Professional Documents
Culture Documents
Recombinant Protein Expression and Purification A Comprehensive Review of Affinity
Recombinant Protein Expression and Purification A Comprehensive Review of Affinity
Uploaded by
Aditi VermaOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Recombinant Protein Expression and Purification A Comprehensive Review of Affinity
Recombinant Protein Expression and Purification A Comprehensive Review of Affinity
Uploaded by
Aditi VermaCopyright:
Available Formats
Biotechnology DOI 10.1002/biot.201100155 Biotechnol. J.
2012, 7, 620–634
Journal
Review
Recombinant protein expression and purification:
A comprehensive review of affinity tags and microbial applications
Carissa L. Young1, Zachary T. Britton1 and Anne S. Robinson1,2
1 Department of Chemical Engineering, University of Delaware, Newark, DE, USA
2 Department of Chemical and Biomolecular Engineering, Tulane University, New Orleans, LA, USA
Protein fusion tags are indispensible tools used to improve recombinant protein expression yields,
Received 22 July 2011
enable protein purification, and accelerate the characterization of protein structure and function. Revised 23 November 2011
Solubility-enhancing tags, genetically engineered epitopes, and recombinant endoproteases have Accepted 29 November 2011
resulted in a versatile array of combinatorial elements that facilitate protein detection and purifi-
cation in microbial hosts. In this comprehensive review, we evaluate the most frequently used sol-
ubility-enhancing and affinity tags. Furthermore, we provide summaries of well-characterized pu-
rification strategies that have been used to increase product yields and have widespread applica-
Supporting information
tion in many areas of biotechnology including drug discovery, therapeutics, and pharmacology.
available online
This review serves as an excellent literature reference for those working on protein fusion tags.
Keywords: Affinity purification · Affinity tags · Proteases · Solubility enhancement
1 Introduction and host cell toxicity are challenges that must be
resolved when microbial hosts are used to express
Recent advances in genomics, proteomics, and recombinant proteins. To address these challenges
bioinformatics have facilitated the use of recombi- of production and purification efficiency, fusion
nant DNA technology in order to evaluate any pro- tags are incorporated to increase expression yields
tein of interest, without prior knowledge of the pro- and influence solubility and native folding; novel
tein’s cellular location or function. The parallel use tags in combination with affinity techniques in-
of affinity tags with recombinant DNA techniques, crease purification yields; and proteases result in
allows the facile modification of proteins of inter- tag removal.
est leading to efficient identification, production, In recent years, numerous fusion tags have been
and isolation from the host system. However, pro- developed for recombinant protein production.
tein insolubility, conformation, stability, and struc- While contemporary reports have included several
tural flexibility, as well as low purification yields overviews of available affinity tags for protein de-
tection or purification [1–4], solubility enhance-
ment [5], tag removal [1], or applications [6, 7] – in
this comprehensive review, we provide an exten-
Correspondence: Dr. Anne S. Robinson, Department of Chemical and
Biomolecular Engineering, Tulane University, 300 Boggs Laboratory, sive summary of various tags and established pu-
New Orleans, LA 70118–5674, USA rification strategies. We describe several design as-
E-mail: asr@tulane.edu pects for these tags that should be considered for
recombinant fusion protein expression, including
Abbreviations: Arg, arginine; DHFR, dihydrofolate reductase; DsbA, protein amino acid composition and size; N- or C-terminal
disulfide isomerase I; GFP, green fluorescent protein; GST, glutathione
fusions, used individually or in tandem; as well as
S-transferase; His, Histidine; IMAC, immobilized metal-affinity chromato-
graphy; KSI, ketosteroid isomerase; MBP, maltose-binding protein;
optimized tags, techniques, and buffer conditions
Ni2+-NTA, Ni(II)-nitrilotriacetic acid; NusA, N-utilization substance A; that lead to purified protein. Additionally, we dis-
SUMO, small ubiquitin related modifier; TAP, tandem affinity purification; cuss the availability of protocols and expression
TEV, tobacco etch virus; TrxA, thioredoxin A vectors through commercial vendors or DNA
620 © 2012 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim
18607314, 2012, 5, Downloaded from https://onlinelibrary.wiley.com/doi/10.1002/biot.201100155, Wiley Online Library on [15/10/2023]. See the Terms and Conditions (https://onlinelibrary.wiley.com/terms-and-conditions) on Wiley Online Library for rules of use; OA articles are governed by the applicable Creative Commons License
Biotechnol. J. 2012, 7, 620–634 www.biotechnology-journal.com
repositories. Details of these plasmid-based de- purified after solubilization with mild detergents
signs and methods are available in the Supporting [14]. GST expression vectors, such as the pGEX se-
information. ries from GE Healthcare, commonly include specif-
ic protease cleavage sites between the tag and part-
ner proteins. Thus, the GST-tag can readily be re-
2 Using protein tags to improve yields of moved from GST fusion proteins after or during
recombinant proteins purification, where affinity for immobilized glu-
tathione greatly simplifies purification of the pro-
2.1 Enhancing protein expression and/or solubility tein of interest from cleavage products. The GST-
tag has been used as an N- or C-terminal tag in a
2.1.1 Fusion proteins affecting solubility variety of expression systems, including bacteria
A common method to overcome expression obsta- [8, 14], yeast [15–17], insect cells [18, 19], and mam-
cles is to use a fusion protein strategy. In this strat- malian cells [20].
egy, a difficult-to-express protein or peptide is In addition to advantages in expression and pu-
fused to one or more other proteins to stabilize ex- rification, GST-tagged fusion proteins have proved
pression in the soluble or insoluble fraction, with useful in studies on protein–DNA interactions [19,
the idea that the fusion protein will drive the re- 21], protein–protein interactions [22, 23], and as
sulting expression. A number of fusion proteins antigens for vaccine studies [24].
have been used for this purpose, including, glu-
tathione S-transferase (GST), maltose-binding 2.1.1.1.2 Maltose-binding protein
protein (MBP), thioredoxin A (TrxA), small ubiqui- Maltose-binding protein (MBP) is a 42 kDa protein
tin related modifier (SUMO), ketosteroid isomerase encoded by the malE gene of Escherichia coli K12
(KSI), and TrpΔLE, which are outlined briefly be- [25]. MBP fusion proteins have been utilized for
low and in Supporting information, Table S1. single-step purification by affinity to cross-linked
While providing potential advantages in protein amylose [26]. MBP fusion proteins bound to immo-
expression and solubility, the fusion protein strate- bilized amylose are eluted under non-denaturing
gy also presents a new set of issues, including the conditions with 10 mM maltose. MBP fused to ei-
removal of the carrier protein and questions re- ther the N- and C-terminus has been shown to in-
garding whether or not the protein of interest re- crease the expression and folding of eukaryotic fu-
tains native structure and activity. For example, car- sion proteins expressed in bacteria [27–31]. Al-
rier proteins may sterically hinder native complex though not completely understood [32], fusion of
formation. Ultimately, parallel analysis of many fu- MBP onto a protein has been shown to enhance the
sion proteins may be required to generate the solubility of the partner protein [31, 33–35]. There
yields and properties of the protein of interest for are a number of vectors for generating MBP fusion
the intended application. proteins that are commercially available, including
the pMAL series from New England Biolabs and
2.1.1.1. Proteins that increase solubility pIVEX series from Roche, which contain a specific
2.1.1.1.1 Glutathione s-transferase protease cleavage site in the region between MBP
Glutathione S-transferase (GST) from Schistosoma and the multiple cloning site. In addition, there are
japonicum is a 26 kDa protein that has been utilized also commercial vectors for the expression of MBP
for single-step purification of fusion proteins [8]. fusion proteins to the non-reducing environment
GST fusion proteins can be purified from crude of the E. coli periplasm (e.g., New England Biolabs
lysate by affinity for immobilized glutathione and pMAL), addition of which may improve the folding
eluted under non-denaturing conditions with of disulfide bond-containing proteins. Although
10 mM reduced glutathione. GST fusion proteins not generally required, MBP has been used in con-
can be detected using an enzymatic assay or im- junction with a small affinity tag to improve purifi-
munoassay. In many cases, the GST-tag protects cation purity [36, 37].
against intracellular proteolysis and stabilizes the
recombinant protein in the soluble fraction as 2.1.1.1.3 Thioredoxin A
monomers or homodimers [9–13]. However, GST is Thioredoxins are universal oxido-reductaces that
considered to be a poor solubility enhancer as some reduce disulfide bonds through thio-disulfide ex-
GST fusion proteins are wholly or partly insoluble. change. One of the E. coli thioredoxins, TrxA, is an
Proteins fused to GST that are enriched in either 11.6 kDa protein that demonstrates high solubility
hydrophobic regions or charged residues or are in the E. coli cytoplasm and inherent thermal sta-
larger than 100 kDa may contribute to insoluble ex- bility, which may be conferred to TrxA fusion pro-
pression. Insoluble GST fusion proteins may be teins. For example, TrxA has been used as an N- or
© 2012 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim 621
Biotechnology
18607314, 2012, 5, Downloaded from https://onlinelibrary.wiley.com/doi/10.1002/biot.201100155, Wiley Online Library on [15/10/2023]. See the Terms and Conditions (https://onlinelibrary.wiley.com/terms-and-conditions) on Wiley Online Library for rules of use; OA articles are governed by the applicable Creative Commons License
Biotechnol. J. 2012, 7, 620–634
Journal
C-terminal fusion protein [38, 39] to increase solu- sult from the biological activity of NusA. NusA
ble protein expression of recombinant proteins slows translation and permits more time for critical
[40]. In addition to enhancing the solubility of fu- folding events to occur [60]. A direct comparison of
sion proteins, TrxA has been shown to aid in the normally aggregation-prone proteins tagged with
crystallization of proteins. Though in most in- either MBP or NusA showed that both MBP and
stances TrxA must be removed prior to structural NusA enhanced solubility overall while not affect-
characterization, TrxA when joined with the linker ing the structure of the aggregation-prone proteins
sequence GSAM aided crystallization of the U2AF [32].
homology motif domain of splicing factor Puf60
[41]. Unlike GST or MBP, TrxA does not facilitate 2.1.1.1.6 Protein disulfide isomerase I (DsbA)
purification on its own; small affinity tags are com- Protein disulfide isomerase I (DsbA) is a 21.1 kDa
monly used in conjunction with TrxA to enable pu- protein that catalyzes the formation of disulfide
rification. bonds in E. coli [62, 63]. When fused to eukaryotic
proteins, an inactive mutant of DsbA that lacked
2.1.1.1.4 Small ubiquitin-related modifier (SUMO) the periplasmic signal sequence was shown to pro-
In its native system, small ubiquitin-like modifier mote the soluble expression of proteins in the
(SUMO) protein, Saccharomyces cerevisiae Smt3, is E. coli cytoplasm [64]. Furthermore, a comparison of
a form of post-translational modification that plays DsbA and thioredoxin fusion proteins showed that
roles in nuclear-cytosolic transport [42], apoptosis DsbA increased the solubility of fusion proteins by
[43], protein activation [44], and stability [45], re- two- to three-fold over thioredoxin [64]. The vec-
sponse to stress [46], and the cell cycle [47]. When tors pET-39 and pET-40 available from EMD Bio-
used as an N-terminal carrier protein during sciences enable expression of DsbA and DsbC, re-
prokaryotic expression, SUMO promotes folding spectively, to the non-reducing environment of the
and structural stability, which leads to enhanced E. coli periplasm, which may result in improved sol-
functional production compared to untagged pro- ubility and folding of the target protein [65].
tein [48–54]. Unlike GST and MBP, SUMO does not This section has outlined a number of fusion
itself serve as a means for purification of fusion proteins that increase the solubility of a protein of
proteins; however, the His6 in series with the SUMO interest and it should be clear that there is no uni-
tag has been established to facilitate purification of versal solution to achieve this end. Oftentimes, par-
fusion proteins. One unique advantage of the allel analysis of different fusion proteins must be
SUMO tag over other expression-enhancing carri- sought, as not only does the fusion protein affect
er proteins is a specific SUMO protease (S. cere- properties of the protein of interest but the protein
visiae UlpI), which recognizes and removes the of interest also affects properties of the carrier pro-
SUMO tag at a Gly–Gly motif. tein. Furthermore, the use of carrier proteins, espe-
The wild-type SUMO tag is an excellent carrier cially for those significantly enhancing solubility of
protein in prokaryotic expression systems but is ef- the fusion, presents obstacles for maintaining the
ficiently removed in eukaryotes in vivo by natural- protein of interest in the soluble fraction after the
ly occurring SUMO proteases [55]. LifeSensors, Inc. carrier protein is removed.
