Professional Documents
Culture Documents
Chemicalkinetics 1
Chemicalkinetics 1
Uploaded by
Cicilia ByabangCopyright:
Available Formats
You might also like
- Generic Solar Sales Opening ScriptDocument5 pagesGeneric Solar Sales Opening ScriptTubi Ad100% (4)
- O Level Biology Practice Questions And Answers EnzymesFrom EverandO Level Biology Practice Questions And Answers EnzymesRating: 5 out of 5 stars5/5 (1)
- Howden Blower Method StatementDocument56 pagesHowden Blower Method StatementBaisel Rahuman0% (1)
- Kinetics Ans Key Master FileDocument10 pagesKinetics Ans Key Master FileJOANA RHEA SAGPAEYNo ratings yet
- Questions On Rate ExpressionDocument1 pageQuestions On Rate ExpressionSai Pranav0% (2)
- CRE - Diagnostic Exam (USA)Document2 pagesCRE - Diagnostic Exam (USA)Kuo SarongNo ratings yet
- PANASONICDocument2 pagesPANASONICJosue Hernandez Gutierrez100% (1)
- 11 Chapter Reaction Kinetics Text Book Exercise PDFDocument14 pages11 Chapter Reaction Kinetics Text Book Exercise PDFBilal KhanNo ratings yet
- Cbse+2 Chemistry 1mark Bits 2023-2024Document41 pagesCbse+2 Chemistry 1mark Bits 2023-2024lama lamaNo ratings yet
- Chemical Kinetics FinalDocument7 pagesChemical Kinetics Finalaxiliya6No ratings yet
- Chemical Reaction KineticsDocument4 pagesChemical Reaction KineticsMichaela BorjaNo ratings yet
- 12 Chemistry23 24 sp01Document14 pages12 Chemistry23 24 sp01bhattkrrish339No ratings yet
- Enzyme Quiz 2013Document2 pagesEnzyme Quiz 2013Fatema MohamedNo ratings yet
- Concordia Colleges BWP: 1 Year Chemistry Chapter#11Document2 pagesConcordia Colleges BWP: 1 Year Chemistry Chapter#11Hafiz ZainNo ratings yet
- Stars Academy Lahore: Stars Entry Test System - 2019Document2 pagesStars Academy Lahore: Stars Entry Test System - 2019Memoona GullNo ratings yet
- ChE Objective Type Questions Compilation-Dean Medina 8-27-10Document177 pagesChE Objective Type Questions Compilation-Dean Medina 8-27-10Clark Ivan Torres100% (1)
- Chemical KineticsDocument16 pagesChemical KineticsOm AgrawalNo ratings yet
- Screenshot 2024-02-18 at 7.36.17 AMDocument1 pageScreenshot 2024-02-18 at 7.36.17 AMrajab25837No ratings yet
- GZB - Xii - WS-8 - Chemistry - Chemical Kinetics - OctoberDocument3 pagesGZB - Xii - WS-8 - Chemistry - Chemical Kinetics - OctoberSaman PanwarNo ratings yet
- Chemical Kinetics Chap 8Document2 pagesChemical Kinetics Chap 8Eliza BethNo ratings yet
- Chemical Kinetics RevisionDocument2 pagesChemical Kinetics RevisionShubham KumarNo ratings yet
- Chemical Kinetics AssignmentDocument3 pagesChemical Kinetics Assignmentjainaba mohamedNo ratings yet
- Solved MCQs6Document2 pagesSolved MCQs6fati maaNo ratings yet
- Chemical Kinetics TestDocument5 pagesChemical Kinetics Testrajneesh kumarNo ratings yet
- 11 Chapter Reaction Kinetics Text Book ExerciseDocument14 pages11 Chapter Reaction Kinetics Text Book ExerciseSajid AzeemNo ratings yet
- Class 12 Ut-1 Question Paper ChemistryDocument19 pagesClass 12 Ut-1 Question Paper ChemistryArun singhNo ratings yet
- Department of Chemistry SUNY/Oneonta Chem 221 - Organic Chemistry I Examination #2 - October 23, 2000Document12 pagesDepartment of Chemistry SUNY/Oneonta Chem 221 - Organic Chemistry I Examination #2 - October 23, 2000Ivy JoyceNo ratings yet
- Q#1 UsaDocument2 pagesQ#1 UsaRomel LeoNo ratings yet
