Professional Documents
Culture Documents
Analysis of Nitrite Ion Contents in Meat Sample by UV Vis Spectroscopy Method Results and Discussion
Analysis of Nitrite Ion Contents in Meat Sample by UV Vis Spectroscopy Method Results and Discussion
Uploaded by
ANH NGUYỄN HUỲNH MINHCopyright:
Available Formats
You might also like
- BCBM 659 - Lab 3Document18 pagesBCBM 659 - Lab 3Nick Morettin0% (1)
- Lab Report (Atomic Absorption Spectroscopy)Document8 pagesLab Report (Atomic Absorption Spectroscopy)Shirley Cheong67% (6)
- ECP 224 Test 2 2020 SolutionDocument6 pagesECP 224 Test 2 2020 SolutionCaleb MunyairiNo ratings yet
- Determination of Manganese in Steel by Flame Atomic Absorption SpectrosDocument7 pagesDetermination of Manganese in Steel by Flame Atomic Absorption Spectrossexycassie100% (1)
- Adsorption - Solved ProblemsDocument5 pagesAdsorption - Solved Problemsshaik mohammed Arshad100% (1)
- Building Lego AtomsDocument3 pagesBuilding Lego Atomsapi-321330579No ratings yet
- AQA A-Level Physics My Revision Notes (Hodder 2017)Document231 pagesAQA A-Level Physics My Revision Notes (Hodder 2017)Petch JakrapatNo ratings yet
- Concentration vs. Absorbance: 1. Standard CurveDocument2 pagesConcentration vs. Absorbance: 1. Standard CurveHee MinNo ratings yet
- Lab AnalysisDocument4 pagesLab AnalysisErnestasBlaževičNo ratings yet
- Chemy 310 Experiment 5Document9 pagesChemy 310 Experiment 5Faisal MumtazNo ratings yet
- Choba 408 EXP 2Document12 pagesChoba 408 EXP 2Choba Tapaphiwa ChobaNo ratings yet
- Aas ManualDocument2 pagesAas ManualCharitra Prakash ChourasiaNo ratings yet
- Ion ChromatographyDocument9 pagesIon ChromatographyOm PhileNo ratings yet
- Experiment.4 Uv/Vis Spectrophotometry: Department of Chemistry University of Bahrain CHEMY310Document6 pagesExperiment.4 Uv/Vis Spectrophotometry: Department of Chemistry University of Bahrain CHEMY310Zahra Al-BasriNo ratings yet
- Uv Spectro PracDocument11 pagesUv Spectro PracLungeloNo ratings yet
- Determination of Composition of Complexes Using Jobs Method (1) NoDocument10 pagesDetermination of Composition of Complexes Using Jobs Method (1) NoCh Safdar FarukhNo ratings yet
- 01.ex Name Spectrophotometric Determination of Iron.Document4 pages01.ex Name Spectrophotometric Determination of Iron.Md Sohel RanaNo ratings yet
- PHY 2042 Experiment #10Document2 pagesPHY 2042 Experiment #10Kelsey WNo ratings yet
- Andres Anal Chem CalibrationDocument5 pagesAndres Anal Chem CalibrationAndres, Andrea Lyn M.No ratings yet
- Experiment 7 (Recovered)Document36 pagesExperiment 7 (Recovered)Manda BaboolalNo ratings yet
- Report 12 InstrumentalDocument3 pagesReport 12 InstrumentalKim Yến PhùngNo ratings yet
- Foster Cole 101230199 Malaïka Zarrouki 2021-01-29Document7 pagesFoster Cole 101230199 Malaïka Zarrouki 2021-01-29Cole FosterNo ratings yet
- CUSO4 PostlabDocument8 pagesCUSO4 PostlabRuwanthika Fernando100% (1)
- Chemistry LabDocument6 pagesChemistry LabOmar Khan100% (2)
- Heat Na DmassDocument8 pagesHeat Na DmassBrennie GohNo ratings yet
- Concentration Absorbance 0 0.0023 2 0.0173 4 0.0290 6 0.0365 8 0.0447 10 0.0642Document3 pagesConcentration Absorbance 0 0.0023 2 0.0173 4 0.0290 6 0.0365 8 0.0447 10 0.0642LOLANANo ratings yet
