Professional Documents
Culture Documents
75%(4)75% found this document useful (4 votes)
3K views1996 AL Chemistry Marking Scheme
1996 AL Chemistry Marking Scheme
Uploaded by
api-3738841Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as PDF or read online from Scribd
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5834)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1093)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (852)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (903)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (541)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (350)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (824)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (405)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- AL 1991 Physics Marking Scheme IIADocument12 pagesAL 1991 Physics Marking Scheme IIAapi-37388410% (3)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- 1998 AL Chemistry Paper 1+2 Marking SchemeDocument36 pages1998 AL Chemistry Paper 1+2 Marking Schemeapi-3738841100% (1)
- 2002 AL Chemistry Paper 1+2 Marking SchemeDocument36 pages2002 AL Chemistry Paper 1+2 Marking Schemeapi-373884138% (8)
- 1998 AL Chemistry Paper 1+2 Marking SchemeDocument36 pages1998 AL Chemistry Paper 1+2 Marking Schemeapi-3738841100% (1)
- AL 1994 Physics Marking Scheme IIBDocument14 pagesAL 1994 Physics Marking Scheme IIBapi-3738841No ratings yet
- AL 1991 Physics Marking SchemeDocument22 pagesAL 1991 Physics Marking Schemeapi-373884150% (2)
- AL 1990 Physics Marking Scheme IIADocument13 pagesAL 1990 Physics Marking Scheme IIAapi-373884167% (3)
- 1993 AL Chemistry Paper I Marking SchemeDocument7 pages1993 AL Chemistry Paper I Marking Schemeapi-373884150% (2)
- 1990 AL Chemistry Paper I Marking SchemeDocument11 pages1990 AL Chemistry Paper I Marking Schemeapi-373884160% (5)
- 2000 AL Chemistry Paper II Marking SchemeDocument15 pages2000 AL Chemistry Paper II Marking Schemeapi-3738841No ratings yet
- 1994 AL Chemistry Paper I Marking SchemeDocument10 pages1994 AL Chemistry Paper I Marking Schemeapi-3738841100% (1)
- 1997 AL Chemistry Paper I Marking SchemeDocument22 pages1997 AL Chemistry Paper I Marking Schemeapi-3738841No ratings yet
- 1995 AL Chemistry Paper 1 Marking SchemeDocument21 pages1995 AL Chemistry Paper 1 Marking Schemeapi-3738841100% (1)
- 1999 AL Chemistry Marking SchemeDocument34 pages1999 AL Chemistry Marking Schemeapi-373884125% (4)
- 1997 AL Chemistry Paper 2 Marking SchemeDocument12 pages1997 AL Chemistry Paper 2 Marking Schemeapi-37388410% (2)
- 2000 AL Chemistry Paper I Marking SchemeDocument20 pages2000 AL Chemistry Paper I Marking Schemeapi-3738841No ratings yet
- 2003 AL Chemistry Paper 1 Marking SchemeDocument16 pages2003 AL Chemistry Paper 1 Marking Schemeapi-3738841100% (1)
- 2001 AL Chemistry Paper II Marking SchemeDocument16 pages2001 AL Chemistry Paper II Marking Schemeapi-373884163% (8)
- AL 2001 Physics Marking SchemeDocument16 pagesAL 2001 Physics Marking Schemeapi-373884125% (8)
- 1999 AL Chemistry Marking SchemeDocument34 pages1999 AL Chemistry Marking Schemeapi-373884125% (4)
- AL 1992 Physics Marking SchemeDocument22 pagesAL 1992 Physics Marking Schemeapi-373884186% (7)
- 1997 AL Chemistry Paper 2 Marking SchemeDocument12 pages1997 AL Chemistry Paper 2 Marking Schemeapi-37388410% (2)
- AL 1996 Physics Marking SchemeDocument14 pagesAL 1996 Physics Marking Schemeapi-3738841100% (4)
- AL 1997 Physics Marking SchemeDocument15 pagesAL 1997 Physics Marking Schemeapi-373884167% (3)
- AL 1995 Physics Marking Scheme IIBDocument7 pagesAL 1995 Physics Marking Scheme IIBapi-3738841100% (1)
- AL 1993 Physics Marking SchemeDocument16 pagesAL 1993 Physics Marking Schemeapi-3738841100% (3)
- AL 1999 Physics Marking SchemeDocument15 pagesAL 1999 Physics Marking Schemeapi-373884150% (2)
- AL 1994 Physics Marking Scheme IADocument14 pagesAL 1994 Physics Marking Scheme IAapi-3738841100% (2)
- AL 2000 Physics Marking SchemeDocument40 pagesAL 2000 Physics Marking Schemeapi-373884180% (10)
- AL 1998 Physics Marking SchemeDocument15 pagesAL 1998 Physics Marking Schemeapi-373884175% (8)
1996 AL Chemistry Marking Scheme
1996 AL Chemistry Marking Scheme
Uploaded by
api-373884175%(4)75% found this document useful (4 votes)
3K views34 pagesCopyright
© Attribution Non-Commercial (BY-NC)
Available Formats
PDF or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as PDF or read online from Scribd
Download as pdf
75%(4)75% found this document useful (4 votes)
3K views34 pages1996 AL Chemistry Marking Scheme
1996 AL Chemistry Marking Scheme
Uploaded by
api-3738841Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as PDF or read online from Scribd
Download as pdf
You are on page 1of 34
4
3
Lo hued
ks
Answer ALL questions in this Sestion. Write your answers in the 5
FRIAR Bal PUN TEA Uw ee
'
SECTION A | b
provided
1. @)—@_ Write dowa the number of neutrons, protons and electrons in one atom of carbon-12,
"2C, and in one atom of carbon-13,-!? C
a S 6p, 6e et
cn, 6p, 60, ° ey
Gi) The isotopic mass of '7C is 12,000 atomic mass unit (a.mu). Calculate the
mass, in kg, of | mol of '*C atoms,
(Lamu, = 1.6605 x 10°” kg; Avogadro constant, L = 6.0221x 10 mol“)
Mass of 1 mole of C= 12,000 x 1.6605 x 10°" x 6.0221 x 10 1
= (0.011996) = 0.0120 kg 1
(Accept answers which could round off to 0.012)
(ii) The following data were obtained from the mass spectrum of a carbon-containing
compound
—————
Ton | Mass/a.m.u. Relative intensity
Ret 12,000 100.00
Ber | 13.003 12
Using the above data, calculate the relative atomic mass of carbon.
