Professional Documents
Culture Documents
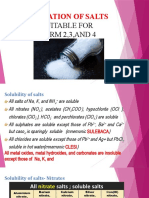
Steps To Making Different Salts - ACTIVITY
Steps To Making Different Salts - ACTIVITY
Uploaded by
zionmills11Copyright:
Available Formats
You might also like
- IGCSE Chemistry - Acids Bases and SaltsDocument13 pagesIGCSE Chemistry - Acids Bases and SaltsChemistryKlipz100% (12)
- Preparation and Purification of Soluble SaltsDocument12 pagesPreparation and Purification of Soluble SaltsJuni FarhanaNo ratings yet
- Preparation of Sodium Ethyl SulfateDocument2 pagesPreparation of Sodium Ethyl SulfateJoan Mas Torrent100% (3)
- Salt Preperation-To Prepare Soluble SaltsDocument4 pagesSalt Preperation-To Prepare Soluble Saltsadetorostephen0% (1)
- Preparation of SaltsDocument9 pagesPreparation of SaltsAkshay ReddyNo ratings yet
- Notes To Study For Chemistry Paper 6Document11 pagesNotes To Study For Chemistry Paper 6sakibsultan_308100% (1)
- Salt PreperationDocument3 pagesSalt PreperationEmaan ShahidNo ratings yet
- Chemistry ATPDocument4 pagesChemistry ATPinspectionNo ratings yet
- Notes To Study For Chemistry Paper 6Document8 pagesNotes To Study For Chemistry Paper 63abood51467% (6)
- Notes To Study For Chemistry Paper 6Document8 pagesNotes To Study For Chemistry Paper 6alibaslamNo ratings yet
- Preparation of SaltsDocument8 pagesPreparation of SaltsRose MusariraNo ratings yet
- Preparation of SaltsDocument8 pagesPreparation of SaltsTAKUNDA MARIMENo ratings yet
- 03 PreparingSaltsDocument3 pages03 PreparingSaltsDiamondNo ratings yet
- Preparing Soluble SaltsDocument10 pagesPreparing Soluble SaltsSuhaan HussainNo ratings yet
- Using A PipetteDocument1 pageUsing A PipetteeeenusNo ratings yet
- Preparing Soluble Salts 1Document9 pagesPreparing Soluble Salts 1Suhaan HussainNo ratings yet
- Preparing Soluble SaltsDocument5 pagesPreparing Soluble SaltsFaris Irfan100% (1)
- Methods of Preparing SaltsDocument6 pagesMethods of Preparing SaltsMahmoud Elsaied SolymanNo ratings yet
- كيمياء OL practical Review on P6 - 240117 - 171619Document110 pagesكيمياء OL practical Review on P6 - 240117 - 171619albasjudyNo ratings yet
- Making Crystals With SaltDocument2 pagesMaking Crystals With SaltNadia BasherNo ratings yet
- Name / Title Topic Learning Objective Key Science Concepts/ Main Messages Props/ MaterialsDocument3 pagesName / Title Topic Learning Objective Key Science Concepts/ Main Messages Props/ MaterialsHaha Hoho TNo ratings yet
- Preparation of SaltsDocument6 pagesPreparation of Saltssakibsultan_308No ratings yet
- Flow Chart For The Preparation of SaltsDocument1 pageFlow Chart For The Preparation of SaltsSaadiah MohammadNo ratings yet
- Preparation of SaltsDocument7 pagesPreparation of Saltsaltygeorge0No ratings yet
- CHWM WorkDocument1 pageCHWM WorkKrishna ShilNo ratings yet
- Salt AnalysisDocument63 pagesSalt Analysisabdullah khalilNo ratings yet
- Preparation of Acids: What Is An Acid?Document7 pagesPreparation of Acids: What Is An Acid?Alas CuatroNo ratings yet
- SaltsDocument17 pagesSaltsmaabelbasheer265No ratings yet
- Chemistry CHP Ter 8Document21 pagesChemistry CHP Ter 8IZIKNo ratings yet
- IGCSE CHEMISTRY Preparation of SaltsDocument4 pagesIGCSE CHEMISTRY Preparation of SaltsNayeemAhmed67% (3)
- Model Answers For PreparationofsaltsDocument2 pagesModel Answers For Preparationofsaltsapi-271128265No ratings yet
- EXP 10 (B) Mohr SaltDocument2 pagesEXP 10 (B) Mohr SaltSarita BhattNo ratings yet
- CHEMISTRY IGCSE REVISION by Aditi PrasadDocument13 pagesCHEMISTRY IGCSE REVISION by Aditi Prasadnksekyicann6No ratings yet