- Experiment 12: Determination of SO As Baso Using Gravimetry and by "Scattering"Document9 pagesExperiment 12: Determination of SO As Baso Using Gravimetry and by "Scattering"mandayiNo ratings yet
- Accuracy Percision LabDocument2 pagesAccuracy Percision LabAlexaNo ratings yet
- Chemical Kinetics: Chapter 14.1-2Document22 pagesChemical Kinetics: Chapter 14.1-2Sagar GurowNo ratings yet
- Study Session 1 AnswersDocument4 pagesStudy Session 1 Answerssayani dasNo ratings yet
- Homework 2 (Ch11) - 2020Document4 pagesHomework 2 (Ch11) - 2020Keiko CheungNo ratings yet
- Exercises of Analytical Chemistry - Part 1: A. Stoichiometry ReminderDocument16 pagesExercises of Analytical Chemistry - Part 1: A. Stoichiometry ReminderTeresa RiosNo ratings yet
- Chem Exp-2Document6 pagesChem Exp-2aanika roshniNo ratings yet
- Uv Vis Spectroscopy Lab Report of The Detection of Analytes From Environmental Samples Using UvDocument13 pagesUv Vis Spectroscopy Lab Report of The Detection of Analytes From Environmental Samples Using UvSaba Naseer100% (1)
- Chapter 8 SolutionsDocument9 pagesChapter 8 SolutionsARSYIAN RIZKI PRATAMANo ratings yet
- Problem Set 6 UV Vis Absorption Spectroscopy1Document10 pagesProblem Set 6 UV Vis Absorption Spectroscopy1Konata OzumiNo ratings yet
- CALCULATIONSDocument3 pagesCALCULATIONSgoabaone kgopaNo ratings yet
- Assignment 5Document8 pagesAssignment 5Adam FaisalNo ratings yet
- Zeroth Order: T, S 25º C Ca M LN (CA0/CA) 1/caDocument4 pagesZeroth Order: T, S 25º C Ca M LN (CA0/CA) 1/caTÙNGNo ratings yet
- Kinetics of The Cis-Trans Isomerization of 4-Anilino-4'-NitroazobenzeneDocument13 pagesKinetics of The Cis-Trans Isomerization of 4-Anilino-4'-NitroazobenzeneMikahNo ratings yet
- AbsorptionDocument7 pagesAbsorptionIrvan DwikiNo ratings yet
- Question & Answer Set-6 PDFDocument3 pagesQuestion & Answer Set-6 PDFhp2020No ratings yet
- Lab 1Document6 pagesLab 1Tiyah TimothyNo ratings yet
- PBRDocument19 pagesPBRdarvyneeNo ratings yet
- Absorbancecoefficient PDFDocument2 pagesAbsorbancecoefficient PDFkofinyameNo ratings yet
- 3 B 3 Print Able VersionDocument2 pages3 B 3 Print Able Versionfernanda boldtNo ratings yet
- Absorbancecoefficient PDFDocument2 pagesAbsorbancecoefficient PDFEduardo GarzaNo ratings yet
- Homework 1 Student Answer WebCTDocument3 pagesHomework 1 Student Answer WebCTTsz Wun CHOWNo ratings yet
- Written Report in ChemistryDocument8 pagesWritten Report in Chemistrybunso padillaNo ratings yet
- Ja6b05345 Si 001Document15 pagesJa6b05345 Si 001HendNo ratings yet
- Exercise 1: Spectrochemical Analysis: BE132P Instrumentation in Biological Engineering 1Document7 pagesExercise 1: Spectrochemical Analysis: BE132P Instrumentation in Biological Engineering 1Bernadette Virola CuevasNo ratings yet
- Flas K# Mass of Charcoa L (G) Initial Concentrati On of Acoh (M) Volume of Naoh Used (ML) Run 1 Run 2Document2 pagesFlas K# Mass of Charcoa L (G) Initial Concentrati On of Acoh (M) Volume of Naoh Used (ML) Run 1 Run 2shaiNo ratings yet
- Exp 1Document8 pagesExp 1Chin RamosNo ratings yet