- Class 12 Science Bihu Holiday HomeworkDocument8 pagesClass 12 Science Bihu Holiday HomeworkSoham RoyNo ratings yet
- Option (Iii) Is The Answer.: NCERT Exemplar Solutions of Class 12 Chemistry Chapter 4 Chemical KineticsDocument15 pagesOption (Iii) Is The Answer.: NCERT Exemplar Solutions of Class 12 Chemistry Chapter 4 Chemical Kineticspriyaranjan singhNo ratings yet
- ChE Objective Type Questions Compilation-Dean Medina 8-19-12Document144 pagesChE Objective Type Questions Compilation-Dean Medina 8-19-12Yul TalaveraNo ratings yet
- 12 Examplar Chapter 4 - MCQ-1 &MCQ-2Document24 pages12 Examplar Chapter 4 - MCQ-1 &MCQ-2Madhurima BoralNo ratings yet
- End Term ALLDocument31 pagesEnd Term ALLJulie Anne CristalesNo ratings yet
- Chelsea Clio Budiman - Equibrium Introductory Conceptual QuestionDocument4 pagesChelsea Clio Budiman - Equibrium Introductory Conceptual Questionpretzel design studio100% (1)
- Chemical EquilibriumDocument44 pagesChemical Equilibriumtarsem jiNo ratings yet
- Senior - 2020 - Class - 12 - Chemistry - Objective Questions - Chemical KineticsDocument5 pagesSenior - 2020 - Class - 12 - Chemistry - Objective Questions - Chemical Kineticsblaise.denzil.rodriguesNo ratings yet
- Unit 04 Rate of Reaction2Document17 pagesUnit 04 Rate of Reaction2Azeem iftikharNo ratings yet
- 1st Year Chemistry Revision Assignment For Test 11Document6 pages1st Year Chemistry Revision Assignment For Test 11Syed Moeen NaqviNo ratings yet
- 11 - Units and Measurement 08.08Document1 page11 - Units and Measurement 08.08sayalis1604No ratings yet
- Chemical Engineering Objective Type Questions Reaction KineticsDocument18 pagesChemical Engineering Objective Type Questions Reaction KineticsSaakshi Sharma67% (3)
- Ans 6.1 (B)Document6 pagesAns 6.1 (B)kailin.kelly528No ratings yet
- Class-12 Chemistry ElectroDocument4 pagesClass-12 Chemistry ElectroHemant ChaudharyNo ratings yet
- CHEMISTRY 12th SubejctiveDocument3 pagesCHEMISTRY 12th SubejctiveVivek SanwalNo ratings yet
- Adama Science and Technology UniversityDocument34 pagesAdama Science and Technology UniversityAme ShumetaNo ratings yet
- Chemical Kinetics - Practice Sheet - VIJETA SERIES CLASS-12THDocument6 pagesChemical Kinetics - Practice Sheet - VIJETA SERIES CLASS-12THrachoudhary9741No ratings yet
- CRE-1 - Mid Sem 5Document2 pagesCRE-1 - Mid Sem 5Aaditya TyagiNo ratings yet
- Chemistry Capsule 30Document32 pagesChemistry Capsule 30Rohith SNo ratings yet
- ChE Objective Type Questions Compilation Dean Medina 8 27 10Document177 pagesChE Objective Type Questions Compilation Dean Medina 8 27 10BEA GEDELYN GARCIANo ratings yet
- Aqa 1 5Document19 pagesAqa 1 5leonidas.wujieweiNo ratings yet
- EquilibriumDocument4 pagesEquilibriumbalramsharmaNo ratings yet
- MCQs For Class XII ChemistryDocument29 pagesMCQs For Class XII Chemistryjkc collegeNo ratings yet
- Suggesion CRE 2022Document14 pagesSuggesion CRE 2022Soumyodeep ChowdhuryNo ratings yet
- HT TP: //qpa Pe R.W But .Ac .In: 2011 Chemistry-IDocument7 pagesHT TP: //qpa Pe R.W But .Ac .In: 2011 Chemistry-IKaushikBoseNo ratings yet
- Chemical KineticsDocument4 pagesChemical KineticsNUCLEAR GAMINGNo ratings yet
- Chemical KineticsDocument32 pagesChemical KineticsTimothy HandokoNo ratings yet
- 16PCH1103Document22 pages16PCH1103MoneeshsabapathiNo ratings yet