has engineered a SUMO-based tag, SUMOstar, that
has been used to enhance protein expression in 2.1.1.1.7 Mistic
yeast [56, 57], insect cells [58], and mammalian Recently, a novel membrane-associating protein
cells [59]. In addition to the SUMOstar carrier pro- from B. subtilis [72] referred to as Mistic has been
tein, LifeSensors, Inc. has also developed a shown to enhance expression levels of eukaryotic
SUMOstar-specific protease. integral membrane proteins in E. coli, when used as
a fusion protein linked to the N-terminus of cargo
2.1.1.1.5 N-utilization substance A (NusA) proteins [73, 74]. Mistic and its orthologs lack an
The transcription termination anti-termination identified signal sequence. Overexpression of these
factor, also known as N-utilization substance A fusion partners does not result in cell toxicity, a
(NusA), is a 55 kDa hydrophilic protein that pro- common result due to overloading the protein
motes the soluble expression of even very hy- translocation machinery. A truncated version of
drophobic fusion proteins in E. coli [60]. In E. coli, Mistic shorter by 25 residues – M110 – expresses
wild-type NusA promotes pauses in DNA tran- primarily in the bacterial membrane [72]. Interest-
scription by RNA polymerase [61]. Because tran- ingly, shorter Mistic proteins maintain comparable
scription and translation are coupled in prokary- levels of expression as M110 even though they are
otes, improvements in soluble expression of NusA soluble in the presence of detergents and are local-
fusion proteins over other fusion proteins may re- ized mainly to the cytoplasm [75]. Mistic’s mecha-
622 © 2012 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim
18607314, 2012, 5, Downloaded from https://onlinelibrary.wiley.com/doi/10.1002/biot.201100155, Wiley Online Library on [15/10/2023]. See the Terms and Conditions (https://onlinelibrary.wiley.com/terms-and-conditions) on Wiley Online Library for rules of use; OA articles are governed by the applicable Creative Commons License
Biotechnol. J. 2012, 7, 620–634 www.biotechnology-journal.com
nism of action remains unclear; however, in some munoprecipitation (IP), co-immunoprecipitation
cases the use of Mistic and its orthologs have sig- (co-IP), immunofluorescence (IF), and affinity pu-
nificantly increased levels of eukaryotic integral rification, epitopes enable the determination of
membrane protein expression in E. coli [73, 74, 76]. protein size, concentration, post-translation modi-
fications, protein interactions, intracellular traf-
2.1.1.2 Proteins that decrease solubility ficking, and in some cases provide a means of ob-
A number of carrier proteins have been utilized to taining pure product (via affinity purification) in
drive expression to inclusion bodies, which pro- order to attain high-resolution crystallographic or
tects them from intracellular degradation, provides NMR structures. The impact of protein engineering
a simple process for recovery, and commonly leads and the extensive use of affinity tags on crystalliza-
to the highest expression yields [66]. Furthermore, tion techniques and structure/function studies
expression to inclusion bodies may be required in have been reviewed elsewhere [77–80]. Described
cases where protein production is toxic to the host below are numerous affinity tags commonly used
cell. in microbial systems, as well as relevant caveats for
Despite its advantages, expression to inclusion their use. Well-characterized purification schemes
bodies requires solubilization with chaotropes or are briefly summarized and relevant protocols for
detergents and subsequent refolding steps that novel purification strategies are appropriately ref-
may ultimately affect biological activity. Therefore, erenced. Additional details are provided in Sup-
use of fusion proteins that direct expression to in- porting information, Tables S2 and S3 with refer-
clusion bodies is generally limited to small easily ences cited therein.
refolded proteins and peptides. Two carrier pro-
teins that direct expression to inclusion bodies in- 3.1.1 c-myc, HA, and FLAG
clude KSI and the gene product of E. coli TrpE. Traditionally, c-myc, hemaglutinin antigen (HA),
and FLAG epitopes have been used primarily
2.1.1.2.1 Ketosteroid isomerase (KSI) as a means of protein detection. An epitope of
Ketosteroid isomerase is an extremely insoluble the human c-myc proto – oncogene product
13-kDa protein [67, 68] that efficiently forms inclu- (EQKLISEEDL) was one of the earliest affinity tags
sion bodies. Insoluble expression KSI fusion pro- developed and is highly specific to the anti-c-myc
teins are commonly employed for the production of antibody 9E10 [81]. Fused to either the N- or C- ter-
recombinant peptides and used in conjunction with minus [82], the c-myc tag is widely used in protein
purification tags. Utilization of KSI as a carrier pro- engineering approaches, including western blot
tein has increased protein production levels by analysis, IP, co-IP, IF, and flow-cytometry [83] in
two- to five-fold when compared to other methods both bacteria and yeast. Although rarely used for
[68]. protein purification, it is possible to couple the
mAb9E10 to divinyl sulfone-activated agarose and
2.1.1.2.2 TrpΔLE purify c-myc-tagged proteins under physiological
The truncated gene product of E. coli TrpE (Trp- conditions; however, elution requires a low pH that
ΔLE), a 27-kDa protein, has also been used to direct may adversely affect protein activity. Despite these
expression of fusion proteins to inclusion bodies concerns, structural studies have been performed
[69–71]. using c-myc fusion proteins purified in this way
[84].
Similarly, as one of the most widely used epitope
3 Facilitating affinity purification and tags in cell biology, the HA tag is a nine amino acid
emerging combinatorial tags sequence (YPYDVPDYA) derived from the human
influenza virus hemaglutinin protein [85, 86]. The
3.1 Selection of affinity tags and utility hemaglutinin epitope may be located within the
DNA coding region of a protein, or at the N- or C-
Affinity tags are highly efficient tools for protein terminus [87]. However, the hemaglutinin epitope
detection, characterization, and purification. An may influence trafficking, folding, and function of
epitope is a short sequence of amino acids that typ- the target protein [88, 89].
ically serves as the antigenic determinant, or the The FLAG epitope is a short, hydrophilic oc-
region to which an antibody binds. Thus, epitope tapeptide (DYKDDDDK) that can be used for anti-
tagging is a technique in which a short sequence body-based purification [90]. A unique aspect of
(i.e., epitope) is added to a protein of interest by re- FLAG utilization is the inherent enterokinase
combinant DNA methods. Used in a variety of ap- cleavage site located within the five C-terminal
plications including western blot analysis, im- residues of the peptide sequence [91], which is ad-
© 2012 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim 623
Biotechnology
18607314, 2012, 5, Downloaded from https://onlinelibrary.wiley.com/doi/10.1002/biot.201100155, Wiley Online Library on [15/10/2023]. See the Terms and Conditions (https://onlinelibrary.wiley.com/terms-and-conditions) on Wiley Online Library for rules of use; OA articles are governed by the applicable Creative Commons License
Biotechnol. J. 2012, 7, 620–634
Journal
vantageous if the recombinant protein of interest is Following purification, the C-terminus of polyArg-
intended for therapeutic use, since the epitope it- tagged proteins can be removed by carboxypepti-
self is immunogenic. The FLAG peptide sequence dase B, although poor cleavage yields and non-
is recognized by the antibody M1, in either a calci- specificity have been reported [104]. It is important
um-dependent [92], or independent [93], manner; to note that the polyArg tag may affect the tertiary
however, additional commercial mAb choices in- structure of proteins whose C-terminus is hy-
clude M2 and M5, which comprise slightly different drophobic [103], despite its use as a standard im-
recognition sites and affinity. FLAG-tagged fusion mobilization method for electron and scanning-
proteins can be purified using an immobilized probe microscopy applications [105]. This is per-
monoclonal antibody matrix under non-denatur- haps not surprising because of the addition of the
ing conditions and eluted by lowering the pH or positive charges under neutral and acidic pH con-
adding chelating agents such as EDTA. However, ditions. This may also contribute to the observation
antibody-based purification matrices are in gener- that the polyArg tag promotes protein internaliza-
al not as stable as either Ni2+-NTA, used with poly- tion [106].
histidine-tagged recombinant proteins, or Strep- In general, the polyArg tail is not the optimal
Tactin, which is used with Strep-tag II fusions, as purification tag in the protein-engineering field
discussed below. and its use is infrequent. In contrast, another
To increase the signal in protein detection ap- polyamino acid construct, the polyhistidine (poly-
plications, a recombinant protein can be designed His) tags are the most widely used affinity tags for
to include multiple epitope tags in series. Examples purifying recombinant proteins that lead to bio-
include multiple copies of the hemaglutinin [94], physical and structural studies. Advantages of the
c-myc [95, 96], and FLAG [97] where typically three polyHis tag include its low immunogenicity and
copies are used, although nine copies of the c-myc small size (0.84 kDa) – with composition ranging
epitope in series have been incorporated in order from 3 to 10 His tags in series. In addition, many
to detect very dilute proteins in yeast [98]. proteins function with the polyHis tag positioned at
either the N- or C-terminus, and purification meth-
3.1.2 1D4 ods can be carried out under both native and dena-
The 1D4 epitope is a short hydrophilic sequence turing conditions [107, 108].
(TETSQVAPA) derived from the C-terminus of To purify recombinant polyHis-tagged prote-
bovine rhodopsin [99, 100]. Combining this epitope ins, immobilized metal-affinity chromatography
and the high affinity rho-1D4 monoclonal antibody (IMAC) is used to isolate the metal-binding pep-
(available from www.flintbox.com) has established tides based on the interaction between the nega-
useful tools in antibody-based purification, local- tively charged His and transition metal ions (Ni2+,
ization studies, and western blot analysis of 1D4- Co2+, Cu2+, Zn2+) immobilized on a matrix [109]. De-
tagged membrane proteins [101, 102]. Additionally, veloped by Hochuli et al. [110], Ni(II)-nitrilotri-
the 1D4 enrichment strategy offers a highly specif- acetic acid (Ni2+-NTA) exhibits high affinity for ad-
ic, non-denaturing method for purifying mem- jacent histidine residues; provides an inexpensive
brane proteins with yields and purities consistent matrix that withstands multiple regeneration cy-
with structural characterization and functional cles under stringent conditions; and permits disso-
proteomics applications [101]. ciation of bound recombinant proteins by an imi-
dazole gradient (20 to 250 mM for polyHis-tagged
3.1.3 polyArg and polyHis fusion proteins; [111, 112]), changes in pH, or met-
The first commercially available affinity tags were al chelation. During purification, non-specific
designed from naturally occurring epitopes and binding of host proteins can occur and may be
used primarily for purification. Yet, during the past overcome by shorter incubation steps (3 h or less),
decade novel engineered tags have been optimized or a low concentration of imidazole, where typical-
for use in many cell types and for purification un- ly 2–50 mM is recommended in column washes. It
der various expression conditions (i.e., inclusion is important to note that the use of imidazole may
bodies, membrane localization). result in protein aggregates and affect activity,
The polyarginine (polyArg) tag was initially competition studies, NMR experiments, or crystal-
used by Sassenfeld and Brewer [103] and typically lographic trials [112]; therefore, dialysis is recom-
consists of five or six consecutive arginines at the mended in order to remove residual imidazole fol-
C-terminal end of a recombinant protein. A protein lowing purification. PolyHis-tagged proteins can be
containing the polyArg tag is purified by absorp- purified by IMAC under denaturing conditions, and
tion to cation exchange resin SP-Sephadex, and then refolded (reviewed in [113]).
eluted via a linear NaCl gradient at alkaline pH.
624 © 2012 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim
18607314, 2012, 5, Downloaded from https://onlinelibrary.wiley.com/doi/10.1002/biot.201100155, Wiley Online Library on [15/10/2023]. See the Terms and Conditions (https://onlinelibrary.wiley.com/terms-and-conditions) on Wiley Online Library for rules of use; OA articles are governed by the applicable Creative Commons License
Biotechnol. J. 2012, 7, 620–634 www.biotechnology-journal.com
Since the first recombinant protein to be suc- rification of a recombinant polyHis-tagged protein
cessfully purified with a Ni2+-NTA matrix – Dihy- resulted in increased yields [120].
drofolate reductase (DHFR) fused to a polyHis-tag
expressed in E. coli [114] – novel matrices (i.e., 3.1.4 Streptavidin binding tags
TALON) have been developed for this affinity tag. The original Strep-tag (WRHPQFGG) was selected
When directly compared to Ni2+-NTA, the use of from a random genetic library [121] based on its
TALON has resulted in decreased levels of non- affinity to the streptavidin core – a proteolytically
specifically bound proteins and consequent yields truncated version of the bacterial protein [122]. To
consisting of increased purity [115, 116]. TALON permit greater flexibility and affinity to an engi-
consists of Co2+-carboxylmethylaspartate (Co2+- neered variant of streptavidin, referred to as Strep-
CMA) that is coupled to resin. Following Co2+-CMA Tactin [123, 124], Strep-tag II is a small affinity
matrix development, a natural 19-residue histidine peptide (WSHPQFEK) that was simultaneously
affinity tag [117] with optimal binding to TALON optimized. Strep-tag II is inert, largely resistant to
[115] has been reported. Overexpression with the cellular proteases, can be used with mild deter-
histidine affinity-tag has been demonstrated in gents, and optimal for the purification of recombi-
bacteria with N-terminal fusions to chlorampheni- nant proteins under physiological conditions. How-
col acetyltransferase (CAT), DHFR, and green flu- ever, Strep-tag II is not well suited for purification
orescent protein (GFP) that were eluted by de- strategies that use denaturing conditions. Dissoci-
creasing pH (5.0) or increasing imidazole (150 mM) ation of Strep-tag II from Strep-Tactin occurs with
concentration [115]. More recently, Dong et al. [118] mild buffer conditions in the presence of D-biotin;
have developed an artificial chaperone-assisted or if resin regeneration is preferred, then D-
IMAC method for the refolding and purification of desthiobiotin is used. An advantage of Strep-tag II,
a polyHis-tagged protein from solubilized inclu- when compared to polyHis-tag purification, is its
sion bodies with increased refolding efficiency. independence from metal ions during purification
IMAC technology combined with polyHis-tag strategies. If metalloproteins are the recombinant
recombinant protein expression continues to dom- protein of choice, then the Strep-tag is optimal
inate generic single-step purification strategies [125].
