if one wears contact
Butanol (C4H10O) lenses.
Observe safety when handling sharp
objects.
OXIDATION AND REDUCTION REACTIONS Wash your hands before you leave the
laboratory
Reduction reaction refers to the gain of Be aware of all the safety devices. Should
electrons by a chemical particle while oxidation know where to find the first aid kit, the
is the loss of electron by another chemical chemical spill kit, the eye wash and the
particle. safety shower
Keep clutter to a minimum. Clean spillage
The particle that loses electrons is said to be of chemicals, and maintain general
oxidized and that one that gains these hygiene immediately after every work.
electrons is said to be reduced. Redox is the Report all spills, injuries, or broken
term that comes from the combination of the equipment to your teacher. Immediately
two words “reduction” and “oxidation”. after a spill or breakage occurs, it must be
reported to your teacher. No matter how
Oxidation and reduction always occur small it may seem.
simultaneously. That is, if one element is Use mitts or tongs with hot materials to
oxidized by losing electron then another element prevent burns.
has to be reduced by taking/ gaining those Work with volatile chemicals under a fume
electrons. hood.
Check glassware for stars or cracks.
Label all chemicals and close the
containers tightly.
Use the appropriate PPEs including hand
METAL AND SOME ASPECTS OF gloves and chemical splash safety
CORROSION googles for the chemicals handled.
All contaminated wastes must be collected
and disposed appropriately as per
Metals are composed of atoms which easily disposal procedure.
lose electrons and form cations. They possess
a lustrous appearance, have high thermal and Don’t (Things to Avoid in a Laboratory…)
electrical conductivities and are malleable and
ductile. Do not touch any equipment, chemicals,
or other materials until told to do so.
Corrosion is a general term applied to the Do not eat or drink, and do not chew gum
process in which uncombined metals, when left in the laboratory.
exposed, eventually combine with the elements When heating liquid, the opening of the
surrounding them (e.g. oxygen from the air) to test tube must NEVER be pointed towards
form compounds. another person or to yourself.
Never blew out the gas flame in the
In this special case of iron, the corrosion Bunsen burner
process is called rusting because the compound Do not put pieces of lab equipment in your
formed is a rust, Fe2O3∙H2O. mouth.
Do not pipet solutions by mouth
Do not use the phone or computer with
gloves on your hands to avoid getting
contaminations.
Never take a direct sniff of the vapor at the
mouth of the container where the
concentration of the vapor is high.
Never leave a heat source unattended
DON’T transfer used chemicals back into
primary container.
DO NOT work with chemicals until you are Lab-safe Refrigerators - Laboratory fridges
sure of their safe handling. This includes are designed to store samples, specimens,
some awareness of their flammability, vaccines and medicines at a very specific
reactivity, toxicity, and disposal. temperature range. They are used to cool
samples or specimens for preservation. inside of
Laboratory Safety Equipment & Personal a lab-safe fridge has no sources of ignition, and
Protective Equipment (PPE) has no internal electrical components which could
trigger an explosion.
Eye Wash Stations - are paramount for every lab.
There should be multiple eyewash stations so
they can be quickly accessed during Laboratory Safety Symbols or Signage
emergencies. Eyewash stations are designed to
flush the eye and face area only. There are
combination units available that contain both
features: a shower and an eyewash.
Safety Goggles - Goggles can be used to protect
the eye against particles, chemicals, water, glare
and from things striking the eyes
Fire Extinguisher - Fires can occur whenever
electrical equipment, and flammable materials
and chemicals are being handled. A fire
extinguisher should be kept in an easily accessed
location, and all laboratory personnel should
know how to use it properly.
Protective Gloves - Laboratory gloves reduce
contamination and protect you while working with
germs, pathogens, or other potentially hazardous
samples.
Lab Coats and Aprons - When there are chemical
spills, these will help prevent dangerous liquids
and particles from ruining clothes and chemical
contact to the skin.
Safety Showers - In the case of hazardous
chemicals coming into contact with skin, it is
extremely important to promptly rinse off the
substances.
First Aid Kits - First aid kits are important to have
initial treatments and care during emergencies.
They can be use to properly sterilize and cover
the exposed area to prevent any dangerous
chemicals from getting into the wound.
Fire Blankets - Fire blankets help smother the
flames if someone’s clothing ignites. After the
person has dropped to the floor and rolled around
to try to extinguish the flames, a fire blanket can
be used as a last resort.