- Salts - Solubilities: E.G. E.GDocument10 pagesSalts - Solubilities: E.G. E.GTraci Yan Yan ChenNo ratings yet
- Salts: Pool 8 ChemistryDocument26 pagesSalts: Pool 8 ChemistryShanna-Loye MckenzieNo ratings yet
- Salts ..Document6 pagesSalts ..rachelNo ratings yet
- Preparation of Salts: Suitable For FORM 2,3, AND 4Document44 pagesPreparation of Salts: Suitable For FORM 2,3, AND 4Richard NestorNo ratings yet
- Paper 6 NotesDocument17 pagesPaper 6 NotesRamY El NahasNo ratings yet
- Chemistry NotesDocument36 pagesChemistry NotesAkshay AroraNo ratings yet
- Topic 8 SaltsDocument29 pagesTopic 8 SaltsNorZahirah Manje Sdo100% (1)
- 12) Acids, Bases and SaltsDocument11 pages12) Acids, Bases and Saltsbushramahmud6468No ratings yet
- Chem O Level NotesDocument2 pagesChem O Level NotesSherylNo ratings yet
- Presentasi Science Grup 1Document12 pagesPresentasi Science Grup 1Chelsica ChelsicaNo ratings yet
- IGCSE Chemistry - Identification of GasesDocument2 pagesIGCSE Chemistry - Identification of GasesNikhil YadavNo ratings yet
- ch18 NotesDocument1 pagech18 NotesOlivia LinNo ratings yet
- LAB Hydrates NumberSIXDocument3 pagesLAB Hydrates NumberSIXTiurma Debora SimatupangNo ratings yet
- Chemistry Atp Igcse RevisionDocument15 pagesChemistry Atp Igcse RevisionYannav NagpalNo ratings yet
- Fire Stone Via KermesDocument4 pagesFire Stone Via Kermesforest ravensonNo ratings yet
- Required Practical Tasks (GCSE) : 1 - Preparation of A Pure, Dry, SaltDocument7 pagesRequired Practical Tasks (GCSE) : 1 - Preparation of A Pure, Dry, Saltastha patelNo ratings yet
- Acids, Alkalis and Salts RevisionDocument19 pagesAcids, Alkalis and Salts RevisionJames EzardNo ratings yet
- Preparation of SaltsDocument6 pagesPreparation of SaltsSeyi IbitoyeNo ratings yet
- Prep NonmetalsDocument5 pagesPrep Nonmetalssiraaju98No ratings yet
- Salts and Their PreparationDocument11 pagesSalts and Their PreparationWafi Bin Hassan The InevitableNo ratings yet
- Ferrous Sulfate (An Official Inorganic Compound) : OccurrenceDocument11 pagesFerrous Sulfate (An Official Inorganic Compound) : Occurrencehumag143No ratings yet
- Salt and SolutionDocument33 pagesSalt and SolutionFarhan Altaf100% (1)
- Chemistry at Home - A Collection of Experiments and Formulas for the Chemistry EnthusiastFrom EverandChemistry at Home - A Collection of Experiments and Formulas for the Chemistry EnthusiastNo ratings yet
- The Chemistry of Soaps and Salts - Chemistry Book for Beginners | Children's Chemistry BooksFrom EverandThe Chemistry of Soaps and Salts - Chemistry Book for Beginners | Children's Chemistry BooksNo ratings yet
- The Chemistry of Fertilisers and Manure - Including Information on the Chemical Constituents and Types of Fertilisers and ManuresFrom EverandThe Chemistry of Fertilisers and Manure - Including Information on the Chemical Constituents and Types of Fertilisers and ManuresRating: 5 out of 5 stars5/5 (1)
Steps To Making Different Salts - ACTIVITY
Steps To Making Different Salts - ACTIVITY
Uploaded by
zionmills11Original Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Steps To Making Different Salts - ACTIVITY
Steps To Making Different Salts - ACTIVITY
Uploaded by
zionmills11Copyright:
Available Formats
The Three Methods for Making Salts
The three methods for making different salts are:
1. Making soluble salts (except sodium, potassium and ammonium salts)
2. Making sodium, potassium and ammonium salts
3. Making insoluble salts
Steps to making salts – they’re jumbled up so put them in order!
