Professional Documents
Culture Documents
HC Copy of Salts
HC Copy of Salts
Uploaded by
ceeernest5310 ratings0% found this document useful (0 votes)
3 views14 pagesCompounds formed from reaction of acids and bases. A salt is a compound containing either a metal ion or ammonium ion and a negative ion from an acid. Normal salts give neutral solutions in water while acid salts give acidic solutions. Solubility depends on the specific salt. Soluble salts are usually prepared by reaction of acids and bases or acids and carbonates. Insoluble salts require double decomposition or direct combination methods. Oxides are compounds of oxygen with another element and can be acidic, basic, neutral, or amphoteric depending on their reactions with acids and bases.
Original Description:
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCompounds formed from reaction of acids and bases. A salt is a compound containing either a metal ion or ammonium ion and a negative ion from an acid. Normal salts give neutral solutions in water while acid salts give acidic solutions. Solubility depends on the specific salt. Soluble salts are usually prepared by reaction of acids and bases or acids and carbonates. Insoluble salts require double decomposition or direct combination methods. Oxides are compounds of oxygen with another element and can be acidic, basic, neutral, or amphoteric depending on their reactions with acids and bases.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
3 views14 pagesHC Copy of Salts
HC Copy of Salts
Uploaded by
ceeernest531Compounds formed from reaction of acids and bases. A salt is a compound containing either a metal ion or ammonium ion and a negative ion from an acid. Normal salts give neutral solutions in water while acid salts give acidic solutions. Solubility depends on the specific salt. Soluble salts are usually prepared by reaction of acids and bases or acids and carbonates. Insoluble salts require double decomposition or direct combination methods. Oxides are compounds of oxygen with another element and can be acidic, basic, neutral, or amphoteric depending on their reactions with acids and bases.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 14
SALTS
Compounds formed from reaction
of acids and bases
Lesson Outcomes
• Be able to define a salt (both normal and
acid)
• Give examples of formulae of salts
(normal and acid)
• List the solubilities of salts
• State the different methods of preparing a
salt and which method is suitable for which
type of salt
• Be able to define an oxide and state the
different types of oxides with examples
Definitions
• A salt is a compound containing either a metal ion OR
an ammonium ion and a negative ion from an acid e.g.
calcium chloride CaCl2, ammonium nitrate NH4NO3
• A normal salt is one in which ALL of the hydrogen ions
from an acid are replaced by metal or ammonium ions
e.g NaCl, MgSO4, Ca3(PO4)2. Normal salts give neutral
solutions when dissolved in water
• An acid salt is one in which SOME of the hydrogen
ions from an acid are replaced by metal or ammonium
ions e.g. sodium hydrogen sulphate NaHSO4,
disodium hydrogen phosphate Na2HPO4. Acid salts
give acidic solutions when dissolved in water
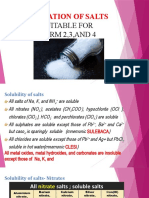
Solubilities of salts
1. All nitrates are soluble
2. All sulphates are soluble except lead sulphate, barium
sulphate and calcium sulphate
3. All chlorides are soluble except lead (II) chloride, silver
chloride and mercury (II) chloride
4. All carbonates are insoluble except sodium carbonate,
potassium carbonate and ammonium carbonate
5. All hydroxides are insoluble except sodium hydroxide,
potassium hydroxide, ammonium hydroxide and calcium
hydroxide (these are not salts but need to be known as well)
Preparation of salts
• Salt preparation depends on the solubility of the salt.
• Soluble salts can be prepared by either:-
• a) reaction of metal and acid (not commonly used)
• b) reaction of base and acid (commonly used)
• c) reaction of carbonate and acid (not as common as b) but
more common than a)
• d) reaction of alkali and acid (only if the base is an alkali i.e.
soluble in water e.g. sodium hydroxide or potassium
hydroxide)
• If the salt must be prepared in the ABSENCE of water (that
is anhydrous), then direct combination or synthesis is used.
• If the salt is insoluble in water, it must be prepared by ionic
precipitation (also called double decomposition)
Soluble salt preparation
• In almost all cases, soluble salts are
prepared by the action of acid & base
OR acid & carbonate.
• The method called titration is used
when the salt being produced is
formed from reaction of
acid and alkali
Preparation of copper(II)
sulphate crystals exercise 1 of 3
Watch the video and answer
the following questions.
a) What are the names of the
TWO substances were used
to make the copper(II)
sulphate crystals?
b) Give a reason why hot
water was used
Preparation of copper(II)
sulphate crystals exercise 2 of 3
Watch the video and answer
the following questions.
c) Write the chemical equation
for the reaction of the two
substances.
d) What is the colour of the
copper(II) sulphate solution?