- Chemical Kinetics MCQ 2Document16 pagesChemical Kinetics MCQ 2vijayresup123No ratings yet
- 12 Chemistry Imp ch4 3 PDFDocument14 pages12 Chemistry Imp ch4 3 PDFrahul gautamNo ratings yet
- The To Prepare For Only App You NeedDocument49 pagesThe To Prepare For Only App You NeedAparajita K SinghNo ratings yet
- 4.chemical Kinetics KCET PYQsDocument2 pages4.chemical Kinetics KCET PYQsPunith kumar100% (4)
- Chemical KineticsDocument49 pagesChemical KineticsS KNo ratings yet
- Final Exam CRIM ADGEDocument9 pagesFinal Exam CRIM ADGEBernard Agcanas FelipeNo ratings yet
- Chemical Kinetics MCQ (Class 12) : - Eart EartDocument4 pagesChemical Kinetics MCQ (Class 12) : - Eart EartBuri MtmNo ratings yet
- Kit - 618XR Parts Required: No Description BOM. QtyDocument8 pagesKit - 618XR Parts Required: No Description BOM. Qtyzarul arjuna0% (1)
- High Density-Pp Low Noise Drainage System: Astral Poly Technik LimitedDocument4 pagesHigh Density-Pp Low Noise Drainage System: Astral Poly Technik LimitedAbdullaKakkadKarumbilNo ratings yet
- Electronics Project List PDFDocument13 pagesElectronics Project List PDFvinothNo ratings yet
- NTC Thermistor RT Curves: WWW - Beta.dk WWW - Beata.se WWW - Betafinland.fiDocument9 pagesNTC Thermistor RT Curves: WWW - Beta.dk WWW - Beata.se WWW - Betafinland.fibasurator2001No ratings yet
- FW6A Manual CuerpoDocument11 pagesFW6A Manual CuerpoHenrry trespalaciosNo ratings yet
- Week 5 Writing Task 1 Line GraphDocument15 pagesWeek 5 Writing Task 1 Line GraphLê Tú QuyênNo ratings yet
- La-Gonave Seminar Presentation-2010Document18 pagesLa-Gonave Seminar Presentation-2010Daniel DaréusNo ratings yet
- Cbse Class - Xi Chemistry Sample Paper 2: Time: 3 Hours Marks: 70 General InstructionsDocument6 pagesCbse Class - Xi Chemistry Sample Paper 2: Time: 3 Hours Marks: 70 General InstructionsBhabaniNo ratings yet
- SUST1000 Interim Report #1Document14 pagesSUST1000 Interim Report #1Kavya Geetha BalajiNo ratings yet
- Ceramic CompositesDocument29 pagesCeramic CompositesAbhey DograNo ratings yet
- Batch19 (DC-DC Converter) PDFDocument56 pagesBatch19 (DC-DC Converter) PDFramNo ratings yet
- SpecificationsDocument8 pagesSpecificationscristianNo ratings yet
- RRF 5Document21 pagesRRF 5Porkkodi SugumaranNo ratings yet
- Bent RuleDocument4 pagesBent RuleHARSHIT KHANNANo ratings yet
- Manual Tehnic ADI-CD - 2013Document79 pagesManual Tehnic ADI-CD - 2013Vlad BalanNo ratings yet
- Current TransformersDocument19 pagesCurrent TransformersyugoplodNo ratings yet
- PT. Anugerah Mega Energi Daily Report: Work Time: 07.00 18.00 Work Time: 07.00 18.00Document1 pagePT. Anugerah Mega Energi Daily Report: Work Time: 07.00 18.00 Work Time: 07.00 18.00heri_prasetyadiNo ratings yet
- Wiring Diagram Outdoor Emergency Lighting Units: Installation Instructions Model: Wgc42FeDocument2 pagesWiring Diagram Outdoor Emergency Lighting Units: Installation Instructions Model: Wgc42FeMario WiryaNo ratings yet
- Presentation On Si and Ci EngineDocument16 pagesPresentation On Si and Ci EngineAman Jain0% (1)
- 4 Amps N-Channel MOSFET: 600voltsDocument5 pages4 Amps N-Channel MOSFET: 600voltsAlir AlichyNo ratings yet
- Bps2021pdf CompressedDocument234 pagesBps2021pdf Compressedkuldeep singhNo ratings yet
- Compressed Gas CylinderDocument4 pagesCompressed Gas Cylinderiwan azaNo ratings yet