12.000 x 100+13003 x 112
relative atomic mass = 12000» 100+ 15008 117 5 1
(100+112)
= 121456336 51 :
ToLi2
(Accept answers which could round off o 12.01)
(Deduct % marks if unit is given)
(S marks)
96-AL-CHEM 1-2
DORR Seen Sees FOR TFACHFRS’ USE ONLY -
; ApRaeaS Ba FOR TEACHERS’ USE ONLY
5
7
/
7 )/ Carbon-14, '*C, is radioactive, emitting B-particles. a
@ ‘Write an equation for the decay of '*C.
: Ho Be IN or He ap+ "IN on 1
| (i) A charcoal sample from the ruins of an ancient settlement was found to have
a 4Cv!2C ratio 0.60 times that found in living organisms,
(Explain why the '*C/!C ratio in the charcoal sample is smaller than that
in living organisms. .
In the piece of charcoal intake of C stops; decay of “ %
“Ccontinues while "°C remainsconstant -. “C/"*C ratio drops %
id 2) Given that the halflife for the decay of Cis 5730 years, calculate the age
= of the charcoal sample,
4 (Note : The integrated form of the rate expression for radioactive decay can
be represented by the following equation :
No) 0301
on) «
where No is the initial number of radioactive nuclei ;
N, is the number of radioactive nuclei at time t
tua is the half-life for the decay)
i
3
a
ret
(Deduct % marks for wrong /no unit)
ai kid
(iii) All radioactive decay has zer0 activation energy. Comment on the effect of
temperature upon the rate of decay.
tooisid Gk
According to Arrhenius Equation, rate of decay is independent of temperature
€ (S marks)
t= A
96-AL-CHEM 1-3
Dre seer snea COR TEACHERS’ USE ONLY
7
oJ LAD
fund G22 tui EQ La Et
Ams FOR TEACHERS’ USE ONLY
(© Explain why
(@ the fist ionization energy of oxygen is greater than that of sulphur’
: | Shas 1 more ¢” she!! IESNRAUMMMIBIIBA han O, the attraction experienced “~
by the outermost electron is smaller hence § has a smaller LE. %
(DO NOT accept explanations in terms of effective nuclear charge; deduct 4 marks for
any misconception) a
(ii) the first ionization energy of oxygen is smaller than that of fluorine.
F has | more proton in the nucleus, the effect of increase in nuclear charge has %
outweighed the! of the additional electron hence F hasa greater LE.
or, Effective nuclear charge experienced by outermost e” of atom increases across a period.
‘outermost e” of O is more easily removed than that of F. o
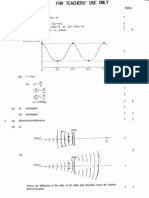
(@ Sketch the pictorial representation of ap orbital and indicate the location of the nucleus in
your diagram, wi
nucleus > (or along any axis) Vo
(1 mark)
(©) At 298 K, $0 cm’ of a solution of In in CCl. were mixed and shaken with 200 cm’ of distilled
water ina separating funnel until equilibrium was attained. The two layers were then
separated, 20.0 cm? of the CCl layer required 12.15 cm? of 0.105 M $,03"(eq) for titration,
‘whereas 50.0 cm? of the aqueous layer required 8.25 cm? of 0.0050 M S:03"(aq),
(Calculate the distribution coefficient of Iz between water and CCl, at 298 K.
