Professional Documents
Culture Documents
Head and Neck Lesions
Head and Neck Lesions
Uploaded by
lunaghilvin20260 ratings0% found this document useful (0 votes)
32 views131 pagesThis document summarizes various head and neck lesions, including both non-neoplastic and neoplastic lesions of the upper aerodigestive tract and salivary glands. Precancerous lesions such as leukoplakia and erythroplakia are described. Non-neoplastic lesions including candidiasis, herpes, lichen planus, and hairy leukoplakia are also summarized. Finally, the document outlines the clinical, microscopic, and immunohistochemical features of various squamous cell carcinomas, salivary gland tumors, sinonasal tumors, and other neoplasms of the head and neck region.
Original Description:
Original Title
Head-and-Neck-Lesions
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document summarizes various head and neck lesions, including both non-neoplastic and neoplastic lesions of the upper aerodigestive tract and salivary glands. Precancerous lesions such as leukoplakia and erythroplakia are described. Non-neoplastic lesions including candidiasis, herpes, lichen planus, and hairy leukoplakia are also summarized. Finally, the document outlines the clinical, microscopic, and immunohistochemical features of various squamous cell carcinomas, salivary gland tumors, sinonasal tumors, and other neoplasms of the head and neck region.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
32 views131 pagesHead and Neck Lesions
Head and Neck Lesions
Uploaded by
lunaghilvin2026This document summarizes various head and neck lesions, including both non-neoplastic and neoplastic lesions of the upper aerodigestive tract and salivary glands. Precancerous lesions such as leukoplakia and erythroplakia are described. Non-neoplastic lesions including candidiasis, herpes, lichen planus, and hairy leukoplakia are also summarized. Finally, the document outlines the clinical, microscopic, and immunohistochemical features of various squamous cell carcinomas, salivary gland tumors, sinonasal tumors, and other neoplasms of the head and neck region.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 131
Head and Neck Lesions
Miles S. Romero, MD, DPSP
Upper Aerodigestive Tract Lesions
Precancerous Lesions
Leukoplakia
• clinical term for a white plaque on a mucous membrane.
• cannot be scraped off and cannot be characterized clinically or pathologically as
any other disease.
Erythroplakia
• clinical term for a red plaque.
• higher risk of dysplasia.
Speckled Erythroplakia
• clinical term for a mixed red
and white lesion.
• have the characteristics of both
leukoplakia and erythroplakia.
Non-neoplastic Lesions
Candidiasis
• Most common oral fungal infection.
• Often appears clinically like a white
plaque.
• Can be highlighted with PASd or GMS
Herpes Simplex Virus
• Virus infects epithelial cells and ganglion
cells.
• Two types classically infecting different
sites:
• Type 1= Oral
• Type 2=Genital
• Infected cells show classic ground glass
intranuclear inclusions with “3 M’s”:
Molding, Margination, Multinucleation.
Lichen Planus
• Chronic, self-limited inflammatory reaction.
• “Band-like” T-cell infiltrate below epithelium
• “Saw-tooth” rete ridges
• NO significant atypia
• Variable thickness and keratinization
• Clinical 5P’s: Purple, Pruritic, Polygonal, Planar,
Papules.
Hairy Leukoplakia
• Epithelial hyperplasia induced by
Epstein-Barr virus (EBV).
• Acanthosis and parakeratosis
• “Balloon” cells in spinous layer with
viral cytopathic effect Often
coinfected with candida.
• Little inflammation.
• No dysplasia.
Geographic Tongue
• “Benign migratory glossitis”
• Multiple, well-defined erythematous
islands with raised whitish yellow
borders that rapidly appear→
migrate around tongue.
• Epithelium with hyperparakeratosis,
acanthosis, spongiosis, elongated
rete ridges, and collections of
neutrophils (Monro abscesses).
Neoplastic Lesions
Squamous Neoplasms, Non-HPV related
Squamous Dysplasia
• Features of nuclear/cellular “atypia”: marked variation in size/shape, marked
hyperchromasia, prominent nucleoli.
• Epithelium may be atrophic or acanthotic, keratinizing or non-keratinizing.
Squamous Dysplasia
Conventional Squamous
Cell Carcinoma
• Malignant epithelial tumor with squamous
differentiation.
• Keratinization (±keratin pearls) and/or
intercellular bridges
• Features of invasion: downward growth of
islands, cords and isolated tumor cells,
irregular interface, desmoplastic
response, lymphovascular invasion,
perineural invasion.
Conventional Squamous
Cell Carcinoma
• Grading is irrespective of
keratinization.
• Well-differentiated: closely
resemble normal squamous mucosa
(matures somewhat normally), few
mitoses.
• Moderately-differentiated: more
pleomorphism and mitoses.
• Poorly-differentiated: basal-type
cells predominate with lots of
mitoses. Often lose expression of
HMWCKs.
Verrucous Squamous Cell
Carcinoma
• Variant of Well-differentiated Squamous Cell Carcinoma
• Dramatic acanthosis with club-shaped projections and
invaginations.
• Marked “church-spire” keratinization.
• Well-defined “Pushing” invasion, often with associated
lymphocytic inflammation.