leading to the biophysical manipulations of Strep-tag II fusion proteins have been incorpo-
polypeptides; however, there are a few caveats to rated and retained for protein crystallography [126,
note. Addition of a polyHis-tag may affect the func- 127] and employed in a variety of hosts, including
tion of the targeted protein or result in weak bind- bacteria [128, 129] and yeast [130–132]. Strept-
ing to select matrices as a result of the change in avidin-binding peptide (SBP) is the larger 38 amino
protein charge; however, these aspects may be cor- acid peptide that was selected based on its in-
rected by changing the location of the polyHis-tag creased affinity to streptavidin [133]. SBP-tagged
(i.e., N- vs. C-terminus), incorporating a flexible proteins have been expressed and purified in bac-
polylinker, or increasing the number of histidine teria [134] under mild elution conditions of biotin
residues. As a consequence of the latter aspect, Lee (2 mM).
and Kim [119] have developed a vector-based strat-
egy using a single insert to optimize the molecular 3.1.5 Additional affinity tags used in microbial hosts
engineering of target genes with different polyHis- Alternative affinity purification tags include the
tag lengths. Furthermore, IMAC should not be em- calmodulin-binding peptide, chitin-binding do-
ployed when purifying proteins with a metal center main, cellulose-binding domain, S-tag, and Softag3.
since the metal itself could be adsorbed to the col- Calmodulin-binding peptide consists of 26
umn; and purification should not be attempted un- amino acids derived from the C-terminus of human
der anaerobic conditions, since Ni2+-NTA will be skeletal muscle myosin light chain kinase [135],
reduced, and therefore ineffective, under that envi- which tightly binds calmodulin in the presence of
ronment. The presence of inherent cysteine- or 0.2 mM CaCl2 [136]. Nanomolar affinity ensures
histidine-rich regions in host proteins may result that stringent washing conditions result in few con-
in non-specific binding, leading to yield contami- taminating proteins and pure yields of recombi-
nation or decreased purification efficiency. Subse- nant proteins. Detergents (up to 0.1%) and reducing
quently, a strategy has been designed to increase agents are compatible during recovery steps of
E. coli product yields by engineering a strain to be calmodulin-binding peptide fusions [137]. Calmod-
deficient in three prevalent host proteins, which ulin-binding peptide is only suitable for expression
under native conditions are a significant fraction of and purification in bacterial hosts since no en-
the eluate [120]; as a result of gene knockouts, pu- dogenous host proteins interact with calmodulin.
In eukaryotes including yeast, this affinity tag may
© 2012 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim 625
Biotechnology
18607314, 2012, 5, Downloaded from https://onlinelibrary.wiley.com/doi/10.1002/biot.201100155, Wiley Online Library on [15/10/2023]. See the Terms and Conditions (https://onlinelibrary.wiley.com/terms-and-conditions) on Wiley Online Library for rules of use; OA articles are governed by the applicable Creative Commons License
Biotechnol. J. 2012, 7, 620–634
Journal
disrupt calcium-signaling pathways and induce ically used as a dual-tagging system that incorpo-
protein–protein interactions in a calcium-depend- rates selective protease cleavage sites.
ent manner [138]. Softag3 is an 8-residue (TKDPSRVG) peptide
Similarly, fusions tags consisting of the chitin- that binds with high affinity to polyol-responsive
binding domain and inteins have been expressed mAb, and is eluted from antibodies under mild con-
and purified in bacterial hosts [139, 140]. The ditions consisting of a low molecular weight poly-
chitin-binding domain consists of 51 residues from hydroxylated compound and non-chaotropic salt
Bacillus circulans [141], and may be fused to either [152]. Although less frequently employed as a re-
the N- or C-terminus. In general, this affinity tag is combinant protein, Softag3 has been used for the
combined in tandem with self-splicing inteins purification of multi-subunit enzyme complexes
[142–144] and is commercially available via the [152, 153] and implemented to study protein inter-
IMPACT™ (Intein-Mediated Purification with an actions [154]. Softag3 is commercially available
Affinity Chitin-binding Tag) system. A chitin ma- through neoclone®.
trix is employed for affinity purification of the re- In contrast to affinity purification, the genetical-
combinant protein, whereas self-cleavage of the ly engineered HaloTag® (Promega) has been de-
thioester bond is induced by a thiol reagent (e.g., signed to enhance expression and solubility of re-
1,4-dithiothreitol or β-mercaptoethanol) or pH ad- combinant proteins in bacterial hosts, as well as to
justment combined with a temperature shift [144, provide an efficient means of protein purification
145]. To reduce non-specific binding, either non- coupled with tag removal by TEV protease-mediat-
ionic detergents or high salt concentrations are ed proteolytic cleavage [155]. Ohana et al. demon-
used. strated the efficacy of HaloTag® for protein expres-
Cellulose-binding domains are non-catalytic sion and purification by evaluating 23 human pro-
domains that vary in size (4–20 kDa) and affinity for teins recombinantly expressed in E. coli. Each
their natural substrate [146]. These domains may heterologous protein was fused to the HaloTag®,
be fused to the N- or C-terminus and bind to cellu- MBP, GST, and His6 at its C-terminus. Results indi-
lose over a wide-range of pH (3.5–9.5). Cellulose cated that an overwhelming 73% of proteins were
matrices are an attractive method for purification produced in soluble form when fused to the
since cellulose inherently maintains a low non- HaloTag®, compared to 52, 39, and 22% of soluble
specific affinity. In general, cellulose-binding do- proteins fused to MBP, GST, or His6, respectively
main interactions with cellulose are so strong that [155]. HaloTag® technology includes a synthetic
they require the use of chaotropic agents, such as linker which covalently binds to a purification ma-
urea or guanidine hydrochloride, for dissociation. trix leading to increased purification yields and
Thus, protein refolding is required following elu- purity when compared to affinity purification
tion. However, ethylene glycol has been used as a schemes. The versatility associated with the
milder elution condition when cellulose-binding HaloTag® includes a variety of ligands applicable
domains of other families have been incorporated for protein visualization, trafficking, and turnover
as an affinity tag and bind to alternative forms of [156, 157], as well as analysis of protein interactions
cellulose matrices with lower affinities. Nahalka et [156].
al. [147] have recently described a purification
strategy that induces cellulose-binding domain- 3.2 Tandem affinity purification and dual-tagging
tagged fusions to aggregate, which results in their methods
selective isolation via differential centrifugation
steps. Unfortunately, no single affinity tag is ideal from
The S-tag is a 15-residue soluble affinity tag every experimental perspective. Therefore, to in-
that binds to the S-protein, derived from RNaseA crease the versatility of tags and downstream ap-
[148, 149]. This interactive complex (S-tag/S-pro- plications, novel dual-tagging methods have been
tein) results in micromolar affinity – dependent developed for different needs. Two affinity tags ex-
upon pH, temperature, and ionic strength [150]. pressed in tandem (i.e., tandem affinity purification
The S-tag itself enables colorimetric detection via (TAP); [158]) enable the use of multiple purifica-
western blot analysis or a rapidly detected assay tion strategies. By incorporating a solubility-en-
dependent upon ribonucleolytic reconstitution and hancing tag in combination with a purification tag
activity. When purified from the S-protein matrix, (e.g., MBP combined with His6; [159]), improve-
the S-tag is eluted under harsh conditions (i.e., ments in solubility and expression yields as well as
pH = 2). The S-tag has enabled the purification of methods for efficient purification are provided. A
recombinant proteins in bacteria [151], and is typ- fusion can be included to evaluate recombinant
protein expression (detected via an epitope or
626 © 2012 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim
18607314, 2012, 5, Downloaded from https://onlinelibrary.wiley.com/doi/10.1002/biot.201100155, Wiley Online Library on [15/10/2023]. See the Terms and Conditions (https://onlinelibrary.wiley.com/terms-and-conditions) on Wiley Online Library for rules of use; OA articles are governed by the applicable Creative Commons License
Biotechnol. J. 2012, 7, 620–634 www.biotechnology-journal.com
complexes where each protein is selectively fused
to a different tag by recombinant DNA methods.
Dual-tagging constructs, such as GFP-His6, of-
fer an additional method of detection using in vivo
fluorescence microscopy instrumentation or in vit-
ro techniques. Recently, the intrinsic fluorescence
of GFP and its variants have been used to rapidly
evaluate positive clones by in-gel fluorescence
techniques [167, 168], in addition to the use of GFP
fluorescent standards and established protocols
[169] permitting the estimation of recombinant
protein concentration via western blot analysis.
Multiple in-depth reviews of TAP tags and pu-
rification schemes are currently available [5, 170,
171]. Detailed elements of combinatorial cassettes
used for dual-tagging and TAP strategies, as well as
vectors available to the bacteria and yeast research
communities, relevant methods, and protocols are
provided in Supporting information, Tables S3 and
S5.
4 Removal of affinity tags: Efficiency of
proteases
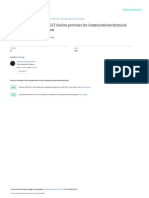
Figure 1. Illustrations of optimized tandem affinity purification (TAP) 4.1 Common proteases
strategy, adapted from the following publications [158]. Overview of the
TAP strategy for a dual-tagging construct consisting of a calmodulin-bind-
ing peptide, TEV protease cleavage site, followed by two IgG-binding units
Removal of carrier proteins and affinity tags is of-
of protein A. The recombinant fusion protein is recovered from cell ex- ten necessary when the presence of the fusion tag
tracts using the protein A tag. Following repetitive wash cycles, the bound affects the structure or biological function of the
IgG material is then released by protease cleavage. Any residual contami- protein of interest. To achieve this end, specific se-
nants are removed by a second purification step that uses the affinity of quences are included between the tag(s) and native
calmodulin-binding peptide for calmodulin beads. The bound material is protein and then cleaved with site-specific pro-
released with EGTA resulting in efficient and high-purity recovery of re-
teases. Common proteases include enterokinase
combinant proteins.
[172], factor Xa [8], SUMO protease [48], tobacco
etch virus (TEV) protease [173], thrombin [174,
GFP) followed by a tag optimal for purification 175], and 3C [176], outlined in Supporting informa-
(e.g., GFP-His6 [160] or GFP-His10 [161]). tion, Table S4.
Tandem affinity purification is an approach Proteases may cleave fusion proteins at unin-
where the protein of interest is fused in-frame to tended sites [151] and the buffer conditions that
two affinity tags, generally separated by a protease promote protease activity and specificity may not
cleavage site. The original TAP tag consisted of a be suitable for fusion protein and product solubili-
calmodulin binding peptide, a TEV cleavage site, ty [177]. It should be noted that protease cleavage
followed by two immunoglobulin binding domains efficiency might vary in an unpredictable manner
of Staphylococcus aureus protein A (ProtA) [158] with each fusion protein. Cleavage efficiency may
and demonstrated maximum protein recovery be improved by applying an increased concentra-
compared to alternative affinity tags such as tion of the protease or by prolonging the digestion.