- De 19977 Marketing EagleBurgmann DMS MSE E5 PDF CatalogMechanicalseals, Magneticcouplings en 19.09.2016Document146 pagesDe 19977 Marketing EagleBurgmann DMS MSE E5 PDF CatalogMechanicalseals, Magneticcouplings en 19.09.2016sanjeevvangeNo ratings yet
- CV 101Document4 pagesCV 101frco1504No ratings yet
- Liebert Ita Datasheet 5kva To 20kva 2Document4 pagesLiebert Ita Datasheet 5kva To 20kva 2HafidGaneshaSecretrdreamholicNo ratings yet
- Lab 3Document7 pagesLab 3jisteeleNo ratings yet
Chemicalkinetics 1
Chemicalkinetics 1
Uploaded by
Cicilia ByabangOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chemicalkinetics 1
Chemicalkinetics 1
Uploaded by
Cicilia ByabangCopyright:
Available Formats
GARDEN DEW RESIDENTIAL SCHOOL GARDEN DEW RESIDENTIAL SCHOOL
CLASS – XII (CHEMISTRY) CLASS – XII (CHEMISTRY)
MM:20 TIME: 45MIN MM:20 TIME: 45MIN
1. Activation energy of a chemical reaction can be determined by 1. Activation energy of a chemical reaction can be determined by
(a) determining the rate constant at standard temperature (a) determining the rate constant at standard temperature
(b) determining the rate constants at two temperatures (b) determining the rate constants at two temperatures
(c) determining probability of collision (c) determining probability of collision
(d) using catalyst (d) using catalyst
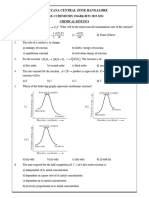
2. According to Arrhenius equation rate constant k is equal to Ae-Ea/RT. 2. According to Arrhenius equation rate constant k is equal to Ae-Ea/RT.
Which of the following options represents the graph of In k versus ? Which of the following options represents the graph of In k versus ?
3. Which of the following statement is not correct about order of a 3. Which of the following statement is not correct about order of a
reaction? reaction?
(a) The order of a reaction can be a fractional number. (a) The order of a reaction can be a fractional number.
(b) Order of a reaction is experimentally determined quantity. (b) Order of a reaction is experimentally determined quantity.
(c) The order of a reaction is always equal to the sum of the (c) The order of a reaction is always equal to the sum of the
stoichiometric coefficients of reactants in the balanced chemical stoichiometric coefficients of reactants in the balanced chemical
equation for a reaction. equation for a reaction.
(d) The order of a reaction is the sum of the powers of molar (d) The order of a reaction is the sum of the powers of molar
concentration of the reactants in the rate law expression. concentration of the reactants in the rate law expression.
4. Which of the following statement is correct? 4. Which of the following statement is correct?
(a) The rate of a reaction decreases with passage of time as the (a) The rate of a reaction decreases with passage of time as the
concentration of reactants decreases. concentration of reactants decreases.
(b) The rate of a reaction is same at any time during the reaction. (b) The rate of a reaction is same at any time during the reaction.
(c) The rate of a reaction is independent of temperature change. (c) The rate of a reaction is independent of temperature change.
(d) The rate of a reaction decreases with increase in concentration of (d) The rate of a reaction decreases with increase in concentration of
reactant(s). reactant(s).