- Engr. Besavilla - Lecture 03 - 10 Nov 2023Document15 pagesEngr. Besavilla - Lecture 03 - 10 Nov 2023Rhowelle TibayNo ratings yet
- Spectroscopy Report FinalDocument4 pagesSpectroscopy Report Finaljlco88No ratings yet
- Chapter 2-Fall-2022-2023-C411Document31 pagesChapter 2-Fall-2022-2023-C411hesham khaledNo ratings yet
- Experiment No: 6: Feed Tanks Batch ReactorDocument5 pagesExperiment No: 6: Feed Tanks Batch Reactorfareeha saeedNo ratings yet
- Lab Report Beer Lambert'S Law Experiment: Determing The Concentration of Unknown Asa SolutionsDocument5 pagesLab Report Beer Lambert'S Law Experiment: Determing The Concentration of Unknown Asa Solutionsumair saleemNo ratings yet
- Atomic Absorption Spectroscopy: CHEMY 313 Analytical ChemistryDocument7 pagesAtomic Absorption Spectroscopy: CHEMY 313 Analytical ChemistryJassim123 SabtNo ratings yet
- Chemistry 2a Form Iv Marking Scheme-1Document4 pagesChemistry 2a Form Iv Marking Scheme-1Mohammed B.S. MakimuNo ratings yet
- Experiment 1: The Visible Spectra of Soft Drinks: A. Pre-Laboratory QuestionsDocument5 pagesExperiment 1: The Visible Spectra of Soft Drinks: A. Pre-Laboratory QuestionsMuhd Mirza HizamiNo ratings yet
- Analytical Modeling of Solute Transport in Groundwater: Using Models to Understand the Effect of Natural Processes on Contaminant Fate and TransportFrom EverandAnalytical Modeling of Solute Transport in Groundwater: Using Models to Understand the Effect of Natural Processes on Contaminant Fate and TransportNo ratings yet
- Spin Orbit CouplingDocument8 pagesSpin Orbit CouplingRajdeep BanerjeeNo ratings yet
- Spectrophotometer: Parts of The SystemDocument4 pagesSpectrophotometer: Parts of The SystemIraqiNo ratings yet
- Phy475 Homework 3Document1 pagePhy475 Homework 3ProfAndré GazotoNo ratings yet
- Assignment 2 GC-MS FFFDocument9 pagesAssignment 2 GC-MS FFFJessie Ctiffany EveNo ratings yet
- Chapter 15: Molecular LuminescenceDocument17 pagesChapter 15: Molecular LuminescenceUsman GhaniNo ratings yet
- Honors ChemistryDocument2 pagesHonors ChemistryLama DebanyNo ratings yet
- Chapter 6 FLSDocument30 pagesChapter 6 FLSnurul najwaNo ratings yet
- Baselec q1 QuestionsDocument5 pagesBaselec q1 QuestionsroiNo ratings yet
- Best Pastpaper For Aqa Oxford ChemsitryDocument25 pagesBest Pastpaper For Aqa Oxford Chemsitryemandurranix09No ratings yet
- Tutorial Mass SpectrometryDocument7 pagesTutorial Mass SpectrometryJasmeetSinghNo ratings yet
- Principles of Pulse Electron Paramagnetic Resonance - Arthur Schweiger, Gunnar Jeschke (2001)Document301 pagesPrinciples of Pulse Electron Paramagnetic Resonance - Arthur Schweiger, Gunnar Jeschke (2001)hippimeNo ratings yet
- BEC-BCS Crossover: Diego Luis Velasco-GonzálezDocument7 pagesBEC-BCS Crossover: Diego Luis Velasco-GonzálezDIEGO LUIS VELASCO GONZALEZNo ratings yet
- Radiation Safety Officers Handbook A PDFDocument100 pagesRadiation Safety Officers Handbook A PDFAlejandro Zubiate100% (1)
- ACTIVITY 2 Formation of Light ElementsDocument6 pagesACTIVITY 2 Formation of Light ElementsWerNo ratings yet
- NMR Info Tables 12-31-09Document48 pagesNMR Info Tables 12-31-09NahdaNo ratings yet