Making soluble salts e.g. CuSO4 (except sodium, potassium and ammonium salts)
Transfer the filtrate to an evaporating dish and heat to boil off some of the solution to make it more
concentrated – do not boil it dry
Add copper oxide until there is some left that will not react (you have added excess so there is no
more acid left)
Filter the excess copper oxide
Measure acid into a beaker and heat it
Allow the mixture to cool slowly at room temperature
Add copper oxide to your acid and continue to heat it.
Pat dry with a paper towel
Making sodium, potassium and ammonium salts (e.g. sodium chloride)
Use the ‘titration’ method
Add dilute hydrochloric acid into the flask from the burette until the indicator turns from yellow to
orange (red is too far)
This solution is heated to evaporate some of the water until a saturated solution is formed
Add NaOH solution to a flask using a pipette, with methyl orange as the indicator
Allow the mixture to cool slowly at room temperature
Making insoluble salts (e.g. lead sulfate)
Transfer the crystals/powder to a dry place (oven if there is one available) so the water evaporates
A precipitate will form
Filter the reaction mixture so you can catch the crystals you made (and are trying to obtain)
Use the precipitate method
Take 25cm3 of lead nitrate in a beaker and add 25cm3 of sodium sulfate
Wash the crystals you have made with distilled water. Repeat this step two more times
You might also like
- IGCSE Chemistry - Acids Bases and SaltsDocument13 pagesIGCSE Chemistry - Acids Bases and SaltsChemistryKlipz100% (12)
- Preparation and Purification of Soluble SaltsDocument12 pagesPreparation and Purification of Soluble SaltsJuni FarhanaNo ratings yet
- Preparation of Sodium Ethyl SulfateDocument2 pagesPreparation of Sodium Ethyl SulfateJoan Mas Torrent100% (3)
- Salt Preperation-To Prepare Soluble SaltsDocument4 pagesSalt Preperation-To Prepare Soluble Saltsadetorostephen0% (1)
- Preparation of SaltsDocument9 pagesPreparation of SaltsAkshay ReddyNo ratings yet
- Notes To Study For Chemistry Paper 6Document11 pagesNotes To Study For Chemistry Paper 6sakibsultan_308100% (1)
- Salt PreperationDocument3 pagesSalt PreperationEmaan ShahidNo ratings yet
- Chemistry ATPDocument4 pagesChemistry ATPinspectionNo ratings yet
- Notes To Study For Chemistry Paper 6Document8 pagesNotes To Study For Chemistry Paper 63abood51467% (6)
- Notes To Study For Chemistry Paper 6Document8 pagesNotes To Study For Chemistry Paper 6alibaslamNo ratings yet
- Preparation of SaltsDocument8 pagesPreparation of SaltsRose MusariraNo ratings yet
- Preparation of SaltsDocument8 pagesPreparation of SaltsTAKUNDA MARIMENo ratings yet
- 03 PreparingSaltsDocument3 pages03 PreparingSaltsDiamondNo ratings yet
- Preparing Soluble SaltsDocument10 pagesPreparing Soluble SaltsSuhaan HussainNo ratings yet
- Using A PipetteDocument1 pageUsing A PipetteeeenusNo ratings yet
- Preparing Soluble Salts 1Document9 pagesPreparing Soluble Salts 1Suhaan HussainNo ratings yet
- Preparing Soluble SaltsDocument5 pagesPreparing Soluble SaltsFaris Irfan100% (1)
- Methods of Preparing SaltsDocument6 pagesMethods of Preparing SaltsMahmoud Elsaied SolymanNo ratings yet
- كيمياء OL practical Review on P6 - 240117 - 171619Document110 pagesكيمياء OL practical Review on P6 - 240117 - 171619albasjudyNo ratings yet
- Making Crystals With SaltDocument2 pagesMaking Crystals With SaltNadia BasherNo ratings yet
- Name / Title Topic Learning Objective Key Science Concepts/ Main Messages Props/ MaterialsDocument3 pagesName / Title Topic Learning Objective Key Science Concepts/ Main Messages Props/ MaterialsHaha Hoho TNo ratings yet
- Preparation of SaltsDocument6 pagesPreparation of Saltssakibsultan_308No ratings yet
- Flow Chart For The Preparation of SaltsDocument1 pageFlow Chart For The Preparation of SaltsSaadiah MohammadNo ratings yet
