Professional Documents
Culture Documents
Is Matter Around Us Pure
Is Matter Around Us Pure
Uploaded by
anweshaasingh22Original Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Is Matter Around Us Pure
Is Matter Around Us Pure
Uploaded by
anweshaasingh22Copyright:
Available Formats
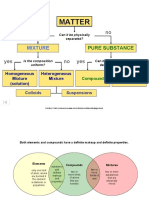
Is Matter Around Us Pure?
Substances
Cannot be broken down further with any physical processes
Made up of only one type of atoms.
Made up of only one element.
Compounds
Cannot be broken down further with any physical processes
Made of more than 1 type of atoms.
Eg : We cannot break the atoms of H2O (water) physically using any physical process.
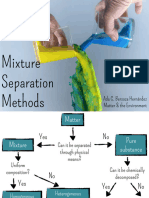
Mixtures
They can be broken down further with physical processes.
Eg : H2O + NaCl can be separated and broken down using evaporation.
When elements are mixed with elements/compounds and compounds are mixed with
compounds/elements without any chemical change, then they can be broken down further with
physical processes.
Matter
Pure Impure
Element True Solution
Compound Suspension
Colloidal Solution
Mixture
Homogenous Heterogenous
True Solution Colloid and Suspension
Solvent is the part of the solution which is larger in quantity and the solute gets dissolved in the
solvent.
Eg - Water sugar solution:
Solute - water
Solvent – Sugar
Homogenous:
Equal proportion of solute and solvent throughout the solution.
Heterogenous
Unequal proportion of solute and solvent throughout the solution.
Tyndall Effect is the light scattering by particles in a colloid or very fine suspension.
Components
Solution = Solvent + Solute
Colloidal Solution = Dispersed medium + Dispersed Phase
Suspension = Medium + Suspended Particle
Solubility is the limit/extent to which the solute can be added to the solvent.
Saturated solution is a solution where the extent to which the solute can be added the solvent has been
reached whereas Unsaturated solution is a solution where the extent to which the solute can be added to the
solvent has not been reached.
Temperature is directly proportional to solubility.
mass/mass = mass of solute/solution (for percentage multiply 100)
mass/volume = mass of solution/volume of solution (for percentage multiply 100)
In a saturated solution, if we mix sugar in x ml water, the volume will not change.
You might also like
- 27 Succession-AnswersDocument8 pages27 Succession-Answersapi-32508966457% (14)
- Ebook PDF Environment Science Issues Solutions by Manuel MollesDocument61 pagesEbook PDF Environment Science Issues Solutions by Manuel Mollesdeborah.sheehan548100% (54)
- A Greener CityDocument7 pagesA Greener CityEric Adams 2021No ratings yet
- Detailed Lesson Plan in Grade 7Document7 pagesDetailed Lesson Plan in Grade 7Jeremy Pomar89% (37)
- Solutions Class 12th Chemistry NotesDocument22 pagesSolutions Class 12th Chemistry NotesSjft6hd Ff100% (12)
- Chemistry Grade 10 Mixtures and SeparationDocument45 pagesChemistry Grade 10 Mixtures and SeparationTrudy- Ann CaineNo ratings yet
- Grade 7 Mixture SubstancesDocument27 pagesGrade 7 Mixture SubstancesRoSs Adrales Areleg100% (1)
- Környezetvédelem TételDocument3 pagesKörnyezetvédelem Tételrencsi0611No ratings yet
- Module - V. CH - 20 .Mixtures & SolutionsDocument27 pagesModule - V. CH - 20 .Mixtures & SolutionsHemant DeshmukhNo ratings yet
