Professional Documents
Culture Documents
Script 1
Script 1
Uploaded by
hUnTer X gaming0 ratings0% found this document useful (0 votes)
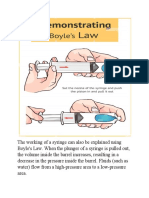
3 views1 pageThe document introduces an experiment to demonstrate Boyle's Law, which states that the volume of a gas is inversely proportional to its pressure. It explains that Robert Boyle discovered this law and defined it as the volume of a gas decreasing with increasing pressure or vice versa. The experiment uses a balloon inside a plastic container to represent lungs, showing how squeezing the container (increasing pressure) decreases the balloon's volume, while releasing the squeeze (decreasing pressure) increases the balloon's volume, illustrating the inverse relationship between pressure and volume defined by Boyle's Law.
Original Description:
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document introduces an experiment to demonstrate Boyle's Law, which states that the volume of a gas is inversely proportional to its pressure. It explains that Robert Boyle discovered this law and defined it as the volume of a gas decreasing with increasing pressure or vice versa. The experiment uses a balloon inside a plastic container to represent lungs, showing how squeezing the container (increasing pressure) decreases the balloon's volume, while releasing the squeeze (decreasing pressure) increases the balloon's volume, illustrating the inverse relationship between pressure and volume defined by Boyle's Law.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
3 views1 pageScript 1
Script 1
Uploaded by
hUnTer X gamingThe document introduces an experiment to demonstrate Boyle's Law, which states that the volume of a gas is inversely proportional to its pressure. It explains that Robert Boyle discovered this law and defined it as the volume of a gas decreasing with increasing pressure or vice versa. The experiment uses a balloon inside a plastic container to represent lungs, showing how squeezing the container (increasing pressure) decreases the balloon's volume, while releasing the squeeze (decreasing pressure) increases the balloon's volume, illustrating the inverse relationship between pressure and volume defined by Boyle's Law.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 1
SCRIPT
Hi guys
Are you guys ready?
So today we will have an experiment about Boyle’s Law
But before that, what is Boyle’s Law and who discovered it?
Robert Boyle
He is the one who discovered that the volume of a gas decreases
with increasing pressure and vice versa – which we call Boyle’s Law.
What is Boyle’s Law
Boyle’s Law is a gas law that states that a gas pressure and
volume are inversely proportional.
Let's do an EXPERIMENTTTTTT
Here is a DIY Respiratory System, the balloon inside represents
our lungs.
When I put pressure inside by pulling this plastic, the balloon
decreases.
Did you see that?
However, if I pull this plastic making the pressure decreases the
volume of the lung or the balloon increases.
This experiment shows how Boyle’s Law works and how pressure is
inversely proportional to volume.
You might also like
- Siyensikula-Boyle's LawDocument3 pagesSiyensikula-Boyle's Lawrolando dumlaoNo ratings yet
- G10 Science Q4 - Boyle's Law (Gas Law)Document13 pagesG10 Science Q4 - Boyle's Law (Gas Law)Sky HadesNo ratings yet
- ScienceDocument17 pagesSciencegones12010No ratings yet
- Gas Laws Boyle'S Law: Presented By: Raymar V. Villarama Althea Lynn B. CallantaDocument17 pagesGas Laws Boyle'S Law: Presented By: Raymar V. Villarama Althea Lynn B. CallantaAlthea Lynn B. CallantaNo ratings yet
- BoyleDocument1 pageBoylePASTEURSYNCH GELBOLINGO, ERNIE IINo ratings yet
- Boyle's LawDocument10 pagesBoyle's Lawja.abarcaa15No ratings yet
- Boyles LawDocument11 pagesBoyles Lawapi-284878968No ratings yet
- Definition of Boyle's Law: What Is An Ideal Gas?Document4 pagesDefinition of Boyle's Law: What Is An Ideal Gas?حسين كاظم ياسينNo ratings yet
- GAS LAWS: Boyle's Law, Charles's Law and Gay-Lussac's Law: Waste-Face-Masks-Ppe-CovidDocument9 pagesGAS LAWS: Boyle's Law, Charles's Law and Gay-Lussac's Law: Waste-Face-Masks-Ppe-CovidMichiiee BatallaNo ratings yet
- Application of BoyleDocument10 pagesApplication of BoyleRuthcel BautistaNo ratings yet
- Boyles LawDocument1 pageBoyles LawRevilloNo ratings yet
- Info GraphicDocument3 pagesInfo GraphicRainNo ratings yet