FLAG-, Strep-, His-, calmodulin-binding protein-, In some cases, the cleavage site is sterically hin-
and cellulose-binding domain-tags [162, 163]. TAP dered and improvements may result by the inclu-
tagging involves two sequential purification steps sion of extra amino acids that flank the cleavage
as illustrated in Fig. 1 and has been implemented in site.
microbial systems. Combinatorial tagging has been
a useful strategy to evaluate the genome scale map- 4.1.1 Enterokinase
ping of protein interaction networks in yeast [96, Enterokinase is a protease that recognizes
164–166] and may also provide a strategic means of DDDDK^X and cleaves at the carboxyl lysine with
purifying heterodimeric or higher-order protein variable efficiencies that depend on the amino acid
© 2012 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim 627
Biotechnology
18607314, 2012, 5, Downloaded from https://onlinelibrary.wiley.com/doi/10.1002/biot.201100155, Wiley Online Library on [15/10/2023]. See the Terms and Conditions (https://onlinelibrary.wiley.com/terms-and-conditions) on Wiley Online Library for rules of use; OA articles are governed by the applicable Creative Commons License
Biotechnol. J. 2012, 7, 620–634
Journal
in the X position. For example, enterokinase cleav- 4.1.5 Thrombin
age efficiency varied from 61% for proline to 88% Thrombin is a protease that recognizes LVPR^GS
for alanine in the X position [178]. Although en- and cleaves between arginine and glycine
terokinase has been shown to cleave at unintended [190–192]. Although thrombin cleavage at the des-
sites [172, 179], it remains useful in some applica- ignated sequence is relatively specific, there have
tions due to the internal enterokinase recognition been reports of thrombin cleavage at alternative
site in the FLAG tag (DYKDDDK). sites [193]. Additionally, impurities in commercial
thrombin preparations, most notably plasmin, have
4.1.2 Factor Xa resulted in non-specific cleavage products in the
Factor Xa is a protease that recognizes I(E/D) past [193, 194], but modern purification methods
GR^X, where X can be any amino acid except argi- have improved thrombin purity [195]. However,
nine or proline [180], and cleaves after the carboxyl thorough characterization of cleaved products
arginine. Ineffective and non-specific proteolysis must be performed following the use of any pro-
has been reported for factor Xa [181, 182], although tease.
factor Xa has been used successfully [183]. Factor Despite these caveats, thrombin may play a spe-
Xa consists of two disulfide-linked chains of 27 and cific role in the preparation of membrane proteins
16 kDa; therefore, the activity of factor Xa may de- for structural characterization as thrombin was de-
crease in buffers with reducing agents. termined to be insensitive to an entire panel of de-
tergents in a recent study by Vergis and Wiener
4.1.3 SUMO protease [177]. In addition to advantages in detergent sensi-
The SUMO protease (S. cerevisiae Ulp1) recognizes tivity, thrombin is efficiently removed from cleav-
the tertiary structure of SUMO and cleaves at the age products by benzamidine sepharose.
N-terminus of the fused protein irrespective of the
sequence with the only exception being proline 4.1.6 3C and PreScission™
[48]. The SUMO protease cleaves efficiently under The 3C protease is derived from human rhinovirus
a wide range of buffer conditions, pH (5.5–10.5) and (HRV 3C) and recognizes the exact residue se-
temperatures (4–37°C). Additionally, SUMO pro- quence (ETLFQ^GP) as the native enzyme where
tease has been shown to efficiently cleave fusion it cleaves between glutamine and glycine [176].
proteins in 2 M urea without yielding non-specific This 22 kDa protease maintains optimal activity at
cleavage products [48], which may facilitate purifi- 4°C and has been constructed in combination with
cation of SUMO-tagged proteins produced in inclu- His-, GST-, NusA, S-, StrepTag II-, and Trx-tags
sion bodies. (EMD4Biosciences). A genetically engineered de-
rivative of HRV 3C, PreScission™ (GE Healthcare)
4.1.4 Tobacco etch virus (TEV) protease has been designed to cleave selectively between
Tobacco etch virus (TEV) protease recognizes the glutamine and glycine of the LEVLFQ^GP
ENLYFQ^S and cleaves between glutamine and recognition site [176, 196].
serine [184–186]. TEV protease is highly specific,
active on a variety of substrates, and cleaves effi-
ciently at low temperatures [173]. One distinct ad- 5 Conclusion
vantage of TEV protease compared to other com-
monly used proteases is that “in house” methods In this post-genomic era, the emergence of bioin-
have been optimized in order to express and puri- formatics combined with proteomic research en-
fy the enzyme [187, 188]. As a direct result, stabiliz- deavors has accelerated the development of fu-
ing mutations and truncations of the enzyme have sions tags. High-throughput protein expression
improved TEV protease activity and expression and purification maintain a pivotal role in structur-
yields. Although wild-type TEV has been shown to al biology (reviewed in [6]). The polyHis-tag IMAC
cleave itself and result in greatly diminished activ- purification process has enabled numerous struc-
ity [188], a mutation (S219V) not only confers re- tural studies; in fact, more than 60% of the all pro-
sistance to unintended cleavage and autoinactiva- tein structures that exist include a polyHis-tag [78].
tion but also increases activity of the enzyme by This well-characterized purification strategy has
two-fold [189]. In addition to the S219V stabilizing been optimized recently for high-throughput on-
mutation, an engineered TEV protease that lacks chip purifications using protein microarrays in or-
the C-terminal residues 238–242 further improved der to evaluate protein function [197]. Moreover, an
expression yields in E. coli, where yields were automated IMAC purification strategy has been de-
nearly 400 mg/L [189]. scribed [198] which is likely to have a significant,
positive impact on the pharmaceutical industry
628 © 2012 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim
18607314, 2012, 5, Downloaded from https://onlinelibrary.wiley.com/doi/10.1002/biot.201100155, Wiley Online Library on [15/10/2023]. See the Terms and Conditions (https://onlinelibrary.wiley.com/terms-and-conditions) on Wiley Online Library for rules of use; OA articles are governed by the applicable Creative Commons License
Biotechnol. J. 2012, 7, 620–634 www.biotechnology-journal.com
and drug design. We anticipate that further ad-
vances in affinity tags and complementary assays Anne Skaja Robinson is the Chair of
integrated with high-throughput technologies will Chemical and Biomolecular Engineer-
provide additional insights into protein-interaction ing at Tulane University and Catherine
networks, accelerate the discovery of new drug tar- and Henry Boh Professor of Engineer-
gets and therapeutics, and increase our ability to ing as of January 2012. Prior to Tulane,
investigate protein structure–function relation- Prof. Robinson was Full Professor and
ships that are presently inaccessible due to techni- Associate Chair at the University of
cal limitations. Delaware, where she started her aca-
demic career. She obtained her Bache-
The authors declare no conflict of interest. lor’s and Master’s degrees in Chemical
Engineering from the Johns Hopkins University. She obtained her PhD
in Chemical Engineering from the University of Illinois at Urbana-
6 References Champaign, and was an NIH Postdoctoral Fellow in the Department
of Biology at MIT before joining the faculty at UD. Her honors include
[1] Arnau, J., Lauritzen, C., Petersen, G. E., Pedersen, J., Current
strategies for the use of affinity tags and tag removal for the a DuPont Young Professor Award, and a National Science Foundation
purification of recombinant proteins. Protein Expr. Purif. Presidential Early Career Award for Science and Engineering (PECASE)
2006, 48, 1–13. Award, and more recently being inducted into AIMBE. She has several
[2] Waugh, D. S., Making the most of affinity tags. Trends patents and over 55 publications in the field of biochemical engineer-
Biotechnol. 2005, 23, 316–320. ing.
[3] Brizzard, B., Epitope tagging. Biotechniques 2008, 44,
693–695.
[4] Terpe, K., Overview of tag protein fusions: From molecular [15] Lu, Q., Bauer, J. C., Greener, A., Using Schizosaccharomyces
and biochemical fundamentals to commercial systems. pombe as a host for expression and purification of eukary-
Appl. Microbiol. Biotechnol. 2003, 60, 523–533. otic proteins. Gene 1997, 200, 135–144.
[5] Walls, D., Loughran, S. T., Tagging recombinant proteins to [16] Mitchell, D. A., Marshall, T. K., Deschenes, R. J., Vectors for
enhance solubility and aid purification. Methods Mol. Biol. the inducible overexpression of glutathione S-transferase
2011, 681, 151–175. fusion proteins in yeast. Yeast 1993, 9, 715–722.
[6] Koehn, J., Hunt, I., High-throughput protein production [17] Rodal, A. A., Duncan, M., Drubin, D., Purification of glu-
(HTPP): A review of enabling technologies to expedite pro- tathione S-transferase fusion proteins from yeast. Methods
tein production. Methods Mol. Biol. 2009, 498, 1–18. Enzymol. 2002, 351, 168–172.
[7] Stevens, R. C., Design of high-throughput methods of pro- [18] Parker, L. L., Atherton-Fessler, S., Piwnica-Worms, H.,
tein production for structural biology. Structure 2000, 8, p107wee1 is a dual-specificity kinase that phosphorylates
R177–R185. p34cdc2 on tyrosine 15. Proc. Natl. Acad. Sci. USA 1992, 89,
[8] Smith, D. B., Johnson, K. S., Single-step purification of 2917–2921.
polypeptides expressed in Escherichia coli as fusions with [19] Beekman, J. M., Cooney, A. J., Elliston, J. F., Tsai, S. Y., Tsai, M.
glutathione S-transferase. Gene 1988, 67, 31–40. J., A rapid one-step method to purify baculovirus-expressed
[9] Parker, M. W., Lo Bello, M., Federici, G., Crystallization of human estrogen receptor to be used in the analysis of the
glutathione S-transferase from human placenta. J. Mol. Biol. oxytocin promoter. Gene 1994, 146, 285–289.
1990, 213, 221–222. [20] Rudert, F., Visser, E., Gradl, G., Grandison, P. et al., pLEF, a
[10] Lim, K., Ho, J. X., Keeling, K., Gilliland, G. L. et al., Three-di- novel vector for expression of glutathione S-transferase fu-
mensional structure of Schistosoma japonicum glutathione sion proteins in mammalian cells. Gene 1996, 169, 281–282.
S-transferase fused with a six-amino acid conserved neu- [21] Lassar, A. B., Buskin, J. N., Lockshon, D., Davis, R. L. et al.,
tralizing epitope of gp41 from HIV. Protein Sci. 1994, 3, MyoD is a sequence-specific DNA binding protein requir-
2233–2244. ing a region of myc homology to bind to the muscle creatine
[11] Ji, X., Zhang, P., Armstrong, R. N., Gilliland, G. L., The three- kinase enhancer. Cell 1989, 58, 823–831.
dimensional structure of a glutathione S-transferase from [22] Ron, D., Dressler, H., pGSTag—a versatile bacterial expres-
the mu gene class. Structural analysis of the binary complex sion plasmid for enzymatic labeling of recombinant pro-
of isoenzyme 3-3 and glutathione at 2.2-A resolution. Bio- teins. Biotechniques 1992, 13, 866–869.
chemistry 1992, 31, 10169–10184. [23] Mayer, B. J., Jackson, P. K., Baltimore, D., The noncatalytic src
[12] Maru, Y., Afar, D. E., Witte, O. N., Shibuya, M., The dimeriza- homology region 2 segment of abl tyrosine kinase binds to
tion property of glutathione S-transferase partially reacti- tyrosine-phosphorylated cellular proteins with high affini-
vates Bcr-Abl lacking the oligomerization domain. J Biol. ty. Proc. Natl. Acad. Sci. USA 1991, 88, 627–631.
Chem. 1996, 271, 15353–15357. [24] McTigue, M. A., Williams, D. R., Tainer, J. A., Crystal struc-
[13] Kaplan, W., Husler, P., Klump, H., Erhardt, J. et al., Confor- tures of a schistosomal drug and vaccine target: Glutathione
mational stability of pGEX-expressed Schistosoma japon- S-transferase from Schistosoma japonica and its complex
icum glutathione S-transferase: A detoxification enzyme with the leading antischistosomal drug praziquantel. J. Mol.
and fusion-protein affinity tag. Protein Sci. 1997, 6, 399–406. Biol. 1995, 246, 21–27.
[14] Frangioni, J. V., Neel, B. G., Solubilization and purification of [25] Duplay, P., Hofnung, M., Two regions of mature periplasmic
enzymatically active glutathione S-transferase (pGEX) fu- maltose-binding protein of Escherichia coli involved in se-
sion proteins. Anal. Biochem. 1993, 210, 179–187. cretion. J. Bacteriol. 1988, 170, 4445–4450.
[26] di Guan, C., Li, P., Riggs, P. D., Inouye, H., Vectors that facili-
tate the expression and purification of foreign peptides in
© 2012 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim 629
Biotechnology
18607314, 2012, 5, Downloaded from https://onlinelibrary.wiley.com/doi/10.1002/biot.201100155, Wiley Online Library on [15/10/2023]. See the Terms and Conditions (https://onlinelibrary.wiley.com/terms-and-conditions) on Wiley Online Library for rules of use; OA articles are governed by the applicable Creative Commons License
Biotechnol. J. 2012, 7, 620–634
Journal
Escherichia coli by fusion to maltose-binding protein. Gene [44] Rajan, S., Plant, L. D., Rabin, M. L., Butler, M. H., Goldstein,
1988, 67, 21–30. S. A., Sumoylation silences the plasma membrane leak K+
[27] Kapust, R. B., Waugh, D. S., Escherichia coli maltose-binding channel K2P1. Cell 2005, 121, 37–47.
protein is uncommonly effective at promoting the solubili- [45] Martin, S., Nishimune, A., Mellor, J. R., Henley, J. M., SUMOy-
ty of polypeptides to which it is fused. Protein Sci. 1999, 8, lation regulates kainate-receptor-mediated synaptic trans-
1668–1674. mission. Nature 2007, 447, 321–325.
[28] Sachdev, D., Chirgwin, J. M., Properties of soluble fusions [46] Mabb, A. M., Wuerzberger-Davis, S. M., Miyamoto, S., PIASy
between mammalian aspartic proteinases and bacterial mediates NEMO sumoylation and NF-kappaB activation in
maltose-binding protein. J. Protein Chem. 1999, 18, 127–136. response to genotoxic stress. Nat. Cell Biol. 2006, 8, 986–993.