Chemical Fume Hoods - Fume hoods are
chemical and fire-resistant enclosures that protect
lab personnel from inhaling dangerous chemicals
by drawing in vapors, gases, and dusts before
ventilating them out of the laboratory.
You might also like
- Chem181: Chemistry For Engineers - Laboratory First Semester, AY 2020-2021 (Cluster 1) Activity No. 1 Common Laboratory ApparatusDocument5 pagesChem181: Chemistry For Engineers - Laboratory First Semester, AY 2020-2021 (Cluster 1) Activity No. 1 Common Laboratory ApparatusMaxine de la TorreNo ratings yet
- Basic Laboratory Glassware and EquipmentDocument5 pagesBasic Laboratory Glassware and EquipmentEj RafaelNo ratings yet
- Dental PulpDocument53 pagesDental Pulpdr parveen bathla67% (3)
- Cloud Computing Basics - T. B. RehmanDocument198 pagesCloud Computing Basics - T. B. Rehmantim.howell8050No ratings yet
- Lab ApparatusDocument10 pagesLab ApparatuslizNo ratings yet
- Laboratory EquipmentDocument3 pagesLaboratory Equipmenttrishamaebosque68No ratings yet
- Common Laboratory ApparatusDocument21 pagesCommon Laboratory ApparatusMaria Kristelle Alejandro PaltaoNo ratings yet
- Lab ShitDocument4 pagesLab ShitpidoghislaineNo ratings yet
- Commonly Encountered Equipment in The Chemistry LaboratoryDocument11 pagesCommonly Encountered Equipment in The Chemistry LaboratoryAudrey MendozaNo ratings yet
- Commonly Encountered Equipment in The Chemistry LaboratoryDocument7 pagesCommonly Encountered Equipment in The Chemistry LaboratoryKIRBY ANN GALABINNo ratings yet
- Commonly Encountered Equipment in The Chemistry LaboratoryDocument9 pagesCommonly Encountered Equipment in The Chemistry Laboratorysadiqah mushtaqNo ratings yet
- Mon Lab ApparatusDocument11 pagesMon Lab ApparatusJustin MenorasNo ratings yet
- Laboratory ApparatusDocument17 pagesLaboratory Apparatuszena100% (1)
- Biochem Laboratory ReviewerDocument6 pagesBiochem Laboratory Reviewer202370092No ratings yet
- Lab Equipment BSCrim Organic - AutosavedDocument130 pagesLab Equipment BSCrim Organic - Autosavedangelo aquinoNo ratings yet
- Lab EquipmentsDocument10 pagesLab Equipmentschouroukbelkacemi236No ratings yet
- Matter and Kinetic Theory of Matter Power PointDocument64 pagesMatter and Kinetic Theory of Matter Power PointAkaNayep ApNo ratings yet
- Chemistry Laboratory: Group 1Document51 pagesChemistry Laboratory: Group 1Rachel Ann De LeonNo ratings yet
- August 19 - Laboratory NotesDocument2 pagesAugust 19 - Laboratory Notesthe horxNo ratings yet
- ACTIVITY 1-Common Lab Apparatus by Group 1 - BSMLS-1PDocument4 pagesACTIVITY 1-Common Lab Apparatus by Group 1 - BSMLS-1PAnne Chelsea Ramirez OritNo ratings yet
- Igoy Ukulan ChemistryDocument8 pagesIgoy Ukulan ChemistryGenesis Ducusin AguilarNo ratings yet
- Laboratory Glassware and Apparatus LSMDocument4 pagesLaboratory Glassware and Apparatus LSMShiina Mashiro100% (1)
- Laboratory Equipment and FunctionsDocument4 pagesLaboratory Equipment and FunctionsDiane DimaalaNo ratings yet
- Acid BuretteDocument8 pagesAcid BuretteLexi LoreNo ratings yet
- Are One of The Most Important Apparatus As They Are Functional From Storing To Mixing Reagents in Any Chemical or Biological ReactionsDocument2 pagesAre One of The Most Important Apparatus As They Are Functional From Storing To Mixing Reagents in Any Chemical or Biological ReactionsManuel Apao BarrunNo ratings yet