- Inductively Coupled PlasmaDocument8 pagesInductively Coupled Plasmagerarjui100% (1)
- Mid Year Paper2 F4 2010Document23 pagesMid Year Paper2 F4 2010Renee YipNo ratings yet
- Physical Science 2112Document3 pagesPhysical Science 2112Cherrylyn DonayreNo ratings yet
- Franck-Hertz Experiment With Hg-TubeDocument4 pagesFranck-Hertz Experiment With Hg-TubeAlexandraFlorentynaNo ratings yet
- Chem 110, Chapter 9 UDLDocument50 pagesChem 110, Chapter 9 UDL5fyqv62kytNo ratings yet
- Chem - June 2022 (R) QPDocument36 pagesChem - June 2022 (R) QPLing JaiNo ratings yet
- Atomic Absorption SpectrosDocument13 pagesAtomic Absorption Spectrosatikah100% (1)
- Topic 12.2 FormativeDocument10 pagesTopic 12.2 FormativeAhmad OmarNo ratings yet
- 2 12Document22 pages2 12Kira BezkorovainaNo ratings yet
- Nuclear Magnetic Resonance: Half-Integer Odd Odd or EvenDocument19 pagesNuclear Magnetic Resonance: Half-Integer Odd Odd or EvenRAJ VYASNo ratings yet
- The Structure of The Atom - Boundless ChemistryDocument13 pagesThe Structure of The Atom - Boundless ChemistrySheena Shane CantelaNo ratings yet
- 8th Grade Science ReviewDocument5 pages8th Grade Science Reviewapi-327567606No ratings yet
Analysis of Nitrite Ion Contents in Meat Sample by UV Vis Spectroscopy Method Results and Discussion
Analysis of Nitrite Ion Contents in Meat Sample by UV Vis Spectroscopy Method Results and Discussion
Uploaded by
ANH NGUYỄN HUỲNH MINHOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Analysis of Nitrite Ion Contents in Meat Sample by UV Vis Spectroscopy Method Results and Discussion
Analysis of Nitrite Ion Contents in Meat Sample by UV Vis Spectroscopy Method Results and Discussion
Uploaded by
ANH NGUYỄN HUỲNH MINHCopyright:
Available Formats
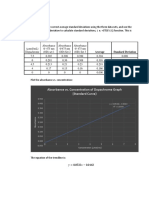
RESULTS AND DISCUSSION:
1.1. Absorption spectrum:
𝝀𝒎𝒂𝒙 = 538 (nm)
Absorbance (A)
Wavelength (nm)
Figure 1: Absorbance spectrum of 0.5 ppm nitrite solution at pH ~ 8.0 – 8.5
From Figure 1, absorbance was measured at wavelength 400 nm to 800 nm. The
curve reaches the peak of 538 (nm), therefore 𝜆𝑚𝑎𝑥 = 538 (nm) will be used to
measure the absorbance of samples at different concentrations for plotting the
standard calibration curve.
1.2. The standard calibration curve:
Using the UV-Vis spectrophotometer to analyze the absorbance of samples
with the concentration 0, 1, 2, 3, 4 (ppm), respectively at 𝜆 = 538 (nm) to obtain
the results in Table 1.
Table 1. Absorbance of water sample at different concentrations
Concentration (ppm) 0 1 2 3 4
Absorbance 0 0.0382 0.0765 0.1052 0.1597
0.16
0.14
0.12
Absorbance (A)
0.1
0.08
0.06
y = 0.0386x - 0.0014
R² = 0.9913
0.04
0.02
0
0 1 2 3 4
Concentration (ppm)
Figure 2: Calibration curve illustrates the relationship between
the absorbance and nitrite concentration
Figure 2 shows the relationship between the absorbance and nitrite
concentration by a straight line with the equation is expressed by: y = 0.0386x –
0.014 in which y is absorbance (A) and x is concentration (ppm). Since R 2 =
0.9913, the equation mentioned above completely follows Lambert – Beer’s law.
Thus, this result can be applied to calculate the concentration of nitrite ion in a
meat sample.