[29] Sachdev, D., Chirgwin, J. M., Fusions to maltose-binding pro- [47] Li, S. J., Hochstrasser, M., A new protease required for cell-
tein: Control of folding and solubility in protein purification. cycle progression in yeast. Nature 1999, 398, 246–251.
Methods Enzymol. 2000, 326, 312–321. [48] Malakhov, M. P., Mattern, M. R., Malakhova, O. A., Drinker, M.
[30] Riggs, P., Expression and purification of recombinant pro- et al., SUMO fusions and SUMO-specific protease for effi-
teins by fusion to maltose-binding protein. Mol. Biotechnol. cient expression and purification of proteins. J. Struct.
2000, 15, 51–63. Funct. Genomics 2004, 5, 75–86.
[31] Dyson, M. R., Shadbolt, S. P., Vincent, K. J., Perera, R. L., Mc- [49] Marblestone, J. G., Edavettal, S. C., Lim, Y., Lim, P. et al., Com-
Cafferty, J., Production of soluble mammalian proteins in parison of SUMO fusion technology with traditional gene
Escherichia coli: Identification of protein features that cor- fusion systems: Enhanced expression and solubility with
relate with successful expression. BMC Biotechnol. 2004, 4, SUMO. Protein Sci. 2006, 15, 182–189.
32. [50] Butt, T. R., Edavettal, S. C., Hall, J. P., Mattern, M. R., SUMO
[32] Nallamsetty, S., Austin, B. P., Penrose, K. J., Waugh, D. S., fusion technology for difficult-to-express proteins. Protein
Gateway vectors for the production of combinatorially Expr. Purif. 2005, 43, 1–9.
tagged His6-MBP fusion proteins in the cytoplasm and [51] Zuo, X., Li, S., Hall, J., Mattern, M. R. et al., Enhanced ex-
periplasm of Escherichia coli. Protein Sci. 2005, 14, pression and purification of membrane proteins by SUMO
2964–2971. fusion in Escherichia coli. J. Struct. Funct. Genomics 2005, 6,
[33] Kataeva, I., Chang, J., Xu, H., Luan, C. H. et al., Improving 103–111.
solubility of Shewanella oneidensis MR-1 and Clostridium [52] Zuo, X., Mattern, M. R., Tan, R., Li, S. et al., Expression and
thermocellum JW-20 proteins expressed into Esherichia coli. purification of SARS coronavirus proteins using SUMO-fu-
J. Proteome Res. 2005, 4, 1942–1951. sions. Protein Expr. Purif. 2005, 42, 100–110.
[34] Busso, D., Delagoutte-Busso, B., Moras, D., Construction of a [53] Guzzo, C. M., Yang, D. C., Systematic analysis of fusion and
set Gateway-based destination vectors for high-throughput affinity tags using human aspartyl-tRNA synthetase ex-
cloning and expression screening in Escherichia coli. Anal. pressed in E. coli. Protein Expr. Purif. 2007, 54, 166–175.
Biochem. 2005, 343, 313–321. [54] Dominy, J. E., Jr., Simmons, C. R., Hirschberger, L. L., Hwang,
[35] Braud, S., Moutiez, M., Belin, P., Abello, N. et al., Dual ex- J. et al., Discovery and characterization of a second mam-
pression system suitable for high-throughput fluorescence- malian thiol dioxygenase, cysteamine dioxygenase. J. Biol.
based screening and production of soluble proteins. J. Pro- Chem. 2007, 282, 25189–25198.
teome Res. 2005, 4, 2137–2147. [55] Hughes, R. S., Dowd, P. F., Hector, R. E., Riedmuller, S. B. et
[36] Hamilton, S. R., O’Donnell, J. B., Jr., Hammet, A., Stapleton, al., Cost-effective high-throughput fully automated con-
D. et al., AMP-activated protein kinase: Detection with re- struction of a multiplex library of mutagenized open read-
combinant AMPK alpha1 subunit. Biochem. Biophys. Res. ing frames for an insecticidal peptide using a plasmid-
Commun. 2002, 293, 892–898. based functional proteomic robotic workcell with improved
[37] Podmore, A. H., Reynolds, P. E., Purification and characteri- vacuum system. J. Assoc. Lab. Autom. 2007, 12, 202–212.
zation of VanXY(C), a D,D-dipeptidase/D,D-carboxypepti- [56] Hughes, S. R., Dowd, P. F., Hector, R. E., Panavas, T. et al., Ly-
dase in vancomycin-resistant Enterococcus gallinarum cotoxin-1 insecticidal peptide optimized by amino acid
BM4174. Eur. J. Biochem. 2002, 269, 2740–2746. scanning mutagenesis and expressed as a coproduct in an
[38] Katti, S. K., LeMaster, D. M., Eklund, H., Crystal structure of ethanologenic Saccharomyces cerevisiae strain. J. Pept. Sci.
thioredoxin from Escherichia coli at 1.68 A resolution. J. Mol. 2008, 14, 1039–1050.
Biol. 1990, 212, 167–184. [57] Hughes, S. R., Sterner, D. E., Bischoff, K. M., Hector, R. E. et
[39] LaVallie, E. R., Lu, Z., Diblasio-Smith, E. A., Collins-Racie, L. al., Engineered Saccharomyces cerevisiae strain for im-
A., McCoy, J. M., Thioredoxin as a fusion partner for produc- proved xylose utilization with a three-plasmid SUMO yeast
tion of soluble recombinant proteins in Escherichia coli. expression system. Plasmid 2009, 61, 22–38.
Methods Enzymol. 2000, 326, 322–340. [58] Liu, L., Spurrier, J., Butt, T. R., Strickler, J. E., Enhanced pro-
[40] LaVallie, E. R., DiBlasio, E. A., Kovacic, S., Grant, K. L. et al., tein expression in the baculovirus/insect cell system using
A thioredoxin gene fusion expression system that circum- engineered SUMO fusions. Protein Expr. Purif. 2008, 62,
vents inclusion body formation in the E. coli cytoplasm. 21–28.
Biotechnology (N Y) 1993, 11, 187–193. [59] Peroutka, R. J., Elshourbagy, N., Piech, T., Butt, T. R., En-
[41] Corsini, L., Hothorn, M., Scheffzek, K., Sattler, M., Stier, G., hanced protein expression in mammalian cells using engi-
Thioredoxin as a fusion tag for carrier-driven crystalliza- neered SUMO fusions: Secreted phospholipase A2. Protein
tion. Protein Sci. 2008, 17, 2070–2079. Sci. 2008, 17, 1586–1595.
[42] Seeler, J. S., Bischof, O., Nacerddine, K., Dejean, A., SUMO, [60] Davis, G. D., Elisee, C., Newham, D. M., Harrison, R. G., New
the three Rs and cancer. Curr. Top. Microbiol. Immunol. 2007, fusion protein systems designed to give soluble expression
313, 49–71. in Escherichia coli. Biotechnol. Bioeng. 1999, 65, 382–388.
[43] Meinecke, I., Cinski, A., Baier, A., Peters, M. A. et al., Modifi- [61] Gusarov, I., Nudler, E., Control of intrinsic transcription ter-
cation of nuclear PML protein by SUMO-1 regulates Fas-in- mination by N and NusA: The basic mechanisms. Cell 2001,
duced apoptosis in rheumatoid arthritis synovial fibrob- 107, 437–449.
lasts. Proc. Natl. Acad. Sci. USA 2007, 104, 5073–5078.
630 © 2012 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim
18607314, 2012, 5, Downloaded from https://onlinelibrary.wiley.com/doi/10.1002/biot.201100155, Wiley Online Library on [15/10/2023]. See the Terms and Conditions (https://onlinelibrary.wiley.com/terms-and-conditions) on Wiley Online Library for rules of use; OA articles are governed by the applicable Creative Commons License
Biotechnol. J. 2012, 7, 620–634 www.biotechnology-journal.com
[62] Martin, J. L., Bardwell, J. C., Kuriyan, J., Crystal structure of [82] Manstein, D. J., Schuster, H. P., Morandini, P., Hunt, D. M.,
the DsbA protein required for disulphide bond formation in Cloning vectors for the production of proteins in Dic-
vivo. Nature 1993, 365, 464–468. tyostelium discoideum. Gene 1995, 162, 129–134.
[63] Bardwell, J. C., McGovern, K., Beckwith, J., Identification of [83] Kipriyanov, S. M., Kupriyanova, O. A., Little, M., Molden-
a protein required for disulfide bond formation in vivo. Cell hauer, G., Rapid detection of recombinant antibody frag-
1991, 67, 581–589. ments directed against cell-surface antigens by flow cytom-
[64] Zhang, Y., Olsen, D. R., Nguyen, K. B., Olson, P. S. et al., Ex- etry. J. Immunol. Methods 1996, 196, 51–62.
pression of eukaryotic proteins in soluble form in Es- [84] McKern, N. Ms., Lou, M., Frenkel, M. J., Verkuylen, A. et al.,
cherichia coli. Protein Expr. Purif. 1998, 12, 159–165. Crystallization of the first three domains of the human in-
[65] Collins-Racie, L. A., McColgan, J. M., Grant, K. L., DiBlasio- sulin-like growth factor-1 receptor. Protein Sci. 1997, 6,
Smith, E. A. et al., Production of recombinant bovine en- 2663–2666.
terokinase catalytic subunit in Escherichia coli using the [85] Wilson, I. A., Niman, H. L., Houghten, R. A., Cherenson, A. R.
novel secretory fusion partner DsbA. Biotechnology (N Y) et al., The structure of an antigenic determinant in a protein.
1995, 13, 982–987. Cell 1984, 37, 767–778.
[66] Lilie, H., Schwarz, E., Rudolph, R., Advances in refolding of [86] Field, J., Nikawa, J., Broek, D., MacDonald, B. et al., Purifica-
proteins produced in E. coli. Curr. Opin. Biotechnol. 1998, 9, tion of a RAS-responsive adenylyl cyclase complex from
497–501. Saccharomyces cerevisiae by use of an epitope addition
[67] Kuliopulos, A., Shortle, D., Talalay, P., Isolation and sequenc- method. Mol. Cell. Biol. 1988, 8, 2159–2165.
ing of the gene encoding delta 5-3-ketosteroid isomerase of [87] Surdej, P., Jacobs-Lorena, M., Strategy for epitope tagging
Pseudomonas testosteroni: Overexpression of the protein. the protein-coding region of any gene. Biotechniques 1994,
Proc. Natl. Acad. Sci. USA 1987, 84, 8893–8897. 17, 560–565.
[68] Kuliopulos, A., Walsh, C. T., Production, purification, and [88] Brothers, S. P., Janovick, J. A., Conn, P. M., Unexpected effects
cleavage of tandem repeats of recombinant peptides. J. Am. of epitope and chimeric tags on gonadotropin-releasing
Chem. Soc. 1994, 116, 4599–4607. hormone receptors: Implications for understanding the mo-
[69] Yansura, D. G., Expression as trpE fusion. Methods Enzymol. lecular etiology of hypogonadotropic hypogonadism. J. Clin.
1990, 185, 161–166. Endocrinol. Metab. 2003, 88, 6107–6112.
[70] Miozzari, G. F., Yanofsky, C., Translation of the leader region [89] Houle, T. D., Ram, M. L., McMurray, W. J., Cala, S. E., Differ-
of the Escherichia coli tryptophan operon. J. Bacteriol. 1978, ent endoplasmic reticulum trafficking and processing path-
133, 1457–1466. ways for calsequestrin (CSQ) and epitope-tagged CSQ. Exp.
[71] Kleid, D. G., Yansura, D., Small, B., Dowbenko, D. et al., Cell. Res. 2006, 312, 4150–4161.
Cloned viral protein vaccine for foot-and-mouth disease: [90] Hopp, T. P., Prickett, K. S., Price, V. L., Libby, R. T. et al., A short
Responses in cattle and swine. Science 1981, 214, 1125–1129. polypeptide marker sequence useful for recombinant pro-
[72] Roosild, T. P., Greenwald, J., Vega, M., Castronovo, S. et al., tein identification and purification. Bio-Technology 1988, 6,
NMR structure of Mistic, a membrane-integrating protein 1204–1210.
for membrane protein expression. Science 2005, 307, [91] Maroux, S., Baratti, J., Desnuelle, P., Purification and speci-
1317–1321. ficity of porcine enterokinase. J. Biol. Chem. 1971, 246,
[73] Roosild, T. P., Vega, M., Castronovo, S., Choe, S., Characteri- 5031–5039.
zation of the family of Mistic homologues. BMC Struct. Biol. [92] Hopp, T. P., Gallis, B., Prickett, K. S., Metal-binding proper-
2006, 6, 10. ties of a calcium-dependent monoclonal antibody. Mol. Im-
[74] Kefala, G., Kwiatkowski, W., Esquivies, L., Maslennikov, I., munol. 1996, 33, 601–608.