- Echeml Chemistry For Engineers - LaboratoryDocument2 pagesEcheml Chemistry For Engineers - LaboratoryJulieAnne DelaCruzNo ratings yet
- GenchemDocument2 pagesGenchemChristian MorfeNo ratings yet
- Mon Lab ApparatusDocument6 pagesMon Lab ApparatusGianni PillejeraNo ratings yet
- Dissilation Apparatus Uses and PartsDocument5 pagesDissilation Apparatus Uses and PartsMa. Lilian Jem MonteroNo ratings yet
- Chemistry ApparatusDocument3 pagesChemistry ApparatusMylz MendozaNo ratings yet
- Laboratory ApparatusDocument3 pagesLaboratory ApparatusLJ Princess Mary MontenegroNo ratings yet
- Dry Lab Inorganic ChemistryDocument7 pagesDry Lab Inorganic ChemistryAbegeil RadimaNo ratings yet
- Activity No 1Document9 pagesActivity No 1Mary Cuba GarciaNo ratings yet
- Common Lab Apparatus and ProcedureDocument12 pagesCommon Lab Apparatus and ProcedureRammohan Balaji PrasadNo ratings yet
- Activity 1Document4 pagesActivity 1Hannah VisitacionNo ratings yet
- SdfsdfsfsDocument5 pagesSdfsdfsfsmwah mwahNo ratings yet
- AN To Laboratory Equipment: By: Ms. Buroker Scott High SchoolDocument28 pagesAN To Laboratory Equipment: By: Ms. Buroker Scott High Schoolningsih rezekiNo ratings yet
- GlasswareDocument28 pagesGlasswareningsih rezeki0% (1)
- BS MTDocument3 pagesBS MTGenrev LisondraNo ratings yet
- Laboratory ApparatusDocument18 pagesLaboratory ApparatusCyrus De LeonNo ratings yet
- Balance Bunsen BurnerDocument5 pagesBalance Bunsen BurnerCharlie NipaNo ratings yet
- Laboratory ApparatusDocument10 pagesLaboratory ApparatusSEAN GAVIN DELOS REYESNo ratings yet
- Common Laboratory EquipmentDocument2 pagesCommon Laboratory EquipmentYuan BrionesNo ratings yet
- Phar 205Document156 pagesPhar 205billhaddNo ratings yet
- Common Laboratory EquipmentDocument16 pagesCommon Laboratory Equipmentbonbonreyes9No ratings yet
- Common Laboratory ToolsDocument4 pagesCommon Laboratory ToolsMargie Ballesteros Manzano50% (2)
- Name Picture Uses Utility Clamp: Used To Secure Glassware To A Ring StandDocument8 pagesName Picture Uses Utility Clamp: Used To Secure Glassware To A Ring StandRyan Jules DaisNo ratings yet
- Triple Beam Balance: - Obtaining The Mass of An ObjectDocument5 pagesTriple Beam Balance: - Obtaining The Mass of An Objectkristle balagtasNo ratings yet
- Apparatus Description UsesDocument6 pagesApparatus Description UsesVenus De GraciaNo ratings yet
- Rara Lab EquipmentDocument4 pagesRara Lab EquipmentHannaNo ratings yet
- Name Description PictureDocument4 pagesName Description PictureHazel AjeroNo ratings yet
- Test Tubes - A Glass Tube Where One End Is Open And: (Type Here)Document5 pagesTest Tubes - A Glass Tube Where One End Is Open And: (Type Here)dean2O6No ratings yet
- Laboratory ToolsDocument2 pagesLaboratory Toolsemmanuelcatayas2009No ratings yet
- Bunsen Burner A Watch GlassDocument4 pagesBunsen Burner A Watch GlassAna Soriano DocoyNo ratings yet
- It Is Used For Mixing, Stirring, and Heating ChemicalsDocument7 pagesIt Is Used For Mixing, Stirring, and Heating ChemicalsDessa GuditoNo ratings yet
- Chemistry Lab ReviewerDocument4 pagesChemistry Lab ReviewernaduasamuelNo ratings yet
- Evaporation: Basic Lab TechniquesDocument4 pagesEvaporation: Basic Lab TechniquesmhemhoryNo ratings yet
- Laboratory ApparatusDocument5 pagesLaboratory ApparatusArantxa Hilario100% (1)
- Chemistry Laboratory: Chem 103 InorganicDocument11 pagesChemistry Laboratory: Chem 103 InorganicShemiah Caminos GeorsuaNo ratings yet
- InorglabequipmentsDocument7 pagesInorglabequipmentsLOUISE RICA LAGAHITNo ratings yet