5. Rate law for the reaction ‘B’ is doubled, keeping the concentration of 5. Rate law for the reaction ‘B’ is doubled, keeping the concentration of
‘A’ ‘A’
constant, the value of rate constant will be constant, the value of rate constant will be
(a) the same (b) doubled (c) quadrupled (d) halved (a) the same (b) doubled (c) quadrupled (d) halved
6. The conversion of molecules X to Y follows second order kinetics. If 6. The conversion of molecules X to Y follows second order kinetics. If
concentration of X is increased to three times how will it affect the rate concentration of X is increased to three times how will it affect the rate
of formation of Y ? of formation of Y ?
OR OR
For a first order reaction, show that time required for 99% completion For a first order reaction, show that time required for 99% completion
is twice the time required for the completion of 90% of reaction. is twice the time required for the completion of 90% of reaction.
7. A reaction is second order in A and first order in B. 7. A reaction is second order in A and first order in B.
(i) How is the rate affected on increasing the concentration of B three (i) How is the rate affected on increasing the concentration of B three
times? times?
(ii) How is the rate affected when the concentrations of both A and B (ii) How is the rate affected when the concentrations of both A and B
are doubled? are doubled?
8. The rate of a reaction quadruples when the temperature changes from 8. The rate of a reaction quadruples when the temperature changes from
293 K to 313 K. Calculate the energy of activation of the reaction 293 K to 313 K. Calculate the energy of activation of the reaction
assuming that it does not change with temperature. assuming that it does not change with temperature.
9. The rate constant for the decomposition of hydrocarbons is 2.418 × 10 – 9. The rate constant for the decomposition of hydrocarbons is 2.418 × 10 –
5s – 1 at 546 K. If the energy of activation is 179.9 kJ/mol, what will be 5s – 1 at 546 K. If the energy of activation is 179.9 kJ/mol, what will be
the value of pre-exponential factor? the value of pre-exponential factor?
10. The following results have been obtained during the kinetic studies of 10. The following results have been obtained during the kinetic studies of
the reaction: the reaction:
2A + B → C + D 2A + B → C + D
Experiment [A]/ [B]/ mol Initial rate of Experiment [A]/ [B]/ mol Initial rate of
mol L-1 L-1 formation of D / mol L-1 L-1 formation of D /
mol L-1 min-1 mol L-1 min-1
I 0.1 0.1 6.0 x 10-3 I 0.1 0.1 6.0 x 10-3
II 0.3 0.2 7.2 x 10-2 II 0.3 0.2 7.2 x 10-2
III 0.3 0.4 2.88 x 10-1 III 0.3 0.4 2.88 x 10-1
IV 0.4 0.1 2.4 x 10-2 IV 0.4 0.1 2.4 x 10-2
Determine the rate law and the rate constant for the reaction. Determine the rate law and the rate constant for the reaction.
You might also like
- Generic Solar Sales Opening ScriptDocument5 pagesGeneric Solar Sales Opening ScriptTubi Ad100% (4)
- O Level Biology Practice Questions And Answers EnzymesFrom EverandO Level Biology Practice Questions And Answers EnzymesRating: 5 out of 5 stars5/5 (1)
- Howden Blower Method StatementDocument56 pagesHowden Blower Method StatementBaisel Rahuman0% (1)
- Kinetics Ans Key Master FileDocument10 pagesKinetics Ans Key Master FileJOANA RHEA SAGPAEYNo ratings yet