- Boyle's Law - SummaryDocument1 pageBoyle's Law - SummaryMichael VegoNo ratings yet
- LoveubabykoDocument2 pagesLoveubabykoSheila Mae NimNo ratings yet
- Boyles LawDocument6 pagesBoyles LawMara TillesNo ratings yet
- Boyle's Law DCDocument1 pageBoyle's Law DChidayatNo ratings yet
- Boyle's Law LESSON PLANDocument5 pagesBoyle's Law LESSON PLANQueencess Ara TorresNo ratings yet
- Boyles LawDocument17 pagesBoyles LawRuss Afuang100% (1)
- Boyles LawDocument9 pagesBoyles Lawanakabah89100% (1)
- Boyles LawDocument12 pagesBoyles LawAlyana Grace GuquibNo ratings yet
- DocumentDocument1 pageDocumentcristinemaelumanao18No ratings yet
- Procedure of Boyle's LawDocument2 pagesProcedure of Boyle's Lawkimmymantos022No ratings yet
- SCIENCE10 Boyles LawDocument33 pagesSCIENCE10 Boyles Lawjelyn aquinoNo ratings yet
- Importance of AirDocument6 pagesImportance of AirJames UgbesNo ratings yet
- Boyles LawDocument10 pagesBoyles LawsibaljodeelynNo ratings yet
- ExamplesDocument12 pagesExamplesMoldir SerikNo ratings yet
- Miguel, John Ryan, D.G Lab Act 7Document3 pagesMiguel, John Ryan, D.G Lab Act 7Simon DeleonNo ratings yet
- Boyles Law CompleteDocument4 pagesBoyles Law CompleteLincoln PiaoanNo ratings yet
- Science10 Q4 Mod1 v2Document47 pagesScience10 Q4 Mod1 v2Zodiac KiluaNo ratings yet
- Reading Article On Gas LawsDocument2 pagesReading Article On Gas LawsJeng JengNo ratings yet
- Boyle's LawDocument24 pagesBoyle's LawRalph Jasil Esparagoza EscanillasNo ratings yet
- GASES and The Kinetic ThoeryDocument5 pagesGASES and The Kinetic ThoeryEnkhbayasgalan SodbayarNo ratings yet
- Boyle's Law, Also Referred To As TheDocument4 pagesBoyle's Law, Also Referred To As TheMaria Lana Grace DiazNo ratings yet
- Boyles Law Lesson PlanDocument2 pagesBoyles Law Lesson PlanFany Fabia60% (5)
- Science ScriptDocument2 pagesScience ScriptTrixie San DiegoNo ratings yet
- Gas LawsDocument4 pagesGas LawspriyaamirthaNo ratings yet
- CATCH Up Friday Chem 1Document1 pageCATCH Up Friday Chem 1Promiseland Christian schoolNo ratings yet
- 3-4 Gas Laws Int - Reader - Study - Guide PDFDocument6 pages3-4 Gas Laws Int - Reader - Study - Guide PDFVara BikkinaNo ratings yet
- Boyle's LawDocument19 pagesBoyle's Lawcabilesrobilyn479No ratings yet
- Tilles, Mara - Science - JHSDocument3 pagesTilles, Mara - Science - JHSMara TillesNo ratings yet
- q4 Las2 g10 Science Boyles-LawDocument9 pagesq4 Las2 g10 Science Boyles-Lawtheobarrios14No ratings yet
- Science10 Q4 Mod1 BoyleslawDocument17 pagesScience10 Q4 Mod1 BoyleslawPrincess PanulayaNo ratings yet
- Application of Gas Laws Ano Ilalagay SaDocument3 pagesApplication of Gas Laws Ano Ilalagay SaB04 Caisip Jonel A.No ratings yet
- Gas Laws: Pressure, Volume, and Hot Air: A Chemistry Lesson For 10 Grade Students Created by Warren MerkelDocument30 pagesGas Laws: Pressure, Volume, and Hot Air: A Chemistry Lesson For 10 Grade Students Created by Warren MerkelDheeDazPequina100% (1)
- Use The Law To Explain The Following ObservationsDocument6 pagesUse The Law To Explain The Following ObservationsCedric Adrian AblogNo ratings yet
- Application of Gas LawsDocument2 pagesApplication of Gas LawsFrancis MuthiguaNo ratings yet
- Empirical: Compression Gas Temperature Robert Boyle Pressure Edme MariotteDocument2 pagesEmpirical: Compression Gas Temperature Robert Boyle Pressure Edme MariotteSaad KhanNo ratings yet
- 3-4 Gas Laws Int - Reader - Study - Guide PDFDocument6 pages3-4 Gas Laws Int - Reader - Study - Guide PDFel graceNo ratings yet
- Lab Report No.-4 Boyle's LawDocument3 pagesLab Report No.-4 Boyle's LawSimply IdinaNo ratings yet
- Avo KashviDocument10 pagesAvo KashviJames Patrick De LeonNo ratings yet
- Boyles Law Homework HelpDocument5 pagesBoyles Law Homework Helpvnutclwlf100% (2)
- L2 Boyle's LawDocument28 pagesL2 Boyle's LawMonosomniacNo ratings yet
- Roselyn 1Document5 pagesRoselyn 1Lecsos clyde Pang-agNo ratings yet
- Charles's LawDocument5 pagesCharles's LawScribdTranslationsNo ratings yet
- Elementary Gas LawsDocument2 pagesElementary Gas LawsZyndee de GuzmanNo ratings yet
- Gas LawsDocument27 pagesGas LawsGenesis Ng0% (3)
- Grade 10fourth Quarter: Boyle's LawDocument1 pageGrade 10fourth Quarter: Boyle's LawRommer PublicoNo ratings yet
- Gas Laws ReviewerDocument12 pagesGas Laws ReviewerMhehycel GadonNo ratings yet
- Real-World Examples: Data AnalysisDocument6 pagesReal-World Examples: Data AnalysisiSmayli (smyle-smayl)No ratings yet