Choe, S., Application of Mistic to improving the expression [93] Einhauer, A., Schuster, M., Wasserbauer, E., Jungbauer, A.,
and membrane integration of histidine kinase receptors Expression and purification of homogenous proteins in
from Escherichia coli. J. Struct. Funct. Genomics 2007, 8, Saccharomyces cerevisiae based on ubiquitin-FLAG fusion.
167–172. Protein Expr. Purif. 2002, 24, 497–504.
[75] Dvir, H., Lundberg, M. E., Maji, S. K., Riek, R., Choe, S., Mist- [94] Ross-Macdonald, P., Sheehan, A., Roeder, G. S., Snyder, M.,
ic: Cellular localization, solution behavior, polymerization, A multipurpose transposon system for analyzing protein
and fibril formation. Protein Sci. 2009, 18, 1564–1570. production, localization, and function in Saccharomyces
[76] Dvir, H., Choe, S., Bacterial expression of a eukaryotic mem- cerevisiae. Proc. Natl. Acad. Sci. USA 1997, 94, 190–195.
brane protein in fusion to various Mistic orthologs. Protein [95] Sharrock, R. A., Clack, T., Heterodimerization of type II phy-
Expr. Purif. 2009, 68, 28–33. tochromes in Arabidopsis. Proc. Natl. Acad. Sci. USA 2004,
[77] Xie, H., Guo, X. M., Chen, H., Making the most of fusion tags 101, 11500–11505.
technology in structural characterization of membrane pro- [96] Graumann, J., Dunipace, L. A., Seol, J. H., McDonald, W. H. et
teins. Mol. Biotechnol. 2009, 42, 135–145. al., Applicability of tandem affinity purification MudPIT to
[78] Derewenda, Z. S., The use of recombinant methods and mo- pathway proteomics in yeast. Mol. Cell. Proteomics 2004, 3,
lecular engineering in protein crystallization. Methods 2004, 226–237.
34, 354–363. [97] Hernan, R., Heuermann, K., Brizzard, B., Multiple epitope
[79] Derewenda, Z. S., Application of protein engineering to en- tagging of expressed proteins for enhanced detection.
hance crystallizability and improve crystal properties. Acta Biotechniques 2000, 28, 789–793.
Crystallogr. D Biol. Crystallogr. 2010, 66, 604–615. [98] Swanson, R., Locher, M., Hochstrasser, M., A conserved
[80] Moon, A. F., Mueller, G. A., Zhong, X., Pedersen, L. C., A syn- ubiquitin ligase of the nuclear envelope/endoplasmic retic-
ergistic approach to protein crystallization: Combination of ulum that functions in both ER-associated and Mat alpha 2
a fixed-arm carrier with surface entropy reduction. Protein repressor degradation. Genes Dev. 2001, 15, 2660–2674.
Sci. 2010, 19, 901–913. [99] Wong, J. P., Reboul, E., Molday, R. S., Kast, J., A carboxy-ter-
[81] Evan, G. I., Lewis, G. K., Ramsay, G., Bishop, J. M., Isolation of minal affinity tag for the purification and mass spectromet-
monoclonal antibodies specific for human c-myc proto- ric characterization of integral membrane proteins. J. Pro-
oncogene product. Mol. Cell Biol. 1985, 5, 3610–3616. teome Res. 2009, 8, 2388–2396.
© 2012 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim 631
Biotechnology
18607314, 2012, 5, Downloaded from https://onlinelibrary.wiley.com/doi/10.1002/biot.201100155, Wiley Online Library on [15/10/2023]. See the Terms and Conditions (https://onlinelibrary.wiley.com/terms-and-conditions) on Wiley Online Library for rules of use; OA articles are governed by the applicable Creative Commons License
Biotechnol. J. 2012, 7, 620–634
Journal
[100] Hodges, R. S., Heaton, R. J., Parker, J. M., Molday, L., Molday, [118] Dong, X. Y., Chen, L. J., Sun, Y., Refolding and purification
R. S., Antigen-antibody interaction. Synthetic peptides de- of histidine-tagged protein by artificial chaperone-assist-
fine linear antigenic determinants recognized by mono- ed metal affinity chromatography. J. Chromatogr. A 2009,
clonal antibodies directed to the cytoplasmic carboxyl ter- 1216, 5207–5213.
minus of rhodopsin. J. Biol. Chem. 1988, 263, 11768–11775. [119] Lee, J., Kim, S. H., High-throughput T7 LIC vector for in-
[101] Zhong, M., Molday, R. S., Binding of retinoids to ABCA4, the troducing C-terminal poly-histidine tags with variable
photoreceptor ABC transporter associated with Stargardt lengths without extra sequences. Protein Expr. Purif. 2009,
macular degeneration. Methods Mol. Biol. 2010, 652, 63, 58–61.
163–176. [120] Liu, Z., Bartlow, P., Varakala, R., Beitle, R. et al., Use of pro-
[102] Farrens, D. L., Dunham, T. D., Fay, J. F., Dews, I. C. et al., De- teomics for design of a tailored host cell for highly efficient
sign, expression, and characterization of a synthetic hu- protein purification. J. Chromatogr. A 2009, 1216, 2433–
man cannabinoid receptor and cannabinoid receptor/G- 2438.
protein fusion protein. J. Pept. Res. 2002, 60, 336–347. [121] Schmidt, T. G., Skerra, A., The random peptide library-as-
[103] Sassenfeld, H. M., Brewer, S. J., A Polypeptide fusion de- sisted engineering of a C-terminal affinity peptide, useful
signed for the purification of recombinant proteins. Bio- for the detection and purification of a functional Ig Fv
Technology 1984, 2, 76–81. fragment. Protein Eng. 1993, 6, 109–122.
[104] Nagai, K., Thogersen, H. C., Synthesis and sequence-spe- [122] Pahler, A., Hendrickson, W. A., Kolks, M. A., Argarana, C. E.,
cific proteolysis of hybrid proteins produced in Escherichia Cantor, C. R., Characterization and crystallization of core
coli. Methods Enzymol. 1987, 153, 461–481. streptavidin. J. Biol. Chem. 1987, 262, 13933–13937.
[105] Nock, S., Spudich, J. A., Wagner, P., Reversible, site-specific [123] Korndorfer, I. P., Skerra, A., Improved affinity of engi-
immobilization of polyarginine-tagged fusion proteins on neered streptavidin for the Strep-tag II peptide is due to a
mica surfaces. FEBS Lett 1997, 414, 233–238. fixed open conformation of the lid-like loop at the binding
[106] Fuchs, S. M., Raines, R. T., Polyarginine as a multifunction- site. Protein Sci. 2002, 11, 883–893.
al fusion tag. Protein Sci. 2005, 14, 1538–1544. [124] Voss, S., Skerra, A., Mutagenesis of a flexible loop in strep-
[107] Chaga, G. S., Twenty-five years of immobilized metal ion tavidin leads to higher affinity for the Strep-tag II peptide
affinity chromatography: past, present and future. J. Bio- and improved performance in recombinant protein purifi-
chem. Biophys. Methods 2001, 49, 313–334. cation. Protein Eng. 1997, 10, 975–982.
[108] Manjasetty, B. A., Turnbull, A. P., Panjikar, S., Bussow, K., [125] Skerra, A., Schmidt, T. G., Use of the Strep-Tag and strep-
Chance, M. R., Automated technologies and novel tech- tavidin for detection and purification of recombinant pro-
niques to accelerate protein crystallography for structural teins. Methods Enzymol. 2000, 326, 271–304.
genomics. Proteomics 2008, 8, 612–625. [126] Breustedt, D. A., Korndorfer, I. P., Redl, B., Skerra, A., The
[109] Porath, J., Carlsson, J., Olsson, I., Belfrage, G., Metal chelate 1.8-A crystal structure of human tear lipocalin reveals an
affinity chromatography, a new approach to protein frac- extended branched cavity with capacity for multiple lig-
tionation. Nature 1975, 258, 598–599. ands. J. Biol. Chem. 2005, 280, 484–493.
[110] Hochuli, E., Dobeli, H., Schacher, A., New metal chelate ad- [127] Korndorfer, I. P., Dommel, M. K., Skerra, A., Structure of the
sorbent selective for proteins and peptides containing periplasmic chaperone Skp suggests functional similarity
neighbouring histidine residues. J. Chromatogr. 1987, 411, with cytosolic chaperones despite differing architecture.
177–184. Nat. Struct. Mol. Biol. 2004, 11, 1015–1020.
[111] Janknecht, R., de Martynoff, G., Lou, J., Hipskind, R. A. et al., [128] Skerra, A., Use of the tetracycline promoter for the tightly
Rapid and efficient purification of native histidine-tagged regulated production of a murine antibody fragment in Es-
protein expressed by recombinant vaccinia virus. Proc. cherichia coli. Gene 1994, 151, 131–135.
Natl. Acad. Sci. USA 1991, 88, 8972–8976. [129] Han, R., Zwiefka, A., Caswell, C. C., Xu, Y. et al., Assessment
[112] Hefti, M. H., Van Vugt-Van der Toorn, C. J., Dixon, R., Ver- of prokaryotic collagen-like sequences derived from
voort, J., A novel purification method for histidine-tagged streptococcal Scl1 and Scl2 proteins as a source of recom-
proteins containing a thrombin cleavage site. Anal. binant GXY polymers. Appl. Microbiol. Biotechnol. 2006, 72,
Biochem. 2001, 295, 180–185. 109–115.
[113] Li, M., Su, Z. G., Janson, J. C., In vitro protein refolding by [130] Lichty, J. J., Malecki, J. L., Agnew, H. D., Michelson-Horowitz,
chromatographic procedures. Protein Expr. Purif. 2004, 33, D. J., Tan, S., Comparison of affinity tags for protein purifi-
1–10. cation. Protein Expr. Purif. 2005, 41, 98–105.
[114] Hochuli, E., Bannwarth, W., Dobeli, H., Gentz, R., Stuber, D., [131] Prinz, B., Schultchen, J., Rydzewski, R., Holz, C. et al., Es-
Genetic approach to facilitate purification of recombinant tablishing a versatile fermentation and purification proce-
proteins with a novel metal chelate adsorbent. Bio-Tech- dure for human proteins expressed in the yeasts Saccha-
nology 1988, 6, 1321–1325. romyces cerevisiae and Pichia pastoris for structural ge-
[115] Chaga, G., Bochkariov, D. E., Jokhadze, G. G., Hopp, J., Nel- nomics. J. Struct. Funct. Genomics 2004, 5, 29–44.
son, P., Natural poly-histidine affinity tag for purification [132] Boettner, M., Prinz, B., Holz, C., Stahl, U., Lang, C., High-
of recombinant proteins on cobalt(II)-carboxymethylas- throughput screening for expression of heterologous pro-
partate crosslinked agarose. J. Chromatogr. A 1999, 864, teins in the yeast Pichia pastoris. J. Biotechnol. 2002, 99,
247–256. 51–62.
[116] Chaga, G., Hopp, J., Nelson, P., Immobilized metal ion affin- [133] Wilson, D. S., Keefe, A. D., Szostak, J. W., The use of mRNA
ity chromatography on Co2+-carboxymethylaspartate- display to select high-affinity protein-binding peptides.
agarose Superflow, as demonstrated by one-step purifica- Proc. Natl. Acad. Sci. USA 2001, 98, 3750–3755.
tion of lactate dehydrogenase from chicken breast muscle. [134] Keefe, A. D., Wilson, D. S., Seelig, B., Szostak, J. W., One-step
Biotechnol. Appl. Biochem. 1999, 29, 19–24. purification of recombinant proteins using a nanomolar-
[117] Chatterjee, D. K., Esposito, D., Enhanced soluble protein affinity streptavidin-binding peptide, the SBP-Tag. Protein
expression using two new fusion tags. Protein Expr. Purif. Expr. Purif. 2001, 23, 440–446.
2006, 46, 122–129. [135] Stofko-Hahn, R. E., Carr, D. W., Scott, J. D., A single step pu-
rification for recombinant proteins. Characterization of a
632 © 2012 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim
18607314, 2012, 5, Downloaded from https://onlinelibrary.wiley.com/doi/10.1002/biot.201100155, Wiley Online Library on [15/10/2023]. See the Terms and Conditions (https://onlinelibrary.wiley.com/terms-and-conditions) on Wiley Online Library for rules of use; OA articles are governed by the applicable Creative Commons License
Biotechnol. J. 2012, 7, 620–634 www.biotechnology-journal.com
microtubule associated protein (MAP 2) fragment which with a polyol-responsive monoclonal antibody. Protein
associates with the type II cAMP-dependent protein ki- Expr. Purif. 2004, 35, 147–155.
nase. FEBS Lett. 1992, 302, 274–278. [153] Burgess, R. R., Thompson, N. E., Advances in gentle im-
[136] Blumenthal, D. K., Takio, K., Edelman, A. M., Charbonneau, munoaffinity chromatography. Curr. Opin. Biotechnol.