Preparation of copper(II)
sulphate crystals exercise 3 of 3
Watch the video and answer the
following questions.
e) Why was excess solid used in
the preparation of the salt?
f) How is the excess solid removed?
g) How is the formation of the
crystals encouraged?
Insoluble salt preparation
• If the salt is required in anhydrous form, direct
combination of the elements may be necessary
like in the production of iron(II) sulphide
• If the insoluble salt can be in the presence of
water, then ionic precipitation or double
decomposition is used like in the production of
lead(II) iodide
Oxides
• Definition: An oxide is a compound containing
oxygen and one other element.
• There are 4 types of oxides:- acidic, basic, neutral
and amphoteric
• Acidic oxides (non-metal oxides) form acids when
dissolved in water e.g. CO2, SO2
• Basic oxides (metal oxides) e.g. CaO act as bases
• Neutral oxides (carbon monoxide CO and nitrogen
monoxide NO have no reaction with either acids or
bases)
• Amphoteric oxides react with BOTH acids and
bases e.g. aluminium oxide Al2O3, zinc oxide ZnO
and lead(II) oxide PbO
Practice Question 1 of 2
Practice Questions 2 of 2
END OF THE TOPIC SALTS
You might also like
- Preparation of Salts PDFDocument24 pagesPreparation of Salts PDFSoniaAlex50% (2)
- Castner Kellner ProcessDocument19 pagesCastner Kellner ProcessRajat Singh Chauhan100% (1)
- Acids and BasesDocument36 pagesAcids and Baseschong5680% (5)
- Antacid Suspension With Oxetacaine & SimethiconeDocument5 pagesAntacid Suspension With Oxetacaine & SimethiconePatricia Joyce Malabanan Sunglao100% (1)
- Acid Bases and SaltsDocument70 pagesAcid Bases and Saltscampbelltyesha4No ratings yet
- S3 Chemistryppt 060323Document27 pagesS3 Chemistryppt 060323helena a.sNo ratings yet
- Notes On SaltsDocument4 pagesNotes On SaltsFelix S100% (1)
- Salts and Salt PreparationDocument36 pagesSalts and Salt PreparationGABRIELLE FOSTER100% (1)
- SALTSDocument7 pagesSALTSabbigailaliNo ratings yet
- 8.6 OxidesDocument6 pages8.6 Oxides29seolaiscooltbhNo ratings yet
- Salt PreparationDocument151 pagesSalt Preparationash kingNo ratings yet
- Salts: Prepared by Alrick MoodieDocument12 pagesSalts: Prepared by Alrick MoodieAllanelevateNo ratings yet
- SALTSDocument6 pagesSALTSlisacarter2468No ratings yet
- Acids, Bases & SaltsDocument31 pagesAcids, Bases & SaltsAndre BirchNo ratings yet
- C16 Neutralisation and Salts%2020230511Document54 pagesC16 Neutralisation and Salts%20202305116gvm4nv2fnNo ratings yet
- AcidsBases - Oxides and SaltsDocument20 pagesAcidsBases - Oxides and SaltsZain AhmadNo ratings yet
- 2.4. Salts 2022Document24 pages2.4. Salts 2022davidnyachieo50No ratings yet
- GCE Study Buddy Chemistry NotesDocument43 pagesGCE Study Buddy Chemistry Notesanwar9602020No ratings yet
- 100L Lecture 4 SaltsDocument6 pages100L Lecture 4 SaltsMichael EhondorNo ratings yet
- Activity 17 (Preparation of Salts)Document4 pagesActivity 17 (Preparation of Salts)Nkemzi Elias NzetengenleNo ratings yet
- 3E5NA Sci Chem Qualitative Analysis Notes Student'sDocument19 pages3E5NA Sci Chem Qualitative Analysis Notes Student'sAditi Ravi kaushikNo ratings yet
- Chapter 11 - Acids, Bases, and Salts PDFDocument7 pagesChapter 11 - Acids, Bases, and Salts PDFAarush SharmaNo ratings yet
- G10 Revised Acids, Bases and SaltsDocument81 pagesG10 Revised Acids, Bases and Saltsandrew.mooreNo ratings yet
- Pak Studies BookDocument10 pagesPak Studies Bookibrahimmkhan777No ratings yet
- Acids, Alkalis and Salts RevisionDocument19 pagesAcids, Alkalis and Salts RevisionJames EzardNo ratings yet
- Salts: Pool 8 ChemistryDocument26 pagesSalts: Pool 8 ChemistryShanna-Loye MckenzieNo ratings yet