- Stress-Free Science: A Visual Guide to Acing Science in Grades 4-8From EverandStress-Free Science: A Visual Guide to Acing Science in Grades 4-8No ratings yet
- Tithi CalculationsDocument15 pagesTithi CalculationsSowmindra RottiNo ratings yet
- Conditional StatementsDocument5 pagesConditional Statementsশেখ মিশারNo ratings yet
- Sanyo ICs STKs PDFDocument117 pagesSanyo ICs STKs PDFFreddy Monge BarbaranNo ratings yet
- Tutorial Fpga Spartan-3eDocument53 pagesTutorial Fpga Spartan-3eCarlos Muñoz BacaNo ratings yet
- Sony Bx1s Chassis Kv-Ar14m50 SMDocument195 pagesSony Bx1s Chassis Kv-Ar14m50 SMCube7 GeronimoNo ratings yet
- Aluminum Alloy DatabaseDocument5 pagesAluminum Alloy Databaserizviabbas2012100% (1)
- Possibility of The Use Polish Soda Lime As The Absorbent in The Canisters of The Oxygen Breathing Apparatus Type Oxy-NGDocument11 pagesPossibility of The Use Polish Soda Lime As The Absorbent in The Canisters of The Oxygen Breathing Apparatus Type Oxy-NGindiomajaderoNo ratings yet
- Multiple Choice Questions Unit 4 PhysicsDocument175 pagesMultiple Choice Questions Unit 4 PhysicsAliya RahmanNo ratings yet
- Msunit 2 GraspsDocument6 pagesMsunit 2 Graspsapi-338845804No ratings yet
- Ambardar Book ChaptersDocument809 pagesAmbardar Book ChaptersJeffrey Hufford100% (1)
- Forces Multiple Choice Questions Set 3 - InteractiveDocument2 pagesForces Multiple Choice Questions Set 3 - InteractiveJoel OkohNo ratings yet
- Manual Variador Allen Bradley 161Document50 pagesManual Variador Allen Bradley 161Cosmin BlagaNo ratings yet
- Unit 5: Central Processing UnitDocument56 pagesUnit 5: Central Processing UnitgobinathNo ratings yet
- Configurar Session-Ttl en FortigateDocument2 pagesConfigurar Session-Ttl en FortigatemasmisemNo ratings yet
- Class Diagram - DrawioDocument24 pagesClass Diagram - DrawioFizba TahirNo ratings yet
- Hookworm: Starting Concept ArtDocument11 pagesHookworm: Starting Concept ArtLewisMaddisonNo ratings yet
- Eee 5543: Random Signal Principles: Vladimir A. PozdinDocument28 pagesEee 5543: Random Signal Principles: Vladimir A. PozdinYasir ButtNo ratings yet
- 26G Radar Level MeterDocument14 pages26G Radar Level Meterilopera1971No ratings yet
- Canon w8400 SM PCDocument235 pagesCanon w8400 SM PCkane123100% (1)
- Electrical Installation and Maintenance (Eim) 5Document55 pagesElectrical Installation and Maintenance (Eim) 5Victor Rosales100% (1)
- FSG000030 500 12 8V Eu5Document12 pagesFSG000030 500 12 8V Eu5jorge luis guevara martinezNo ratings yet
- Fundamentals of Highway Bridge Demolition: Conference PaperDocument10 pagesFundamentals of Highway Bridge Demolition: Conference PaperDenver PlayletsNo ratings yet
- COA - Potassium IodateDocument2 pagesCOA - Potassium IodateMechem EurofinsNo ratings yet
- Homework 1Document10 pagesHomework 1شمس صبيح عبد الرحيمNo ratings yet
- Ph302 Tutorial Sheet 1Document2 pagesPh302 Tutorial Sheet 1Manik MalhotraNo ratings yet
- FlywheelDocument10 pagesFlywheelKasam SantoshrishiNo ratings yet
- CPS 104 Computer Organization and Programming Lecture-22: Single Cycle Datapath, ControlDocument36 pagesCPS 104 Computer Organization and Programming Lecture-22: Single Cycle Datapath, ControlprachesNo ratings yet
- Bacteriology Lab 2 - Instruments Used in Bacteriology LaboratoryDocument1 pageBacteriology Lab 2 - Instruments Used in Bacteriology LaboratoryJiro Anderson EscañaNo ratings yet