- Questions On Rate ExpressionDocument1 pageQuestions On Rate ExpressionSai Pranav0% (2)
- CRE - Diagnostic Exam (USA)Document2 pagesCRE - Diagnostic Exam (USA)Kuo SarongNo ratings yet
- PANASONICDocument2 pagesPANASONICJosue Hernandez Gutierrez100% (1)
- 11 Chapter Reaction Kinetics Text Book Exercise PDFDocument14 pages11 Chapter Reaction Kinetics Text Book Exercise PDFBilal KhanNo ratings yet
- Cbse+2 Chemistry 1mark Bits 2023-2024Document41 pagesCbse+2 Chemistry 1mark Bits 2023-2024lama lamaNo ratings yet
- Chemical Kinetics FinalDocument7 pagesChemical Kinetics Finalaxiliya6No ratings yet
- Chemical Reaction KineticsDocument4 pagesChemical Reaction KineticsMichaela BorjaNo ratings yet
- 12 Chemistry23 24 sp01Document14 pages12 Chemistry23 24 sp01bhattkrrish339No ratings yet
- Enzyme Quiz 2013Document2 pagesEnzyme Quiz 2013Fatema MohamedNo ratings yet
- Concordia Colleges BWP: 1 Year Chemistry Chapter#11Document2 pagesConcordia Colleges BWP: 1 Year Chemistry Chapter#11Hafiz ZainNo ratings yet
- Stars Academy Lahore: Stars Entry Test System - 2019Document2 pagesStars Academy Lahore: Stars Entry Test System - 2019Memoona GullNo ratings yet
- ChE Objective Type Questions Compilation-Dean Medina 8-27-10Document177 pagesChE Objective Type Questions Compilation-Dean Medina 8-27-10Clark Ivan Torres100% (1)
- Chemical KineticsDocument16 pagesChemical KineticsOm AgrawalNo ratings yet
- Screenshot 2024-02-18 at 7.36.17 AMDocument1 pageScreenshot 2024-02-18 at 7.36.17 AMrajab25837No ratings yet
- GZB - Xii - WS-8 - Chemistry - Chemical Kinetics - OctoberDocument3 pagesGZB - Xii - WS-8 - Chemistry - Chemical Kinetics - OctoberSaman PanwarNo ratings yet
- Chemical Kinetics Chap 8Document2 pagesChemical Kinetics Chap 8Eliza BethNo ratings yet
- Chemical Kinetics RevisionDocument2 pagesChemical Kinetics RevisionShubham KumarNo ratings yet
- Chemical Kinetics AssignmentDocument3 pagesChemical Kinetics Assignmentjainaba mohamedNo ratings yet
- Solved MCQs6Document2 pagesSolved MCQs6fati maaNo ratings yet
- Chemical Kinetics TestDocument5 pagesChemical Kinetics Testrajneesh kumarNo ratings yet
- 11 Chapter Reaction Kinetics Text Book ExerciseDocument14 pages11 Chapter Reaction Kinetics Text Book ExerciseSajid AzeemNo ratings yet
- Class 12 Ut-1 Question Paper ChemistryDocument19 pagesClass 12 Ut-1 Question Paper ChemistryArun singhNo ratings yet
- Department of Chemistry SUNY/Oneonta Chem 221 - Organic Chemistry I Examination #2 - October 23, 2000Document12 pagesDepartment of Chemistry SUNY/Oneonta Chem 221 - Organic Chemistry I Examination #2 - October 23, 2000Ivy JoyceNo ratings yet
- Q#1 UsaDocument2 pagesQ#1 UsaRomel LeoNo ratings yet
- Class 12 Science Bihu Holiday HomeworkDocument8 pagesClass 12 Science Bihu Holiday HomeworkSoham RoyNo ratings yet
- Option (Iii) Is The Answer.: NCERT Exemplar Solutions of Class 12 Chemistry Chapter 4 Chemical KineticsDocument15 pagesOption (Iii) Is The Answer.: NCERT Exemplar Solutions of Class 12 Chemistry Chapter 4 Chemical Kineticspriyaranjan singhNo ratings yet
- ChE Objective Type Questions Compilation-Dean Medina 8-19-12Document144 pagesChE Objective Type Questions Compilation-Dean Medina 8-19-12Yul TalaveraNo ratings yet