- Acid, Salt and Salt PreparationDocument13 pagesAcid, Salt and Salt PreparationLiton DasNo ratings yet
- 12) Acids, Bases and SaltsDocument11 pages12) Acids, Bases and Saltsbushramahmud6468No ratings yet
- Chemistry 10Document29 pagesChemistry 10Javed QasimNo ratings yet
- Grade 10 Chemistry Week 12 Lesson 1Document4 pagesGrade 10 Chemistry Week 12 Lesson 1nesiaroberts903No ratings yet
- Chapter 8: SaltsDocument14 pagesChapter 8: SaltsLynn HengNo ratings yet
- Hsbc2103 Topic 8Document29 pagesHsbc2103 Topic 8Rohani Abdul ShukorNo ratings yet
- Acids, Bases and Salts 3BDocument34 pagesAcids, Bases and Salts 3Bkesiangeorge07No ratings yet
- 5.3 and 5.4 SaltsDocument19 pages5.3 and 5.4 SaltsLandau StudentNo ratings yet
- Acid Base Neutralisation SaltsDocument5 pagesAcid Base Neutralisation SaltsGaurav YadavNo ratings yet
- 2.4. SaltsDocument27 pages2.4. Saltsgabrielsuva6No ratings yet
- 6.1 Role of Water in Showing Chemical Properties of Acid and AlkaliDocument29 pages6.1 Role of Water in Showing Chemical Properties of Acid and AlkaliNur Shahirah100% (1)
- Acid Base and SaltsDocument19 pagesAcid Base and SaltslindaoeghagharaNo ratings yet
- Acids, Bases, Salts-IG ChemistryDocument16 pagesAcids, Bases, Salts-IG ChemistryRashi GhadiyaNo ratings yet
- Unit 7 Salts PDFDocument20 pagesUnit 7 Salts PDFFatima KabirNo ratings yet
- Chemistry Chapter 8 SaltsDocument32 pagesChemistry Chapter 8 SaltsnorlieyNo ratings yet
- Preparation of Salts: Suitable For FORM 2,3, AND 4Document44 pagesPreparation of Salts: Suitable For FORM 2,3, AND 4Richard NestorNo ratings yet
- Acids, Bases and SaltsDocument16 pagesAcids, Bases and SaltsRhea FrancisNo ratings yet
- Notes Salts (Chemistry)Document32 pagesNotes Salts (Chemistry)Darishana100% (1)
- HC Copy of 3rd Form Acids and BasesDocument32 pagesHC Copy of 3rd Form Acids and Basesceeernest531No ratings yet
- Topic 8 SaltsDocument29 pagesTopic 8 SaltsNorZahirah Manje Sdo100% (1)
- CH 3Document2 pagesCH 3danish12mciNo ratings yet
- ABS Complete - ChemisteryDocument71 pagesABS Complete - ChemisterymitaNo ratings yet
- 9.1 Acid and BasesDocument32 pages9.1 Acid and BasesUmida ZaylobiddinovaNo ratings yet
- Acids and Bases Lesson 2Document9 pagesAcids and Bases Lesson 2Waleed SaifNo ratings yet
- 8.1 Definitions of SaltsDocument5 pages8.1 Definitions of Saltsscta94No ratings yet
- Experiment Number: One Degree of Dissociation of Double Salts and Complex Compounds 1. ObjectiveDocument24 pagesExperiment Number: One Degree of Dissociation of Double Salts and Complex Compounds 1. ObjectiveMosisa DugasaNo ratings yet
- How To Test For Acids and Bases, Netralisation ReactionsDocument14 pagesHow To Test For Acids and Bases, Netralisation Reactionsshelter musasaNo ratings yet
- Chapter 8 SALTSDocument75 pagesChapter 8 SALTSSiti Hajar Abd HamidNo ratings yet
- Notes Updates SaltsDocument32 pagesNotes Updates SaltsLim Jing YeeNo ratings yet
- Acids and BasesDocument98 pagesAcids and BasesLaziNo ratings yet
- Acids Bases and SaltsDocument6 pagesAcids Bases and SaltsHanaa AbouziedNo ratings yet
- Ch-2 Part-2Document4 pagesCh-2 Part-2Kartik BhardwajNo ratings yet
- Third Term Chemistry SS1Document75 pagesThird Term Chemistry SS1Sunday Ozovehe100% (1)
- SaltsDocument34 pagesSaltscar_yii100% (1)
- Std10 Science EM 3 PDFDocument90 pagesStd10 Science EM 3 PDFVivek AnandanNo ratings yet
- Abbott's American Watchmaker: An Encyclopedia for the Horologist, Jeweler, Gold and SilversmithFrom EverandAbbott's American Watchmaker: An Encyclopedia for the Horologist, Jeweler, Gold and SilversmithNo ratings yet
- Acids and AlkalisDocument17 pagesAcids and AlkalisSumi VjNo ratings yet
