Professional Documents
Culture Documents
Electrochemical Series
Electrochemical Series
Uploaded by
Leslie LamCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Electrochemical Series
Electrochemical Series
Uploaded by
Leslie LamCopyright:
Available Formats
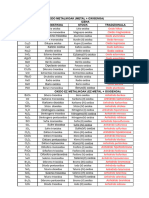
ELECTROCHEMICAL SERIES
Oxidizing Reducing
Oxidizing agent Reducing agent
power power
Weakest Strongest
K+(aq) + e- ⇌ K(s)
oxidizing reducing
agent agent
Ca2+(aq) + 2e- ⇌ Ca(s)
Na+(aq) + e- ⇌ Na(s)
Mg2+(aq) + 2e- ⇌ Mg(s)
Al3+(aq) + 3e- ⇌ Al(s)
Zn2+(aq) + 2e- ⇌ Zn(s)
Fe2+(aq) + 2e- ⇌ Fe(s)
Pb2+(aq) + 2e- ⇌ Pb(s)
2H+(aq) + 2e- ⇌ H2(g)
SO42-(aq) + 4H+(aq) + 2e- ⇌ SO2(g) + 2H2O(l)
Cu2+(aq) + 2e- ⇌ Cu(s)
O2(g) + 2H2O(l) + 4e- ⇌ 4OH-(aq)
I2(aq) + 2e- ⇌ 2I-(aq)
Fe3+(aq) + e- ⇌ Fe2+(aq)
Ag+(aq) + e- ⇌ Ag(s)
2H+(aq) + NO3-(aq) + e- ⇌ NO2(g) + H2O(l)
Br2(aq/g/l) + 2e- ⇌ 2Br-(aq)
Cr2O72-(aq) + 14H+ + 6e- ⇌ 2Cr3+(aq) + 7H2O(l)
Cl2(aq/g) + 2e- ⇌ 2Cl-(aq)
MnO4-(aq) + 8H+(aq) + 5e- ⇌ Mn2+(aq) + 4H2O(l)
Strongest Weakest
oxidizing reducing
F2(aq/g) + 2e- ⇌ 2F-(aq)
agent agent
You might also like
- Formulazio AriketakDocument3 pagesFormulazio AriketakAlfonso HernangilNo ratings yet
- Formulazio Ez OrganikoaDocument8 pagesFormulazio Ez OrganikoaJara EspumosaNo ratings yet
- Potenciales Estandar de ReduccionDocument5 pagesPotenciales Estandar de ReduccionlisandroNo ratings yet
- Standard Reduction Potentials at 25°C, 1 Atm: Half-Reaction /V Half-Reaction /V Half-Reaction /VDocument1 pageStandard Reduction Potentials at 25°C, 1 Atm: Half-Reaction /V Half-Reaction /V Half-Reaction /VNurathirah HussinNo ratings yet
- Standard Reduction PotentialsDocument3 pagesStandard Reduction PotentialsjeonghanniejsvtNo ratings yet
- Ariketak Estekiometria 2Document30 pagesAriketak Estekiometria 2Soukaina El machhourNo ratings yet
- Erreakzio Kimikoen DoiketaDocument1 pageErreakzio Kimikoen DoiketagarlegorNo ratings yet
- Kast RakoDocument85 pagesKast RakoAndreas Eduard LerrickNo ratings yet
- PDF Serie Electroquimica de Los Metales CompressDocument2 pagesPDF Serie Electroquimica de Los Metales CompressLuissin RamirezNo ratings yet
- 5 Gaia - Erredox EUSK 2021-2022-1Document38 pages5 Gaia - Erredox EUSK 2021-2022-1MarinaNo ratings yet
- LampiranDocument90 pagesLampiranfullsunNo ratings yet
- 2018 Ekaina B AukeraDocument1 page2018 Ekaina B AukeraGarazi IDIAKEZ IRASTORZANo ratings yet
- 2018 Ekaina B AukeraDocument1 page2018 Ekaina B AukeraMaddi Iñarra SarobeNo ratings yet
- Erreakzio Kimikoen DoiketaDocument6 pagesErreakzio Kimikoen DoiketaOdon Armas ZalbideaNo ratings yet
- Nahasturik 3 ZuzendutaDocument2 pagesNahasturik 3 ZuzendutavitoNo ratings yet
- Formulazioa Eta Nomenklatura AzterketaDocument2 pagesFormulazioa Eta Nomenklatura AzterketaEJAIRAM5No ratings yet
- LampiranDocument31 pagesLampiranNo NameNo ratings yet
- 2016 BIOENERGETIKAko ARIKETAKDocument3 pages2016 BIOENERGETIKAko ARIKETAKitxasoNo ratings yet
- Nahastuta 2 ZuzendutaDocument3 pagesNahastuta 2 ZuzendutavitoNo ratings yet
- 4 Gaia Formulazioa 4Document11 pages4 Gaia Formulazioa 4Ivan García BerasateguiNo ratings yet
- Formulazio KimikaDocument3 pagesFormulazio KimikaAnaNo ratings yet
- 17-18 NOMENKLATURA DBH 4 (Ebatzita)Document11 pages17-18 NOMENKLATURA DBH 4 (Ebatzita)ESTHERNo ratings yet
- Formulazioa Ariketak NahastutaDocument3 pagesFormulazioa Ariketak NahastutaIKER GARCIA MARTÍNEZNo ratings yet