1.3. Calculation:
𝐴1 + 𝐴2 + ⋯ + 𝐴𝑛
𝐴̅ =
𝑛
𝐴̅ + 0.014 6 (25 × 10−3 ) × 69 × 10−3 𝑚𝑔
𝐶 𝑁𝑎𝑁𝑂2 𝑖𝑛 𝑠𝑎𝑚𝑝𝑙𝑒 = × × (𝑝𝑝𝑚 𝑜𝑟 )
0.0386 100 5.000 × 10−3 𝑘𝑔
You might also like
- BCBM 659 - Lab 3Document18 pagesBCBM 659 - Lab 3Nick Morettin0% (1)
- Lab Report (Atomic Absorption Spectroscopy)Document8 pagesLab Report (Atomic Absorption Spectroscopy)Shirley Cheong67% (6)
- ECP 224 Test 2 2020 SolutionDocument6 pagesECP 224 Test 2 2020 SolutionCaleb MunyairiNo ratings yet
- Determination of Manganese in Steel by Flame Atomic Absorption SpectrosDocument7 pagesDetermination of Manganese in Steel by Flame Atomic Absorption Spectrossexycassie100% (1)
- Adsorption - Solved ProblemsDocument5 pagesAdsorption - Solved Problemsshaik mohammed Arshad100% (1)
- Building Lego AtomsDocument3 pagesBuilding Lego Atomsapi-321330579No ratings yet
- AQA A-Level Physics My Revision Notes (Hodder 2017)Document231 pagesAQA A-Level Physics My Revision Notes (Hodder 2017)Petch JakrapatNo ratings yet
- Concentration vs. Absorbance: 1. Standard CurveDocument2 pagesConcentration vs. Absorbance: 1. Standard CurveHee MinNo ratings yet
- Lab AnalysisDocument4 pagesLab AnalysisErnestasBlaževičNo ratings yet
- Chemy 310 Experiment 5Document9 pagesChemy 310 Experiment 5Faisal MumtazNo ratings yet
- Choba 408 EXP 2Document12 pagesChoba 408 EXP 2Choba Tapaphiwa ChobaNo ratings yet
- Aas ManualDocument2 pagesAas ManualCharitra Prakash ChourasiaNo ratings yet
- Ion ChromatographyDocument9 pagesIon ChromatographyOm PhileNo ratings yet
- Experiment.4 Uv/Vis Spectrophotometry: Department of Chemistry University of Bahrain CHEMY310Document6 pagesExperiment.4 Uv/Vis Spectrophotometry: Department of Chemistry University of Bahrain CHEMY310Zahra Al-BasriNo ratings yet
- Uv Spectro PracDocument11 pagesUv Spectro PracLungeloNo ratings yet
- Determination of Composition of Complexes Using Jobs Method (1) NoDocument10 pagesDetermination of Composition of Complexes Using Jobs Method (1) NoCh Safdar FarukhNo ratings yet
- 01.ex Name Spectrophotometric Determination of Iron.Document4 pages01.ex Name Spectrophotometric Determination of Iron.Md Sohel RanaNo ratings yet
- PHY 2042 Experiment #10Document2 pagesPHY 2042 Experiment #10Kelsey WNo ratings yet
- Andres Anal Chem CalibrationDocument5 pagesAndres Anal Chem CalibrationAndres, Andrea Lyn M.No ratings yet
- Experiment 7 (Recovered)Document36 pagesExperiment 7 (Recovered)Manda BaboolalNo ratings yet
- Report 12 InstrumentalDocument3 pagesReport 12 InstrumentalKim Yến PhùngNo ratings yet
- Foster Cole 101230199 Malaïka Zarrouki 2021-01-29Document7 pagesFoster Cole 101230199 Malaïka Zarrouki 2021-01-29Cole FosterNo ratings yet
- CUSO4 PostlabDocument8 pagesCUSO4 PostlabRuwanthika Fernando100% (1)
- Chemistry LabDocument6 pagesChemistry LabOmar Khan100% (2)
- Heat Na DmassDocument8 pagesHeat Na DmassBrennie GohNo ratings yet
- Concentration Absorbance 0 0.0023 2 0.0173 4 0.0290 6 0.0365 8 0.0447 10 0.0642Document3 pagesConcentration Absorbance 0 0.0023 2 0.0173 4 0.0290 6 0.0365 8 0.0447 10 0.0642LOLANANo ratings yet
- Experiment 12: Determination of SO As Baso Using Gravimetry and by "Scattering"Document9 pagesExperiment 12: Determination of SO As Baso Using Gravimetry and by "Scattering"mandayiNo ratings yet
- Accuracy Percision LabDocument2 pagesAccuracy Percision LabAlexaNo ratings yet
- Chemical Kinetics: Chapter 14.1-2Document22 pagesChemical Kinetics: Chapter 14.1-2Sagar GurowNo ratings yet