- Lesson2 MixturesDocument28 pagesLesson2 Mixturesapi-432159577No ratings yet
- Journal: - Describe The Difference Between A Mixture and A CompoundDocument19 pagesJournal: - Describe The Difference Between A Mixture and A Compoundnatalie_iris_delgado100% (1)
- IPC-Solutions PPT-BordersDocument30 pagesIPC-Solutions PPT-BordersJhen BonNo ratings yet
- Is Matter Around Us PureDocument13 pagesIs Matter Around Us PureNANDITA NAYAK BNo ratings yet
- WWW Askiitians Com Revision Notes Class 9 Science Is Matter Around Us PureDocument17 pagesWWW Askiitians Com Revision Notes Class 9 Science Is Matter Around Us PureDeepak Kumar SharmaNo ratings yet
- Is Matter Around Us Pure Notes PDFDocument15 pagesIs Matter Around Us Pure Notes PDFgkclubakshayaNo ratings yet
- Lecture 5Document30 pagesLecture 5ziaifzaNo ratings yet
- Physical Properties of SolutionsDocument19 pagesPhysical Properties of SolutionsJescil Ann OriolNo ratings yet
- G7 Science Q1 - Week 7-Concentration of SolutionDocument39 pagesG7 Science Q1 - Week 7-Concentration of SolutionChristian Matthew AguilarNo ratings yet
- Reviewer Module 3 ChemDocument7 pagesReviewer Module 3 Chemidieh ligNo ratings yet
- Science LessonsDocument8 pagesScience LessonsAnita PoshNo ratings yet
- Chapter 2 - Matter Around Us PureDocument14 pagesChapter 2 - Matter Around Us PureSwathi KarunakaranNo ratings yet
- Elements Compounds MixturesDocument45 pagesElements Compounds MixturesPaige PolkNo ratings yet
- ChemistryDocument26 pagesChemistryfanumohd1010No ratings yet
- Diversity of Materials NewDocument37 pagesDiversity of Materials Newmichameilromero13No ratings yet
- Classifying Matter: Elements, Compounds, and MixturesDocument45 pagesClassifying Matter: Elements, Compounds, and Mixturesaini.mohamat.zoomNo ratings yet
- Solubility of DrugsDocument147 pagesSolubility of Drugsharshagadia234No ratings yet
- Is Matter Around Us Pure NotesDocument11 pagesIs Matter Around Us Pure NotesMahima varshneyNo ratings yet
- Chapter 11 Properties of Solutions (Sulaiman Al-Isaee's Conflicted Copy)Document64 pagesChapter 11 Properties of Solutions (Sulaiman Al-Isaee's Conflicted Copy)iB13eNo ratings yet
- Saturated and UnsaturatedDocument20 pagesSaturated and UnsaturatedBernadette Sta. AnaNo ratings yet
- Chapter 12-SolutionsDocument32 pagesChapter 12-SolutionsNada MeselhyNo ratings yet
- Notes-Science-is Matter Around Us PureDocument10 pagesNotes-Science-is Matter Around Us PureHina SharmaNo ratings yet
- Matterclass PresDocument12 pagesMatterclass PresFitz BaniquedNo ratings yet
- Kimia Larutan: Moondra Zubir, PH.DDocument23 pagesKimia Larutan: Moondra Zubir, PH.DmaudysakinahNo ratings yet
- Chemistry: Classification of MatterDocument29 pagesChemistry: Classification of MatterRamzen Raphael DomingoNo ratings yet
- Matter ClassifiedDocument20 pagesMatter ClassifiedShefa CapurasNo ratings yet
- A.3 Classification of Matter by CompositionDocument44 pagesA.3 Classification of Matter by CompositionMA. HAZEL TEOLOGONo ratings yet
- Chapter-2 - IS MATTER AROUND US PUREDocument25 pagesChapter-2 - IS MATTER AROUND US PURESATYAM RATHOURNo ratings yet
- Matter ClassifiedDocument20 pagesMatter Classifiedsandeep.pandeyNo ratings yet
- Mixture Separation Methods-1Document31 pagesMixture Separation Methods-1Mariana Martínez RangelNo ratings yet