H. et al., Identification of the calmodulin-binding domain 2002, 13, 304–308.
of skeletal muscle myosin light chain kinase. Proc. Natl. [154] Thompson, N. E., Arthur, T. M., Burgess, R. R., Development
Acad. Sci. USA 1985, 82, 3187–3191. of an epitope tag for the gentle purification of proteins by
[137] Vaillancourt, P., Zheng, C. F., Hoang, D. Q., Breister, L., immunoaffinity chromatography: Application to epitope-
Affinity purification of recombinant proteins fused to tagged green fluorescent protein. Anal. Biochem. 2003, 323,
calmodulin or to calmodulin-binding peptides. Methods 171–179.
Enzymol. 2000, 326, 340–362. [155] Ohana, R. F., Hurst, R., Vidugiriene, J., Slater, M. R. et al.,
[138] Head, J. F., A better grip on calmodulin. Curr. Biol. 1992, 2, HaloTag-based purification of functional human kinases
609–611. from mammalian cells. Protein Expr. Purif. 2011, 76,
[139] Szweda, P., Pladzyk, R., Kotlowski, R., Kur, J., Cloning, ex- 154–164.
pression, and purification of the Staphylococcus simulans [156] Los, G. V., Encell, L. P., McDougall, M. G., Hartzell, D. D. et
lysostaphin using the intein-chitin-binding domain (CBD) al., HaloTag: A novel protein labeling technology for cell
system. Protein Expr. Purif. 2001, 22, 467–471. imaging and protein analysis. ACS Chem. Biol. 2008, 3,
[140] Wiese, A., Wilms, B., Syldatk, C., Mattes, R., Altenbuchner, 373–382.
J., Cloning, nucleotide sequence and expression of a hy- [157] Los, G. V., Wood, K., The HaloTag: A novel technology for
dantoinase and carbamoylase gene from Arthrobacter au- cell imaging and protein analysis. Methods Mol. Biol. 2007,
rescens DSM 3745 in Escherichia coli and comparison with 356, 195–208.
the corresponding genes from Arthrobacter aurescens [158] Rigaut, G., Shevchenko, A., Rutz, B., Wilm, M. et al., A
DSM 3747. Appl. Microbiol. Biotechnol. 2001, 55, 750–757. generic protein purification method for protein complex
[141] Watanabe, T., Ito, Y., Yamada, T., Hashimoto, M. et al., The characterization and proteome exploration. Nat. Biotech-
roles of the C-terminal domain and type III domains of nol. 1999, 17, 1030–1032.
chitinase A1 from Bacillus circulans WL-12 in chitin degra- [159] Tropea, J. E., Cherry, S., Nallamsetty, S., Bignon, C., Waugh,
dation. J. Bacteriol. 1994, 176, 4465–4472. D. S., A generic method for the production of recombinant
[142] Chong, S., Mersha, F. B., Comb, D. G., Scott, M. E. et al., Sin- proteins in Escherichia coli using a dual hexahistidine-
gle-column purification of free recombinant proteins us- maltose-binding protein affinity tag. Methods Mol. Biol.
ing a self-cleavable affinity tag derived from a protein 2007, 363, 1–19.
splicing element. Gene 1997, 192, 271–281. [160] Liu, H., Naismith, J. H., A simple and efficient expression
[143] Chong, S., Shao, Y., Paulus, H., Benner, J. et al., Protein splic- and purification system using two newly constructed vec-
ing involving the Saccharomyces cerevisiae VMA intein. tors. Protein Expr. Purif. 2009, 63, 102–111.
The steps in the splicing pathway, side reactions leading to [161] O’Malley, M. A., Lazarova, T., Britton, Z. T., Robinson, A. S.,
protein cleavage, and establishment of an in vitro splicing High-level expression in Saccharomyces cerevisiae en-
system. J. Biol. Chem. 1996, 271, 22159–22168. ables isolation and spectroscopic characterization of func-
[144] Xu, M. Q., Paulus, H., Chong, S., Fusions to self-splicing in- tional human adenosine A2a receptor. J. Struct. Biol. 2007,
teins for protein purification. Methods Enzymol. 2000, 326, 159, 166–178.
376–418. [162] Ford, C. F., Suominen, I., Glatz, C. E., Fusion tails for the re-
[145] Evans, T. C., Jr., Xu, M. Q., Intein-mediated protein ligation: covery and purification of recombinant proteins. Protein
Harnessing nature’s escape artists. Biopolymers 1999, 51, Expr. Purif. 1991, 2, 95–107.
333–342. [163] Sheibani, N., Prokaryotic gene fusion expression systems
[146] Carrard, G., Koivula, A., Soderlund, H., Beguin, P., Cellu- and their use in structural and functional studies of pro-
lose-binding domains promote hydrolysis of different sites teins. Prep. Biochem. Biotechnol. 1999, 29, 77–90.
on crystalline cellulose. Proc. Natl. Acad. Sci. USA 2000, 97, [164] Ho, Y., Gruhler, A., Heilbut, A., Bader, G. D. et al., Systemat-
10342–10347. ic identification of protein complexes in Saccharomyces
[147] Nahalka, J., Nidetzky, B., Fusion to a pull-down domain: A cerevisiae by mass spectrometry. Nature 2002, 415, 180–183.
novel approach of producing Trigonopsis variabilisD- [165] Ghaemmaghami, S., Huh, W. K., Bower, K., Howson, R. W. et
amino acid oxidase as insoluble enzyme aggregates. al., Global analysis of protein expression in yeast. Nature
Biotechnol. Bioeng. 2007, 97, 454–461. 2003, 425, 737–741.
[148] Karpeisky, M., Senchenko, V. N., Dianova, M. V., Kanevsky, [166] Gavin, A. C., Bosche, M., Krause, R., Grandi, P. et al., Func-
V., Formation and properties of S-protein complex with tional organization of the yeast proteome by systematic
S-peptide-containing fusion protein. FEBS Lett. 1994, 339, analysis of protein complexes. Nature 2002, 415, 141–147.
209–212. [167] Newstead, S., Kim, H., von Heijne, G., Iwata, S., Drew, D.,
[149] Kim, J. S., Raines, R. T., A misfolded but active dimer of High-throughput fluorescent-based optimization of eu-
bovine seminal ribonuclease. Eur. J. Biochem. 1994, 224, karyotic membrane protein overexpression and purifica-
109–114. tion in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA
[150] Connelly, P. R., Varadarajan, R., Sturtevant, J. M., Richards, 2007, 104, 13936–13941.
F. M., Thermodynamics of protein–peptide interactions in [168] Young, C. L., Yuraszeck, T., Robinson, A. S., Decreased se-
the ribonuclease S system studied by titration calorimetry. cretion and unfolded protein response upregulation.
Biochemistry 1990, 29, 6108–6114. Methods Enzymol. 2011, 491, 235–260.
[151] Lellouch, A. C., Geremia, R. A., Expression and study of re- [169] Drew, D., Newstead, S., Sonoda, Y., Kim, H. et al., GFP-
combinant ExoM, a beta1-4 glucosyltransferase involved based optimization scheme for the overexpression and pu-
in succinoglycan biosynthesis in Sinorhizobium meliloti. rification of eukaryotic membrane proteins in Saccha-
J. Bacteriol. 1999, 181, 1141–1148. romyces cerevisiae. Nat. Protoc. 2008, 3, 784–798.
[152] Duellman, S. J., Thompson, N. E., Burgess, R. R., An epitope [170] Xu, X., Song, Y., Li, Y., Chang, J. et al., The tandem affinity
tag derived from human transcription factor IIB that reacts purification method: An efficient system for protein com-
© 2012 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim 633
Biotechnology
18607314, 2012, 5, Downloaded from https://onlinelibrary.wiley.com/doi/10.1002/biot.201100155, Wiley Online Library on [15/10/2023]. See the Terms and Conditions (https://onlinelibrary.wiley.com/terms-and-conditions) on Wiley Online Library for rules of use; OA articles are governed by the applicable Creative Commons License
Biotechnol. J. 2012, 7, 620–634
Journal
plex purification and protein interaction identification. [184] Dougherty, W. G., Parks, T. D., Cary, S. M., Bazan, J. F., Flett-
Protein Expr. Purif. 2010, 72, 149–156. erick, R. J., Characterization of the catalytic residues of the
[171] Puig, O., Caspary, F., Rigaut, G., Rutz, B. et al., The tandem tobacco etch virus 49-kDa proteinase. Virology 1989, 172,
affinity purification (TAP) method: A general procedure of 302–310.
protein complex purification. Methods 2001, 24, 218–229. [185] Dougherty, W. G., Carrington, J. C., Cary, S. M., Parks, T. D.,
[172] Choi, S. I., Song, H. W., Moon, J. W., Seong, B. L., Recombi- Biochemical and mutational analysis of a plant virus
nant enterokinase light chain with affinity tag: Expression polyprotein cleavage site. Embo J. 1988, 7, 1281–1287.
from Saccharomyces cerevisiae and its utilities in fusion [186] Carrington, J. C., Dougherty, W. G., A viral cleavage site cas-
protein technology. Biotechnol. Bioeng. 2001, 75, 718–724. sette: Identification of amino acid sequences required for
[173] Parks, T. D., Leuther, K. K., Howard, E. D., Johnston, S. A., tobacco etch virus polyprotein processing. Proc. Natl. Acad.
Dougherty, W. G., Release of proteins and peptides from fu- Sci. USA 1988, 85, 3391–3395.
sion proteins using a recombinant plant virus proteinase. [187] Tropea, J. E., Cherry, S., Waugh, D. S., Expression and pu-
Anal. Biochem. 1994, 216, 413–417. rification of soluble His(6)-tagged TEV protease. Methods
[174] Sticha, K. R., Sieg, C. A., Bergstrom, C. P., Hanna, P. E., Wag- Mol. Biol. 2009, 498, 297–307.
ner, C. R., Overexpression and large-scale purification of [188] Blommel, P. G., Fox, B. G., A combined approach to improv-
recombinant hamster polymorphic arylamine N-acetyl- ing large-scale production of tobacco etch virus protease.
transferase as a dihydrofolate reductase fusion protein. Protein Expr. Purif. 2007, 55, 53–68.
Protein Expr. Purif. 1997, 10, 141–153. [189] Kapust, R. B., Tozser, J., Fox, J. D., Anderson, D. E. et al., To-
[175] Vothknecht, U. C., Kannangara, C. G., von Wettstein, D., Ex- bacco etch virus protease: Mechanism of autolysis and ra-
pression of catalytically active barley glutamyl tRNAGlu tional design of stable mutants with wild-type catalytic
reductase in Escherichia coli as a fusion protein with glu- proficiency. Protein Eng. 2001, 14, 993–1000.
tathione S-transferase. Proc. Natl. Acad. Sci. USA 1996, 93, [190] Chang, J. Y., Alkan, S. S., Hilschmann, N., Braun, D. G.,
9287–9291. Thrombin specificity. Selective cleavage of antibody light
[176] Cordingley, M. G., Callahan, P. L., Sardana, V. V., Garsky, V. chains at the joints of variable with joining regions and
M., Colonno, R. J., Substrate requirements of human rhi- joining with constant regions. Eur. J. Biochem. 1985, 151,
novirus 3C protease for peptide cleavage in vitro. J. Biol. 225–230.
Chem. 1990, 265, 9062–9065. [191] Chang, J. Y., Thrombin specificity. Requirement for apolar
[177] Vergis, J. M., Wiener, M. C., The variable detergent sensitiv- amino acids adjacent to the thrombin cleavage site of
ity of proteases that are utilized for recombinant protein polypeptide substrate. Eur. J. Biochem. 1985, 151, 217–224.
affinity tag removal. Protein Expr. Purif. 78, 139–142. [192] Haun, R. S., Moss, J., Ligation-independent cloning of glu-
[178] Hosfield, T., Lu, Q., Influence of the amino acid residue tathione S-transferase fusion genes for expression in Es-
downstream of (Asp)4Lys on enterokinase cleavage of a cherichia coli. Gene 1992, 112, 37–43.
fusion protein. Anal. Biochem. 1999, 269, 10–16. [193] Koehl, C., Abecassis, J., Determination of oxalic acid in
[179] Liew, O. W., Ching Chong, J. P., Yandle, T. G., Brennan, S. O., urine by atomic absorption spectrophotometry. Clin. Chim.
Preparation of recombinant thioredoxin fused N-terminal Acta 1976, 70, 71–77.
proCNP: Analysis of enterokinase cleavage products re- [194] Donaldson, V. H., Kleniewski, J., The role of plasmin in
veals new enterokinase cleavage sites. Protein Expr. Purif. kinin-release by preparations of human thrombin. Thromb
2005, 41, 332–340. Res. 1979, 16, 401–406.
[180] Nagai, K., Thogersen, H. C., Generation of beta-globin by [195] Litwiller, R. D., Jenny, R. J., Mann, K. G., Identification and
sequence-specific proteolysis of a hybrid protein pro- isolation of vitamin K-dependent proteins by HPLC. Anal.
duced in Escherichia coli. Nature 1984, 309, 810–812. Biochem. 1986, 158, 355–360.