82500050
ratio of concentration ofa: TO * TET OOF 1
20
= 0.012934 = 0.013 - 1
(Accept distribution coefficient expressed as {lx(CCL)V/(la(@q)], in which case
the value calculated is 77.3 (77)
(Award 14 marks for a correct expression of Ky )
Gi) the 200 em’ of distilled water contain some dissolved KI, will this affect the value
of the distribution coefficient ? Briefly explain
Nochange + %
because distribution coefficient is a function of temperature only %
(Also accept K will change because there is a change in ionic strength/activity (1/0))
G marks)
96-AL-CHEM I++
=e eer ee ee ran TrerucDe? Tee ANI V
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5834)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1093)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (852)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (903)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (541)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (350)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (824)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (405)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- AL 1991 Physics Marking Scheme IIADocument12 pagesAL 1991 Physics Marking Scheme IIAapi-37388410% (3)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- 1998 AL Chemistry Paper 1+2 Marking SchemeDocument36 pages1998 AL Chemistry Paper 1+2 Marking Schemeapi-3738841100% (1)
- 2002 AL Chemistry Paper 1+2 Marking SchemeDocument36 pages2002 AL Chemistry Paper 1+2 Marking Schemeapi-373884138% (8)
- 1998 AL Chemistry Paper 1+2 Marking SchemeDocument36 pages1998 AL Chemistry Paper 1+2 Marking Schemeapi-3738841100% (1)
- AL 1994 Physics Marking Scheme IIBDocument14 pagesAL 1994 Physics Marking Scheme IIBapi-3738841No ratings yet
- AL 1991 Physics Marking SchemeDocument22 pagesAL 1991 Physics Marking Schemeapi-373884150% (2)
- AL 1990 Physics Marking Scheme IIADocument13 pagesAL 1990 Physics Marking Scheme IIAapi-373884167% (3)
- 1993 AL Chemistry Paper I Marking SchemeDocument7 pages1993 AL Chemistry Paper I Marking Schemeapi-373884150% (2)
- 1990 AL Chemistry Paper I Marking SchemeDocument11 pages1990 AL Chemistry Paper I Marking Schemeapi-373884160% (5)
- 2000 AL Chemistry Paper II Marking SchemeDocument15 pages2000 AL Chemistry Paper II Marking Schemeapi-3738841No ratings yet
- 1994 AL Chemistry Paper I Marking SchemeDocument10 pages1994 AL Chemistry Paper I Marking Schemeapi-3738841100% (1)
- 1997 AL Chemistry Paper I Marking SchemeDocument22 pages1997 AL Chemistry Paper I Marking Schemeapi-3738841No ratings yet
- 1995 AL Chemistry Paper 1 Marking SchemeDocument21 pages1995 AL Chemistry Paper 1 Marking Schemeapi-3738841100% (1)
- 1999 AL Chemistry Marking SchemeDocument34 pages1999 AL Chemistry Marking Schemeapi-373884125% (4)
- 1997 AL Chemistry Paper 2 Marking SchemeDocument12 pages1997 AL Chemistry Paper 2 Marking Schemeapi-37388410% (2)
- 2000 AL Chemistry Paper I Marking SchemeDocument20 pages2000 AL Chemistry Paper I Marking Schemeapi-3738841No ratings yet
- 2003 AL Chemistry Paper 1 Marking SchemeDocument16 pages2003 AL Chemistry Paper 1 Marking Schemeapi-3738841100% (1)
- 2001 AL Chemistry Paper II Marking SchemeDocument16 pages2001 AL Chemistry Paper II Marking Schemeapi-373884163% (8)
- AL 2001 Physics Marking SchemeDocument16 pagesAL 2001 Physics Marking Schemeapi-373884125% (8)
- 1999 AL Chemistry Marking SchemeDocument34 pages1999 AL Chemistry Marking Schemeapi-373884125% (4)
- AL 1992 Physics Marking SchemeDocument22 pagesAL 1992 Physics Marking Schemeapi-373884186% (7)
- 1997 AL Chemistry Paper 2 Marking SchemeDocument12 pages1997 AL Chemistry Paper 2 Marking Schemeapi-37388410% (2)
- AL 1996 Physics Marking SchemeDocument14 pagesAL 1996 Physics Marking Schemeapi-3738841100% (4)
- AL 1997 Physics Marking SchemeDocument15 pagesAL 1997 Physics Marking Schemeapi-373884167% (3)
- AL 1995 Physics Marking Scheme IIBDocument7 pagesAL 1995 Physics Marking Scheme IIBapi-3738841100% (1)
- AL 1993 Physics Marking SchemeDocument16 pagesAL 1993 Physics Marking Schemeapi-3738841100% (3)
- AL 1999 Physics Marking SchemeDocument15 pagesAL 1999 Physics Marking Schemeapi-373884150% (2)
- AL 1994 Physics Marking Scheme IADocument14 pagesAL 1994 Physics Marking Scheme IAapi-3738841100% (2)
- AL 2000 Physics Marking SchemeDocument40 pagesAL 2000 Physics Marking Schemeapi-373884180% (10)
- AL 1998 Physics Marking SchemeDocument15 pagesAL 1998 Physics Marking Schemeapi-373884175% (8)