- Physics (Electric Flux)Document3 pagesPhysics (Electric Flux)Charline Lucille BanoNo ratings yet
- Chemistry (Solutions)Document3 pagesChemistry (Solutions)Charline Lucille BanoNo ratings yet
- What Is Mixture: Is Matter Around Us PureDocument19 pagesWhat Is Mixture: Is Matter Around Us PureAditya Kumar SinghNo ratings yet
- Week 5 - Pure Substance and MixtureDocument48 pagesWeek 5 - Pure Substance and MixtureGalang AlphaNo ratings yet
- Classification of MatterDocument30 pagesClassification of MatterCharl Rey PilonNo ratings yet
- What Are Mixtures and SolutionsDocument4 pagesWhat Are Mixtures and SolutionsWacoomo TaroomaNo ratings yet
- Solutions (Autosaved)Document42 pagesSolutions (Autosaved)joiechristinemarie28sarsonasNo ratings yet
- Notes CH - Is Matter Around Us PureDocument4 pagesNotes CH - Is Matter Around Us Pureadityamanik.121No ratings yet
- Class 9 Chemistry Notes Chapter 2 and 4Document10 pagesClass 9 Chemistry Notes Chapter 2 and 4ap4618720No ratings yet
- DiffusionDocument62 pagesDiffusionHamza KhanNo ratings yet
- Mixtures and SubstanceDocument6 pagesMixtures and SubstanceRajin Fritz ArmaNo ratings yet
- Solubility of Drugs PDFDocument66 pagesSolubility of Drugs PDFPrabhas MeherNo ratings yet
- Solutions Stoichiometry EquilibriumDocument79 pagesSolutions Stoichiometry EquilibriumKat JornadalNo ratings yet
- Solubility and Distribution PhenomenaDocument89 pagesSolubility and Distribution Phenomenadesekar sejati100% (2)
- Solubility and Distribution Phenomena: Aseel SamaroDocument89 pagesSolubility and Distribution Phenomena: Aseel Samaroveneta gizdakovaNo ratings yet
- Classification of MatterDocument14 pagesClassification of MattermichelleNo ratings yet
- растворы и концентрация 1Document21 pagesрастворы и концентрация 1Dorama AikaNo ratings yet
- Is Matter Around Us PureDocument9 pagesIs Matter Around Us Pureella groverNo ratings yet
- Topic 1 Stoichiometric RelationshipsDocument49 pagesTopic 1 Stoichiometric RelationshipsMohammad Andrew GhaniNo ratings yet
- Physical Properties of SolutionsDocument15 pagesPhysical Properties of SolutionsShiela Mae PortilloNo ratings yet
- Physically CombinedDocument82 pagesPhysically CombinedRonalyn CariñoNo ratings yet
- IV. Properties of SolutionDocument11 pagesIV. Properties of SolutionHania ABDULNo ratings yet
- SolutionsDocument32 pagesSolutionsMayuresh PanseNo ratings yet
- Topic 7-Properties of Solutions CopyDocument37 pagesTopic 7-Properties of Solutions CopyKenneth DalionNo ratings yet
- The Big Chemistry Book on Solutions - Chemistry for 4th Graders | Children's Chemistry BooksFrom EverandThe Big Chemistry Book on Solutions - Chemistry for 4th Graders | Children's Chemistry BooksNo ratings yet
- Oil and Water Won't Mix and Other Mixture Separation Techniques - Chemistry Book for Kids 8-10 | Children's Chemistry BooksFrom EverandOil and Water Won't Mix and Other Mixture Separation Techniques - Chemistry Book for Kids 8-10 | Children's Chemistry BooksNo ratings yet
- 2009 HKCEE Physics P2 SolutionsDocument20 pages2009 HKCEE Physics P2 Solutionssnoopysnoopy1990No ratings yet
- The Swine Flu Pandemic: You Should Spend About 20 Minutes On Questions 1-13 Which Are Based On Reading Passage 1 BelowDocument7 pagesThe Swine Flu Pandemic: You Should Spend About 20 Minutes On Questions 1-13 Which Are Based On Reading Passage 1 BelowrobinNo ratings yet