- 12 Examplar Chapter 4 - MCQ-1 &MCQ-2Document24 pages12 Examplar Chapter 4 - MCQ-1 &MCQ-2Madhurima BoralNo ratings yet
- End Term ALLDocument31 pagesEnd Term ALLJulie Anne CristalesNo ratings yet
- Chelsea Clio Budiman - Equibrium Introductory Conceptual QuestionDocument4 pagesChelsea Clio Budiman - Equibrium Introductory Conceptual Questionpretzel design studio100% (1)
- Chemical EquilibriumDocument44 pagesChemical Equilibriumtarsem jiNo ratings yet
- Senior - 2020 - Class - 12 - Chemistry - Objective Questions - Chemical KineticsDocument5 pagesSenior - 2020 - Class - 12 - Chemistry - Objective Questions - Chemical Kineticsblaise.denzil.rodriguesNo ratings yet
- Unit 04 Rate of Reaction2Document17 pagesUnit 04 Rate of Reaction2Azeem iftikharNo ratings yet
- 1st Year Chemistry Revision Assignment For Test 11Document6 pages1st Year Chemistry Revision Assignment For Test 11Syed Moeen NaqviNo ratings yet
- 11 - Units and Measurement 08.08Document1 page11 - Units and Measurement 08.08sayalis1604No ratings yet
- Chemical Engineering Objective Type Questions Reaction KineticsDocument18 pagesChemical Engineering Objective Type Questions Reaction KineticsSaakshi Sharma67% (3)
- Ans 6.1 (B)Document6 pagesAns 6.1 (B)kailin.kelly528No ratings yet
- Class-12 Chemistry ElectroDocument4 pagesClass-12 Chemistry ElectroHemant ChaudharyNo ratings yet
- CHEMISTRY 12th SubejctiveDocument3 pagesCHEMISTRY 12th SubejctiveVivek SanwalNo ratings yet
- Adama Science and Technology UniversityDocument34 pagesAdama Science and Technology UniversityAme ShumetaNo ratings yet
- Chemical Kinetics - Practice Sheet - VIJETA SERIES CLASS-12THDocument6 pagesChemical Kinetics - Practice Sheet - VIJETA SERIES CLASS-12THrachoudhary9741No ratings yet
- CRE-1 - Mid Sem 5Document2 pagesCRE-1 - Mid Sem 5Aaditya TyagiNo ratings yet
- Chemistry Capsule 30Document32 pagesChemistry Capsule 30Rohith SNo ratings yet
- ChE Objective Type Questions Compilation Dean Medina 8 27 10Document177 pagesChE Objective Type Questions Compilation Dean Medina 8 27 10BEA GEDELYN GARCIANo ratings yet
- Aqa 1 5Document19 pagesAqa 1 5leonidas.wujieweiNo ratings yet
- EquilibriumDocument4 pagesEquilibriumbalramsharmaNo ratings yet
- MCQs For Class XII ChemistryDocument29 pagesMCQs For Class XII Chemistryjkc collegeNo ratings yet
- Suggesion CRE 2022Document14 pagesSuggesion CRE 2022Soumyodeep ChowdhuryNo ratings yet
- HT TP: //qpa Pe R.W But .Ac .In: 2011 Chemistry-IDocument7 pagesHT TP: //qpa Pe R.W But .Ac .In: 2011 Chemistry-IKaushikBoseNo ratings yet
- Chemical KineticsDocument4 pagesChemical KineticsNUCLEAR GAMINGNo ratings yet
- Chemical KineticsDocument32 pagesChemical KineticsTimothy HandokoNo ratings yet
- 16PCH1103Document22 pages16PCH1103MoneeshsabapathiNo ratings yet
- Chemical Kinetics MCQ 2Document16 pagesChemical Kinetics MCQ 2vijayresup123No ratings yet
- 12 Chemistry Imp ch4 3 PDFDocument14 pages12 Chemistry Imp ch4 3 PDFrahul gautamNo ratings yet
- The To Prepare For Only App You NeedDocument49 pagesThe To Prepare For Only App You NeedAparajita K SinghNo ratings yet
- 4.chemical Kinetics KCET PYQsDocument2 pages4.chemical Kinetics KCET PYQsPunith kumar100% (4)
- Chemical KineticsDocument49 pagesChemical KineticsS KNo ratings yet
- Final Exam CRIM ADGEDocument9 pagesFinal Exam CRIM ADGEBernard Agcanas FelipeNo ratings yet