- My TestDocument6 pagesMy TestMarin PesicNo ratings yet
- Learning Activity Sheets in Grade 12 General Chemistry 1: (WEEK2)Document6 pagesLearning Activity Sheets in Grade 12 General Chemistry 1: (WEEK2)johnnymar edemNo ratings yet
- Sample Paper-Ftre-2022-Class-X-P2-PcmDocument20 pagesSample Paper-Ftre-2022-Class-X-P2-Pcmhipakin454100% (1)
- 24-09-23 - JR - Iit - Star Co-Sc (Model-A) - Jee Adv - Nm-I (P-I) - Wat-21 - QPDocument18 pages24-09-23 - JR - Iit - Star Co-Sc (Model-A) - Jee Adv - Nm-I (P-I) - Wat-21 - QPnamratagolechhaNo ratings yet
- Science - Form 4 - Chapter 5Document12 pagesScience - Form 4 - Chapter 5Marcia PattersonNo ratings yet
- IB Chemistry SL ReviewDocument120 pagesIB Chemistry SL ReviewShamwow_12389% (9)
- Science Most Important Questions by PKM For 2023Document241 pagesScience Most Important Questions by PKM For 2023HIMANK BOOBNo ratings yet
- Nsejs PapersDocument197 pagesNsejs PapersAjiteshNo ratings yet
- Chemical Resistance Chart: What We Need To KnowDocument7 pagesChemical Resistance Chart: What We Need To Knowcipta karyaNo ratings yet
- All NCERT Books PDF From WWW - Ncert.onlineDocument16 pagesAll NCERT Books PDF From WWW - Ncert.onlineKaushik RajNo ratings yet
- GCSE Triple Chemistry 2023Document70 pagesGCSE Triple Chemistry 2023Ramy MohamedNo ratings yet
- Extraction of Scandium From Lateritic Nickel-Cobalt Ore Leach Solution by Ion Exchange: A Special Study and Literature Review On Previous WorksDocument10 pagesExtraction of Scandium From Lateritic Nickel-Cobalt Ore Leach Solution by Ion Exchange: A Special Study and Literature Review On Previous WorksmtanaydinNo ratings yet
- PHARMACEUTICAL INORGANIC CHEMISTRY: Gastrointestinal Agents (Antacid)Document9 pagesPHARMACEUTICAL INORGANIC CHEMISTRY: Gastrointestinal Agents (Antacid)MD REFATNo ratings yet
- NCERT Class 10 Science Lab Manual Materials PDFDocument80 pagesNCERT Class 10 Science Lab Manual Materials PDFMadhu MithaNo ratings yet
- Carbon Steel Billets, Blooms, Slabs and Bars For ForgingsDocument9 pagesCarbon Steel Billets, Blooms, Slabs and Bars For ForgingsHizkia Yarden SinagaNo ratings yet
- Bag. 2 Stabilitas Dan KetidakstabilanDocument33 pagesBag. 2 Stabilitas Dan KetidakstabilanAesyah FadhilahNo ratings yet
- Chemistry Lab ManualDocument112 pagesChemistry Lab ManualSanthana SethuramanNo ratings yet
- NSTSE Class 7 Solved Paper 2009Document23 pagesNSTSE Class 7 Solved Paper 2009swainanjanNo ratings yet
- NCERT-Solution-CBSE-Class-10-Science-Chapter-2 Tyrtgrtrtgrn BBBBGHGHGHGHDocument8 pagesNCERT-Solution-CBSE-Class-10-Science-Chapter-2 Tyrtgrtrtgrn BBBBGHGHGHGHNew Day RocksNo ratings yet
- Campbell Lecture Notes Chemistry of LifeDocument42 pagesCampbell Lecture Notes Chemistry of LifeSophia Andrei VillalunaNo ratings yet
- Polyetherether Ketone (PEEK) Chemical Compatibility ChartDocument14 pagesPolyetherether Ketone (PEEK) Chemical Compatibility Chartteban09No ratings yet
- G2 SolubilityDocument3 pagesG2 SolubilityBryan AliNo ratings yet
- Calculation in ChemistryDocument4 pagesCalculation in ChemistryCHONG PEI SI MoeNo ratings yet
- 5070 s07 QP 4Document20 pages5070 s07 QP 4mstudy123456No ratings yet
- I I Formularies Hard Surface Cleaning Industrial Metal Cleaners 110-12-009 USDocument8 pagesI I Formularies Hard Surface Cleaning Industrial Metal Cleaners 110-12-009 USbexigaobrother100% (5)
- Parjanya Kumar Shukla Dr. Amita VermaDocument23 pagesParjanya Kumar Shukla Dr. Amita VermaPrathiNo ratings yet
- Methods of Sampling and Test (Physical and Chemical) For Water and Waste WaterDocument2 pagesMethods of Sampling and Test (Physical and Chemical) For Water and Waste WaterAnish kumarNo ratings yet