- DLA TLE 8 Week 6Document2 pagesDLA TLE 8 Week 6Joyce Dela Rama JulianoNo ratings yet
- Atoms and Atomic Theory: Essential Questions: How Can We Describe TH e Molecular Motion of TH e States of Matter?Document29 pagesAtoms and Atomic Theory: Essential Questions: How Can We Describe TH e Molecular Motion of TH e States of Matter?Anonymous eMOb79RNt5No ratings yet
- A. Inoue (Auth.), Professor Akihisa Inoue, Professor Koji Hashimoto (Eds.) - Amorphous and Nanocrystalline Materials - Preparation, Properties, and Applications-SpringeDocument216 pagesA. Inoue (Auth.), Professor Akihisa Inoue, Professor Koji Hashimoto (Eds.) - Amorphous and Nanocrystalline Materials - Preparation, Properties, and Applications-Springeunknown1711No ratings yet
- Lecture 3: Rock Forming Minerals: Ardhi UniversityDocument61 pagesLecture 3: Rock Forming Minerals: Ardhi UniversityInnocent RevocatusNo ratings yet
- Inter HWKDocument5 pagesInter HWKMohammed Elias AlamNo ratings yet
- Mathew Dzingai Design 2016-17 PDFDocument309 pagesMathew Dzingai Design 2016-17 PDFAuliverKayMHNo ratings yet
- Report On Hvac Design of A BuildingDocument66 pagesReport On Hvac Design of A BuildingHemani SinghNo ratings yet
- Energy Profile of SomaliaDocument4 pagesEnergy Profile of SomaliaEngineerAnoj KumarNo ratings yet
- Earth and Life Science: Quarter 1 - Module 3: MineralsDocument26 pagesEarth and Life Science: Quarter 1 - Module 3: MineralsAdonis Besa92% (53)
- Electrostatic Analysis of Power Line: Author: Electromagneticworks Company: Electromagneticworks IncDocument12 pagesElectrostatic Analysis of Power Line: Author: Electromagneticworks Company: Electromagneticworks IncCNEMWNo ratings yet
- Physical Science: Quarter 1 - Module 2: The Atomic Number and The Synthesis of New ElementsDocument24 pagesPhysical Science: Quarter 1 - Module 2: The Atomic Number and The Synthesis of New ElementsArthur LaurelNo ratings yet
- Forensic Aspects of Arson and Explosion InvestigationsDocument26 pagesForensic Aspects of Arson and Explosion InvestigationsSyeda Noor FatimaNo ratings yet
- Electrostatics: OutlineDocument27 pagesElectrostatics: OutlineAndrei AlidoNo ratings yet
- What Is A CellDocument2 pagesWhat Is A Celljose de jesus sanchezNo ratings yet
- Forest Service National Prescribed Fire Program Review-2022Document107 pagesForest Service National Prescribed Fire Program Review-2022Albuquerque JournalNo ratings yet
- Tutorial 4 QuestionsDocument2 pagesTutorial 4 QuestionsJiNx AngNo ratings yet
- Science 8 Q4 Week 7 NewDocument19 pagesScience 8 Q4 Week 7 NewROGIE GALAGAR SAZNo ratings yet
- Ac71656 1996 Coldweatheroperations PDFDocument290 pagesAc71656 1996 Coldweatheroperations PDFKebede Michael100% (3)
- ASCE Load Combination and Wind CalculationDocument4 pagesASCE Load Combination and Wind Calculationyugandhar singhNo ratings yet
- Lesson 5Document10 pagesLesson 5Michaela Joice BalayanNo ratings yet
- Active and Passive Methods of Avalanche Hazard MitigationDocument3 pagesActive and Passive Methods of Avalanche Hazard Mitigationshanmuka dinesh gangapuramNo ratings yet
- Environmental Impact-Earth Atmospheric LayersDocument22 pagesEnvironmental Impact-Earth Atmospheric LayersShirley J MoralesNo ratings yet
- Heavy Mineral Concentration in BangladeshDocument2 pagesHeavy Mineral Concentration in BangladeshApu DeyNo ratings yet