[181] Ko, Y. H., Thomas, P. J., Pedersen, P. L., The cystic fibrosis [196] Walker, P. A., Leong, L. E., Ng, P. W., Tan, S. H. et al., Efficient
transmembrane conductance regulator. Nucleotide bind- and rapid affinity purification of proteins using recombi-
ing to a synthetic peptide segment from the second pre- nant fusion proteases. Biotechnology (N Y) 1994, 12,
dicted nucleotide binding fold. J. Biol. Chem. 1994, 269, 601–605.
14584–14588. [197] Kwon, K., Grose, C., Pieper, R., Pandya, G. A. et al., High
[182] Jenny, R. J., Mann, K. G., Lundblad, R. L., A critical review quality protein microarray using in situ protein purifica-
of the methods for cleavage of fusion proteins with throm- tion. BMC Biotechnol. 2009, 9, 72.
bin and factor Xa. Protein Expr. Purif. 2003, 31, 1–11. [198] Steen, J., Uhlen, M., Hober, S., Ottosson, J., High-through-
[183] Pryor, K. D., Leiting, B., High-level expression of soluble put protein purification using an automated set-up for
protein in Escherichia coli using a His6-tag and maltose- high-yield affinity chromatography. Protein Expr. Purif.
binding-protein double-affinity fusion system. Protein 2006, 46, 173–178.
Expr. Purif. 1997, 10, 309–319.
634 © 2012 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim
You might also like
- 2013fall IndianpolisORGN AbstractsDocument368 pages2013fall IndianpolisORGN AbstractstinperasovicNo ratings yet
- Young 2012 Recombinant Protein Expression andDocument15 pagesYoung 2012 Recombinant Protein Expression andMuôngNo ratings yet
- Gopal 2013Document7 pagesGopal 2013Humberto FreitasNo ratings yet
- Affinity ChromatographyDocument32 pagesAffinity ChromatographyRONAK LASHKARINo ratings yet
- 4GeneticDesign Exp ColiDocument15 pages4GeneticDesign Exp ColiLuis Adan Martínez AvilaNo ratings yet
- BMC BiotechnologyDocument18 pagesBMC Biotechnologyfather45No ratings yet
- Arnau2006 PDFDocument13 pagesArnau2006 PDFMarisol BravoNo ratings yet
- Fbioe 07 00139Document9 pagesFbioe 07 00139aliNo ratings yet
- Báo số 1 - han2017Document19 pagesBáo số 1 - han2017Võ Hoài QuyênNo ratings yet
- No 4 LagiDocument14 pagesNo 4 Laginur qomariyahNo ratings yet
- 1 s2.0 S0141813023002933 MainDocument22 pages1 s2.0 S0141813023002933 MainDiego RosilesNo ratings yet
- Agyei2018 Article ProspectsInTheUseOfAptamersForDocument10 pagesAgyei2018 Article ProspectsInTheUseOfAptamersForHiiHiengKokNo ratings yet
- Making The Most of Affinity Tags: David S. WaughDocument5 pagesMaking The Most of Affinity Tags: David S. WaughmudapakajNo ratings yet
- Means To Optimize Protein Expression in Transgenic Plants: SciencedirectDocument7 pagesMeans To Optimize Protein Expression in Transgenic Plants: Sciencedirectluluatul maghfirohNo ratings yet
- Recombinant Protein Expression ThesisDocument5 pagesRecombinant Protein Expression ThesisOnlinePaperWritersCanada100% (2)
- Kato 2007Document7 pagesKato 2007gwenstacy551No ratings yet
- As91159 Gene ExpressionDocument3 pagesAs91159 Gene Expressionapi-323107386No ratings yet
- How To Obtain Labeled Proteins and What To Do With ThemDocument11 pagesHow To Obtain Labeled Proteins and What To Do With Thempál locNo ratings yet
- Zao, Et Al (2016) Recombinant Production of Medium ToDocument9 pagesZao, Et Al (2016) Recombinant Production of Medium Totantry puspitasariNo ratings yet
- Ysab 029Document13 pagesYsab 029ibrikcheeNo ratings yet
- Review Affinity Fusion Strategies For Detection, Purification, and Immobilization of Recombinant ProteinsDocument16 pagesReview Affinity Fusion Strategies For Detection, Purification, and Immobilization of Recombinant ProteinsPrasad KulkarniNo ratings yet
- Proteomics Lab ReportDocument4 pagesProteomics Lab Reportnursah.aslanNo ratings yet
- Toxiproteomics MerrickDocument2 pagesToxiproteomics MerrickJuanCarlosDíazConejeroNo ratings yet
- E2017005 PDFDocument6 pagesE2017005 PDFComputational and Structural Biotechnology JournalNo ratings yet
- Functional Characterization of Pathway Inhibitors For The Ubiquitin-Proteasome System (UPS) As Tool Compounds For CRBN and VHL-mediated Targeted Protein DegradationDocument23 pagesFunctional Characterization of Pathway Inhibitors For The Ubiquitin-Proteasome System (UPS) As Tool Compounds For CRBN and VHL-mediated Targeted Protein DegradationAndy HackettNo ratings yet
- Advances On Size Exclusion Chromatography and Applications On The Analysis of Protein Biopharmaceuticals and Protein Aggregates: A Mini ReviewDocument21 pagesAdvances On Size Exclusion Chromatography and Applications On The Analysis of Protein Biopharmaceuticals and Protein Aggregates: A Mini ReviewLesly MartinezNo ratings yet
- Expression of Lignocellulolytic Enzymes in Pichia PastorisDocument11 pagesExpression of Lignocellulolytic Enzymes in Pichia PastorisRoberta B.No ratings yet
- 10.1038@s41570 020 00223 8Document22 pages10.1038@s41570 020 00223 8Khaira Rusdi NumlilNo ratings yet
- Guia Practica para Proteinas en Cuerpos de InclusionDocument15 pagesGuia Practica para Proteinas en Cuerpos de InclusionMarco Ku CenturionNo ratings yet
- Grammel2013 PDFDocument10 pagesGrammel2013 PDFdupuytrenNo ratings yet
- plphys_v179_3_907Document11 pagesplphys_v179_3_907dafbeNo ratings yet
- Strategies To Maximize Heterologous Protein ExpressionDocument10 pagesStrategies To Maximize Heterologous Protein ExpressionElías Octavio Gómez MontesNo ratings yet
- Improved YieldDocument22 pagesImproved YieldLuis Antonio FloresNo ratings yet
- 39-Ahsan (FINAL CUT)Document12 pages39-Ahsan (FINAL CUT)خاک نشینNo ratings yet
- Affinity Purification of ProteinDocument7 pagesAffinity Purification of ProteinRONAK LASHKARINo ratings yet
- Capacidade de Ligação de Ligases E3 para Aplicações de Degradação de Proteínas DirecionadasDocument13 pagesCapacidade de Ligação de Ligases E3 para Aplicações de Degradação de Proteínas DirecionadasRenan Guilherme de Oliveira GuihNo ratings yet
- Promega HaloTag Fusion Protein GuideDocument3 pagesPromega HaloTag Fusion Protein GuideMoritz ListNo ratings yet
- Metabolic Labeling of Proteins For Proteomics : Robert J. Beynon and Julie M. PrattDocument16 pagesMetabolic Labeling of Proteins For Proteomics : Robert J. Beynon and Julie M. PrattNidhi JaisNo ratings yet
- Module 7-Lecture 1 Microbial Biotechnology: Genetic ManipulationDocument37 pagesModule 7-Lecture 1 Microbial Biotechnology: Genetic ManipulationAakanksha RaulNo ratings yet
- COSTBI 2018 196 Accepted RefcorrectedDocument14 pagesCOSTBI 2018 196 Accepted RefcorrectedShatanik MukherjeeNo ratings yet
- SILACDocument11 pagesSILACMaihafizah Mohd ZahariNo ratings yet
- Glycopp: A Webserver For Prediction of N-And O - Glycosites in Prokaryotic Protein SequencesDocument13 pagesGlycopp: A Webserver For Prediction of N-And O - Glycosites in Prokaryotic Protein SequencesAadil Hussain BhatNo ratings yet
- Trends in Food Science & Technology: Mehdi Akbari, Seyed Hadi Razavi, Marek KieliszekDocument12 pagesTrends in Food Science & Technology: Mehdi Akbari, Seyed Hadi Razavi, Marek KieliszekAdellia Rahmanda SyaputriNo ratings yet
- 2022 Article 1882Document16 pages2022 Article 1882BRUNA COELHO DE ANDRADENo ratings yet
- Wright 2015Document12 pagesWright 2015LasaroNo ratings yet
- Lee 2009Document11 pagesLee 2009Jordan VillalobosNo ratings yet
- Love Et Al. - 2012 - Systematic Single-Cell Analysis of Pichia PastorisDocument11 pagesLove Et Al. - 2012 - Systematic Single-Cell Analysis of Pichia PastorisAnaNo ratings yet
- Protein Purification by Affinity Chromatography: January 2012Document21 pagesProtein Purification by Affinity Chromatography: January 2012Maria Villamizar GuerreroNo ratings yet
- 1-s2.0-S2666246922000027-Main Expanding The Landscape of E3 Ligases For Targeted Protein DegradationDocument5 pages1-s2.0-S2666246922000027-Main Expanding The Landscape of E3 Ligases For Targeted Protein DegradationTsung-Shing WangNo ratings yet
- Purifying His Tag ProteinDocument9 pagesPurifying His Tag ProteinRONAK LASHKARINo ratings yet
- Method 4 - e ColiDocument9 pagesMethod 4 - e ColiHarithpriya KannanNo ratings yet
- Protein Body-Inducing Fusions For High-Level Production and Purification of Recombinant Proteins in PlantsDocument15 pagesProtein Body-Inducing Fusions For High-Level Production and Purification of Recombinant Proteins in Plantsdengboff96No ratings yet
- Rampado2022 - Lysis BufferDocument11 pagesRampado2022 - Lysis BufferRamonaTecucianuNo ratings yet
- Protein EngineeringDocument14 pagesProtein Engineeringzamannakibur3No ratings yet
- 11protein Purification and AnalysisDocument26 pages11protein Purification and AnalysisFidiya Septi Kusma WardaniNo ratings yet
- Mehmeti Et Al, 2011Document8 pagesMehmeti Et Al, 2011olindacabralNo ratings yet
- Docking and Binding Free Energy Calculations of Sirtuin InhibitorsDocument15 pagesDocking and Binding Free Energy Calculations of Sirtuin InhibitorsHenrique HolandaNo ratings yet
- Ijn 3 487 PDFDocument10 pagesIjn 3 487 PDFYean-baptiste Dupas DecallaisNo ratings yet
- Protein & Metabolic EngineeringDocument10 pagesProtein & Metabolic EngineeringADITYAROOP PATHAKNo ratings yet
- Recombinant Protein Technology 2023Document36 pagesRecombinant Protein Technology 2023Ana Laura Mendoza AriasNo ratings yet
- Ubiquitin and UBL SignalingDocument40 pagesUbiquitin and UBL SignalingpankajmrigNo ratings yet
- 1841846791Document600 pages1841846791Mohammed Hussein100% (1)
- Tmp146a TMPDocument9 pagesTmp146a TMPFrontiersNo ratings yet
- Mammalian Hibernation: Differential Gene Expression and Novel Application of Epigenetic ControlsDocument12 pagesMammalian Hibernation: Differential Gene Expression and Novel Application of Epigenetic ControlsaufaaNo ratings yet
- Plant Proteostasis Methods and Protocols (L. Maria Lois, Marco Trujillo)Document408 pagesPlant Proteostasis Methods and Protocols (L. Maria Lois, Marco Trujillo)Archibald BoxleitnerNo ratings yet
- Proteins and Cell Regulation Vol 10 - Sirtuins, 1E (2016)Document291 pagesProteins and Cell Regulation Vol 10 - Sirtuins, 1E (2016)DiahaNo ratings yet
- Viruses 02 00189Document24 pagesViruses 02 00189kishorechandraNo ratings yet
- Ubiquitin and Protein Degradation, Part A by Raymond J. Deshaies (Eds.)Document654 pagesUbiquitin and Protein Degradation, Part A by Raymond J. Deshaies (Eds.)Andonis AngelovNo ratings yet
- A Comprehensive View of The Epigenetic Landscape Part IIDocument26 pagesA Comprehensive View of The Epigenetic Landscape Part IIBetzabethNo ratings yet
- Histone ModificationDocument2 pagesHistone ModificationHarshin TNo ratings yet
- Molecular Aspects of MedicineDocument20 pagesMolecular Aspects of MedicineLorena RamosNo ratings yet
- Ubiquitin Family Modifiers and The Proteasome Reviews and Protocols by Alexander Varshavsky (Auth.), R. Jürgen Dohmen, Martin Scheffner (Eds.)Document660 pagesUbiquitin Family Modifiers and The Proteasome Reviews and Protocols by Alexander Varshavsky (Auth.), R. Jürgen Dohmen, Martin Scheffner (Eds.)Andonis AngelovNo ratings yet