- Preparation of SaltsDocument7 pagesPreparation of Saltsaltygeorge0No ratings yet
- CHWM WorkDocument1 pageCHWM WorkKrishna ShilNo ratings yet
- Salt AnalysisDocument63 pagesSalt Analysisabdullah khalilNo ratings yet
- Preparation of Acids: What Is An Acid?Document7 pagesPreparation of Acids: What Is An Acid?Alas CuatroNo ratings yet
- SaltsDocument17 pagesSaltsmaabelbasheer265No ratings yet
- Chemistry CHP Ter 8Document21 pagesChemistry CHP Ter 8IZIKNo ratings yet
- IGCSE CHEMISTRY Preparation of SaltsDocument4 pagesIGCSE CHEMISTRY Preparation of SaltsNayeemAhmed67% (3)
- Model Answers For PreparationofsaltsDocument2 pagesModel Answers For Preparationofsaltsapi-271128265No ratings yet
- EXP 10 (B) Mohr SaltDocument2 pagesEXP 10 (B) Mohr SaltSarita BhattNo ratings yet
- CHEMISTRY IGCSE REVISION by Aditi PrasadDocument13 pagesCHEMISTRY IGCSE REVISION by Aditi Prasadnksekyicann6No ratings yet
- Salts - Solubilities: E.G. E.GDocument10 pagesSalts - Solubilities: E.G. E.GTraci Yan Yan ChenNo ratings yet
- Salts: Pool 8 ChemistryDocument26 pagesSalts: Pool 8 ChemistryShanna-Loye MckenzieNo ratings yet
- Salts ..Document6 pagesSalts ..rachelNo ratings yet
- Preparation of Salts: Suitable For FORM 2,3, AND 4Document44 pagesPreparation of Salts: Suitable For FORM 2,3, AND 4Richard NestorNo ratings yet
- Paper 6 NotesDocument17 pagesPaper 6 NotesRamY El NahasNo ratings yet
- Chemistry NotesDocument36 pagesChemistry NotesAkshay AroraNo ratings yet
- Topic 8 SaltsDocument29 pagesTopic 8 SaltsNorZahirah Manje Sdo100% (1)
- 12) Acids, Bases and SaltsDocument11 pages12) Acids, Bases and Saltsbushramahmud6468No ratings yet
- Chem O Level NotesDocument2 pagesChem O Level NotesSherylNo ratings yet
- Presentasi Science Grup 1Document12 pagesPresentasi Science Grup 1Chelsica ChelsicaNo ratings yet
- IGCSE Chemistry - Identification of GasesDocument2 pagesIGCSE Chemistry - Identification of GasesNikhil YadavNo ratings yet
- ch18 NotesDocument1 pagech18 NotesOlivia LinNo ratings yet
- LAB Hydrates NumberSIXDocument3 pagesLAB Hydrates NumberSIXTiurma Debora SimatupangNo ratings yet
- Chemistry Atp Igcse RevisionDocument15 pagesChemistry Atp Igcse RevisionYannav NagpalNo ratings yet
- Fire Stone Via KermesDocument4 pagesFire Stone Via Kermesforest ravensonNo ratings yet
- Required Practical Tasks (GCSE) : 1 - Preparation of A Pure, Dry, SaltDocument7 pagesRequired Practical Tasks (GCSE) : 1 - Preparation of A Pure, Dry, Saltastha patelNo ratings yet
- Acids, Alkalis and Salts RevisionDocument19 pagesAcids, Alkalis and Salts RevisionJames EzardNo ratings yet
- Preparation of SaltsDocument6 pagesPreparation of SaltsSeyi IbitoyeNo ratings yet
- Prep NonmetalsDocument5 pagesPrep Nonmetalssiraaju98No ratings yet
- Salts and Their PreparationDocument11 pagesSalts and Their PreparationWafi Bin Hassan The InevitableNo ratings yet
- Ferrous Sulfate (An Official Inorganic Compound) : OccurrenceDocument11 pagesFerrous Sulfate (An Official Inorganic Compound) : Occurrencehumag143No ratings yet
- Salt and SolutionDocument33 pagesSalt and SolutionFarhan Altaf100% (1)
- Chemistry at Home - A Collection of Experiments and Formulas for the Chemistry EnthusiastFrom EverandChemistry at Home - A Collection of Experiments and Formulas for the Chemistry EnthusiastNo ratings yet
- The Chemistry of Soaps and Salts - Chemistry Book for Beginners | Children's Chemistry BooksFrom EverandThe Chemistry of Soaps and Salts - Chemistry Book for Beginners | Children's Chemistry BooksNo ratings yet
- The Chemistry of Fertilisers and Manure - Including Information on the Chemical Constituents and Types of Fertilisers and ManuresFrom EverandThe Chemistry of Fertilisers and Manure - Including Information on the Chemical Constituents and Types of Fertilisers and ManuresRating: 5 out of 5 stars5/5 (1)