- Study Session 1 AnswersDocument4 pagesStudy Session 1 Answerssayani dasNo ratings yet
- Homework 2 (Ch11) - 2020Document4 pagesHomework 2 (Ch11) - 2020Keiko CheungNo ratings yet
- Exercises of Analytical Chemistry - Part 1: A. Stoichiometry ReminderDocument16 pagesExercises of Analytical Chemistry - Part 1: A. Stoichiometry ReminderTeresa RiosNo ratings yet
- Chem Exp-2Document6 pagesChem Exp-2aanika roshniNo ratings yet
- Uv Vis Spectroscopy Lab Report of The Detection of Analytes From Environmental Samples Using UvDocument13 pagesUv Vis Spectroscopy Lab Report of The Detection of Analytes From Environmental Samples Using UvSaba Naseer100% (1)
- Chapter 8 SolutionsDocument9 pagesChapter 8 SolutionsARSYIAN RIZKI PRATAMANo ratings yet
- Problem Set 6 UV Vis Absorption Spectroscopy1Document10 pagesProblem Set 6 UV Vis Absorption Spectroscopy1Konata OzumiNo ratings yet
- CALCULATIONSDocument3 pagesCALCULATIONSgoabaone kgopaNo ratings yet
- Assignment 5Document8 pagesAssignment 5Adam FaisalNo ratings yet
- Zeroth Order: T, S 25º C Ca M LN (CA0/CA) 1/caDocument4 pagesZeroth Order: T, S 25º C Ca M LN (CA0/CA) 1/caTÙNGNo ratings yet
- Kinetics of The Cis-Trans Isomerization of 4-Anilino-4'-NitroazobenzeneDocument13 pagesKinetics of The Cis-Trans Isomerization of 4-Anilino-4'-NitroazobenzeneMikahNo ratings yet
- AbsorptionDocument7 pagesAbsorptionIrvan DwikiNo ratings yet
- Question & Answer Set-6 PDFDocument3 pagesQuestion & Answer Set-6 PDFhp2020No ratings yet
- Lab 1Document6 pagesLab 1Tiyah TimothyNo ratings yet
- PBRDocument19 pagesPBRdarvyneeNo ratings yet
- Absorbancecoefficient PDFDocument2 pagesAbsorbancecoefficient PDFkofinyameNo ratings yet
- 3 B 3 Print Able VersionDocument2 pages3 B 3 Print Able Versionfernanda boldtNo ratings yet
- Absorbancecoefficient PDFDocument2 pagesAbsorbancecoefficient PDFEduardo GarzaNo ratings yet
- Homework 1 Student Answer WebCTDocument3 pagesHomework 1 Student Answer WebCTTsz Wun CHOWNo ratings yet
- Written Report in ChemistryDocument8 pagesWritten Report in Chemistrybunso padillaNo ratings yet
- Ja6b05345 Si 001Document15 pagesJa6b05345 Si 001HendNo ratings yet
- Exercise 1: Spectrochemical Analysis: BE132P Instrumentation in Biological Engineering 1Document7 pagesExercise 1: Spectrochemical Analysis: BE132P Instrumentation in Biological Engineering 1Bernadette Virola CuevasNo ratings yet
- Flas K# Mass of Charcoa L (G) Initial Concentrati On of Acoh (M) Volume of Naoh Used (ML) Run 1 Run 2Document2 pagesFlas K# Mass of Charcoa L (G) Initial Concentrati On of Acoh (M) Volume of Naoh Used (ML) Run 1 Run 2shaiNo ratings yet
- Exp 1Document8 pagesExp 1Chin RamosNo ratings yet
- Engr. Besavilla - Lecture 03 - 10 Nov 2023Document15 pagesEngr. Besavilla - Lecture 03 - 10 Nov 2023Rhowelle TibayNo ratings yet
- Spectroscopy Report FinalDocument4 pagesSpectroscopy Report Finaljlco88No ratings yet
- Chapter 2-Fall-2022-2023-C411Document31 pagesChapter 2-Fall-2022-2023-C411hesham khaledNo ratings yet
- Experiment No: 6: Feed Tanks Batch ReactorDocument5 pagesExperiment No: 6: Feed Tanks Batch Reactorfareeha saeedNo ratings yet
- Lab Report Beer Lambert'S Law Experiment: Determing The Concentration of Unknown Asa SolutionsDocument5 pagesLab Report Beer Lambert'S Law Experiment: Determing The Concentration of Unknown Asa Solutionsumair saleemNo ratings yet
- Atomic Absorption Spectroscopy: CHEMY 313 Analytical ChemistryDocument7 pagesAtomic Absorption Spectroscopy: CHEMY 313 Analytical ChemistryJassim123 SabtNo ratings yet