- Oxidoak Eta HidruroakDocument10 pagesOxidoak Eta HidruroakidoialazaroNo ratings yet
- Emaitzak Formulazio Ez OrganikoaDocument6 pagesEmaitzak Formulazio Ez OrganikoaRiad MaalmineNo ratings yet
- Hautaproba 19992000 Ekaina EBATZITADocument8 pagesHautaproba 19992000 Ekaina EBATZITAaneaneaneNo ratings yet
- Formulazioa Birpasoko Ariketak. ZuzenketaDocument2 pagesFormulazioa Birpasoko Ariketak. ZuzenketaTelmo Martinez AbaigarNo ratings yet
- Teoria Formulazio Ez-OrganikoaDocument20 pagesTeoria Formulazio Ez-Organikoa01- Joseba Zabala Martin100% (1)
- Taula Dena ZuzendutaDocument13 pagesTaula Dena ZuzendutaDonaNo ratings yet
- 2 Oxigenoaren Konbinazio Bitarrak EmaitzakDocument1 page2 Oxigenoaren Konbinazio Bitarrak EmaitzakcristinaNo ratings yet
- 運算放大器Document34 pages運算放大器黃偉No ratings yet
- 4.3.1 Erredox ErreakzioakDocument25 pages4.3.1 Erredox ErreakzioakOskarNo ratings yet
- Formulazio Ariketak (1) ZUZENKETAKDocument5 pagesFormulazio Ariketak (1) ZUZENKETAKIker Moreno RojasNo ratings yet
- Birpasatzeko Ariketak (1eba) 2021 22Document2 pagesBirpasatzeko Ariketak (1eba) 2021 22Laura HernándezNo ratings yet
- CHP 03 AnsDocument11 pagesCHP 03 AnschemiekenNo ratings yet
- Ariketak (Hidroxidoak)Document1 pageAriketak (Hidroxidoak)anderguti2008No ratings yet
- 2018 Ekaina A AukeraDocument1 page2018 Ekaina A AukeraMaddi Iñarra SarobeNo ratings yet
- Elementu Eta Konposatu KimikoakDocument8 pagesElementu Eta Konposatu KimikoakEJAIRAM5No ratings yet
- Páginas Desdekimika - Ez2013-2Document2 pagesPáginas Desdekimika - Ez2013-2eskolaNo ratings yet
- Supliment 10Document3 pagesSupliment 10Gigi HamburgerNo ratings yet
- Formulazioa3 DBHDocument18 pagesFormulazioa3 DBHgartxi2008No ratings yet
- Kalkuluestekiometrikoak II SoluzioakDocument6 pagesKalkuluestekiometrikoak II SoluzioakRosendo VivancoNo ratings yet
- Formulazioa TeoriaDocument13 pagesFormulazioa TeoriaXabiNo ratings yet
- Formazio-Entalpia EstandarrakDocument1 pageFormazio-Entalpia EstandarrakAratz EgurenNo ratings yet
- Umol BZ Adsorbed/ G Sio2 PBZ (Atm) 343 K 363 K 383 K 403 K: P/NVSPDocument7 pagesUmol BZ Adsorbed/ G Sio2 PBZ (Atm) 343 K 363 K 383 K 403 K: P/NVSPpaula peñaililloNo ratings yet
- Formulazio Ariketak (2) ZUZENKETAKDocument3 pagesFormulazio Ariketak (2) ZUZENKETAKPaula GimenoNo ratings yet
- Páginas Desdekimika Extr. 2014-2Document1 pagePáginas Desdekimika Extr. 2014-2eskolaNo ratings yet
- 04 Peroxidoak emDocument1 page04 Peroxidoak emjosu mañarikuaNo ratings yet
- Formulazio Ariketak 1Document7 pagesFormulazio Ariketak 1IKER GARCIA MARTÍNEZNo ratings yet
- Erreakzio KimikoakDocument3 pagesErreakzio KimikoakapartiedaNo ratings yet
- ARIKETAK 1 - EmaitzakDocument4 pagesARIKETAK 1 - Emaitzakanderguti2008No ratings yet
- Hidruro BitarrakDocument2 pagesHidruro Bitarrakmaiteuranga98No ratings yet
- Formulazio Ezorganikoa PDFDocument14 pagesFormulazio Ezorganikoa PDFUXUE PORRASNo ratings yet
- A - Nukleo EmaitzakDocument2 pagesA - Nukleo EmaitzakOier Jurado MartinNo ratings yet
- HEDocument42 pagesHEFebri5awalsyah100% (1)
- 01-ERREAKZIO KIMIKOA-Erreakzio Kimikoak - Ariketa EbatziakDocument7 pages01-ERREAKZIO KIMIKOA-Erreakzio Kimikoak - Ariketa EbatziakXabiNo ratings yet
- PEMISAHANDocument2 pagesPEMISAHANNatalia FransiscaNo ratings yet
- Ariketak 1 (Emaitzak)Document5 pagesAriketak 1 (Emaitzak)d.laraNo ratings yet