- Chemical Kinetics MCQ (Class 12) : - Eart EartDocument4 pagesChemical Kinetics MCQ (Class 12) : - Eart EartBuri MtmNo ratings yet
- Kit - 618XR Parts Required: No Description BOM. QtyDocument8 pagesKit - 618XR Parts Required: No Description BOM. Qtyzarul arjuna0% (1)
- High Density-Pp Low Noise Drainage System: Astral Poly Technik LimitedDocument4 pagesHigh Density-Pp Low Noise Drainage System: Astral Poly Technik LimitedAbdullaKakkadKarumbilNo ratings yet
- Electronics Project List PDFDocument13 pagesElectronics Project List PDFvinothNo ratings yet
- NTC Thermistor RT Curves: WWW - Beta.dk WWW - Beata.se WWW - Betafinland.fiDocument9 pagesNTC Thermistor RT Curves: WWW - Beta.dk WWW - Beata.se WWW - Betafinland.fibasurator2001No ratings yet
- FW6A Manual CuerpoDocument11 pagesFW6A Manual CuerpoHenrry trespalaciosNo ratings yet
- Week 5 Writing Task 1 Line GraphDocument15 pagesWeek 5 Writing Task 1 Line GraphLê Tú QuyênNo ratings yet
- La-Gonave Seminar Presentation-2010Document18 pagesLa-Gonave Seminar Presentation-2010Daniel DaréusNo ratings yet
- Cbse Class - Xi Chemistry Sample Paper 2: Time: 3 Hours Marks: 70 General InstructionsDocument6 pagesCbse Class - Xi Chemistry Sample Paper 2: Time: 3 Hours Marks: 70 General InstructionsBhabaniNo ratings yet
- SUST1000 Interim Report #1Document14 pagesSUST1000 Interim Report #1Kavya Geetha BalajiNo ratings yet
- Ceramic CompositesDocument29 pagesCeramic CompositesAbhey DograNo ratings yet
- Batch19 (DC-DC Converter) PDFDocument56 pagesBatch19 (DC-DC Converter) PDFramNo ratings yet
- SpecificationsDocument8 pagesSpecificationscristianNo ratings yet
- RRF 5Document21 pagesRRF 5Porkkodi SugumaranNo ratings yet
- Bent RuleDocument4 pagesBent RuleHARSHIT KHANNANo ratings yet
- Manual Tehnic ADI-CD - 2013Document79 pagesManual Tehnic ADI-CD - 2013Vlad BalanNo ratings yet
- Current TransformersDocument19 pagesCurrent TransformersyugoplodNo ratings yet
- PT. Anugerah Mega Energi Daily Report: Work Time: 07.00 18.00 Work Time: 07.00 18.00Document1 pagePT. Anugerah Mega Energi Daily Report: Work Time: 07.00 18.00 Work Time: 07.00 18.00heri_prasetyadiNo ratings yet
- Wiring Diagram Outdoor Emergency Lighting Units: Installation Instructions Model: Wgc42FeDocument2 pagesWiring Diagram Outdoor Emergency Lighting Units: Installation Instructions Model: Wgc42FeMario WiryaNo ratings yet
- Presentation On Si and Ci EngineDocument16 pagesPresentation On Si and Ci EngineAman Jain0% (1)
- 4 Amps N-Channel MOSFET: 600voltsDocument5 pages4 Amps N-Channel MOSFET: 600voltsAlir AlichyNo ratings yet
- Bps2021pdf CompressedDocument234 pagesBps2021pdf Compressedkuldeep singhNo ratings yet
- Compressed Gas CylinderDocument4 pagesCompressed Gas Cylinderiwan azaNo ratings yet
- De 19977 Marketing EagleBurgmann DMS MSE E5 PDF CatalogMechanicalseals, Magneticcouplings en 19.09.2016Document146 pagesDe 19977 Marketing EagleBurgmann DMS MSE E5 PDF CatalogMechanicalseals, Magneticcouplings en 19.09.2016sanjeevvangeNo ratings yet
- CV 101Document4 pagesCV 101frco1504No ratings yet
- Liebert Ita Datasheet 5kva To 20kva 2Document4 pagesLiebert Ita Datasheet 5kva To 20kva 2HafidGaneshaSecretrdreamholicNo ratings yet
- Lab 3Document7 pagesLab 3jisteeleNo ratings yet