- Chemistry 2a Form Iv Marking Scheme-1Document4 pagesChemistry 2a Form Iv Marking Scheme-1Mohammed B.S. MakimuNo ratings yet
- Experiment 1: The Visible Spectra of Soft Drinks: A. Pre-Laboratory QuestionsDocument5 pagesExperiment 1: The Visible Spectra of Soft Drinks: A. Pre-Laboratory QuestionsMuhd Mirza HizamiNo ratings yet
- Analytical Modeling of Solute Transport in Groundwater: Using Models to Understand the Effect of Natural Processes on Contaminant Fate and TransportFrom EverandAnalytical Modeling of Solute Transport in Groundwater: Using Models to Understand the Effect of Natural Processes on Contaminant Fate and TransportNo ratings yet
- Spin Orbit CouplingDocument8 pagesSpin Orbit CouplingRajdeep BanerjeeNo ratings yet
- Spectrophotometer: Parts of The SystemDocument4 pagesSpectrophotometer: Parts of The SystemIraqiNo ratings yet
- Phy475 Homework 3Document1 pagePhy475 Homework 3ProfAndré GazotoNo ratings yet
- Assignment 2 GC-MS FFFDocument9 pagesAssignment 2 GC-MS FFFJessie Ctiffany EveNo ratings yet
- Chapter 15: Molecular LuminescenceDocument17 pagesChapter 15: Molecular LuminescenceUsman GhaniNo ratings yet
- Honors ChemistryDocument2 pagesHonors ChemistryLama DebanyNo ratings yet
- Chapter 6 FLSDocument30 pagesChapter 6 FLSnurul najwaNo ratings yet
- Baselec q1 QuestionsDocument5 pagesBaselec q1 QuestionsroiNo ratings yet
- Best Pastpaper For Aqa Oxford ChemsitryDocument25 pagesBest Pastpaper For Aqa Oxford Chemsitryemandurranix09No ratings yet
- Tutorial Mass SpectrometryDocument7 pagesTutorial Mass SpectrometryJasmeetSinghNo ratings yet
- Principles of Pulse Electron Paramagnetic Resonance - Arthur Schweiger, Gunnar Jeschke (2001)Document301 pagesPrinciples of Pulse Electron Paramagnetic Resonance - Arthur Schweiger, Gunnar Jeschke (2001)hippimeNo ratings yet
- BEC-BCS Crossover: Diego Luis Velasco-GonzálezDocument7 pagesBEC-BCS Crossover: Diego Luis Velasco-GonzálezDIEGO LUIS VELASCO GONZALEZNo ratings yet
- Radiation Safety Officers Handbook A PDFDocument100 pagesRadiation Safety Officers Handbook A PDFAlejandro Zubiate100% (1)
- ACTIVITY 2 Formation of Light ElementsDocument6 pagesACTIVITY 2 Formation of Light ElementsWerNo ratings yet
- NMR Info Tables 12-31-09Document48 pagesNMR Info Tables 12-31-09NahdaNo ratings yet
- Inductively Coupled PlasmaDocument8 pagesInductively Coupled Plasmagerarjui100% (1)
- Mid Year Paper2 F4 2010Document23 pagesMid Year Paper2 F4 2010Renee YipNo ratings yet
- Physical Science 2112Document3 pagesPhysical Science 2112Cherrylyn DonayreNo ratings yet
- Franck-Hertz Experiment With Hg-TubeDocument4 pagesFranck-Hertz Experiment With Hg-TubeAlexandraFlorentynaNo ratings yet
- Chem 110, Chapter 9 UDLDocument50 pagesChem 110, Chapter 9 UDL5fyqv62kytNo ratings yet
- Chem - June 2022 (R) QPDocument36 pagesChem - June 2022 (R) QPLing JaiNo ratings yet
- Atomic Absorption SpectrosDocument13 pagesAtomic Absorption Spectrosatikah100% (1)
- Topic 12.2 FormativeDocument10 pagesTopic 12.2 FormativeAhmad OmarNo ratings yet
- 2 12Document22 pages2 12Kira BezkorovainaNo ratings yet
- Nuclear Magnetic Resonance: Half-Integer Odd Odd or EvenDocument19 pagesNuclear Magnetic Resonance: Half-Integer Odd Odd or EvenRAJ VYASNo ratings yet
- The Structure of The Atom - Boundless ChemistryDocument13 pagesThe Structure of The Atom - Boundless ChemistrySheena Shane CantelaNo ratings yet
- 8th Grade Science ReviewDocument5 pages8th Grade Science Reviewapi-327567606No ratings yet