• No significant cytologic atypia.
• NO infiltrative growth.
Basaloid Squamous Cell Carcinoma
• Basaloid, hyperchromatic appearance
(high N:C ratio) often with a
conventional component.
• HPV-negative.
• Rounded nests with peripheral
palisading and admixed hyalinized
stroma.
• Frequent mitoses and
comedonecrosis.
• More aggressive.
Papillary Squamous Cell Carcinoma
• Exophytic papillary growth
pattern with thin fibrovascular
cores covered by severely
dysplastic basaloid cells.
• Uncommon.
• May be HPV-related in
oropharynx, but not
elsewhere.
• Better prognosis
• Adenosquamous Carcinoma • Lymphoepithelial Carcinoma
• Arises from squamous epithelium but • Sheets of pleomorphic cells with a
shows both squamous and glandular prominent intratumor chronic
differentiation. inflammatory infiltrate.
HPV-related Squamous Lesions
Verruca Vulgaris
• Benign squamous proliferation in oral
cavity
• Caused by low-risk HPV (e.g., Type 2
and 4)
• Exophytic and papillomatous.
• Hyperkeratosis and acanthosis.
• Elongated and “cup-like” rete ridges.
• Cytologically bland with prominent
granular layer and occasional
koilocytes.
Squamous Papilloma
• Benign exophytic squamous
proliferations with branching
fibrovascular cores.
• Usually associated with HPV types 6 or
11.
• Variable koilocytes.
• Often solitary.
• Malignant transformation is very rare.
Squamous Cell Carcinoma,
HPV-Positive
• >90% caused by HPV type 16 → associated
with oral sex
• Incidence rising: Frequently white men in 50’s
• Strong predilection to oropharynx: Base of
Tongue (BOT) and Tonsils
• Distinct morphology:
• Non-keratinizing, high N:C ratios→ basaloid
appearance.
• Frequent mitoses and/or apoptotic figures.
• Frequent associated lymphocytes/lymphoid
stroma.
Squamous Cell Carcinoma, HPV-Positive
• Grading is NOT applicable!!
• HPV can be detected by: In situ hybridization or PCR.
• Diffuse “block positive” staining with p16 is used as a reliable surrogate
marker
• for the presence of high-risk HPV in oropharyngeal carcinomas.
• Significantly better prognosis than conventional SCC
Important Staging Details
Sinonasal/Nasopharyngeal Tumors
Benign
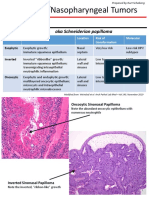
Sinonasal Papillomas
Exophytic Sinonasal Papilloma
Immature squamous epithelium
Inverted Sinonasal Papilloma
Respiratory Epithelial Adenomatoid Hamartoma
(REAH)
• Sinonasal glandular proliferation
arising from the surface epithelium
(i.e., in continuity with the
surface).
• Invaginations of small to medium-
sized glands surrounded by
hyalinized stroma with
characteristic thickened,
eosinophilic basement membrane
Inflammatory Polyp
• Surface ciliated, sinonasal mucosa, possibly with
squamous metaplasia.
• Edematous stroma (without a proliferation of
seromucinous glands).
• Mixed inflammation (usu. Lymphocytes, plasma cells,
and eosinophils)
Malignant
Squamous cell carcinoma
• Most common carcinoma
• Keratinizing or Non-
keratinizing
• Associated with tobacco
exposure.
Sinonasal Undifferentiated Carcinoma (SNUC)
• Poorly differentiated
carcinoma
• Monotonous, hyperchromatic
nuclei, often with prominent
nucleoli.
• CK+, but squamous markers
negative.
• Aggressive high-grade
malignancy→ poor outcome
NUT (Midline) Carcinoma
• Poorly-differentiated
carcinoma.
• Often with “abrupt
keratinization” or squamous
differentiation.
• Younger patients.
• In the midline, often in the
head and neck.
• Aggressive high-grade
malignancy→ poor outcome
Lymphoepithelial Carcinoma
• Non-keratinizing nasopharyngeal
carcinoma, undifferentiated type
• Sheets of malignant cells with
vesicular chromatin, indistinct
cytoplasm, and abundant tumor
infiltrating lymphocytes.
• EBV-positive.
• Positive for CK, CK5/6, p40, p63
• More common in Asians.
Neuroendocrine Carcinoma
• Divided into:
• Small cell neuroendocrine carcinoma
• Large cell neuroendocrine carcinoma
• Strong staining with a neuroendocrine
stain (e.g., synaptophysin or
chromogranin).
• Often perinuclear “dot-like” keratin
expression.
Olfactory Neuroblastoma
• aka “Esthesioneuroblastoma”
• Malignant neuroectodermal
neoplasm
• Confined to the cribriform plate
• Lobulated, nests to sheets of cells
with speckled chromatin.
• High N:C ratio
• IHC: Diffuse
Synaptophysin/Chromogranin, S100,
CK negative.
Mesenchymal Tumors
Glomangiopericytoma
• Patternless proliferation of
regular, spindled cells with ovoid
nuclei.
• Prominent vascularity with
perivascular hyalinization.
• Can see “staghorn” vessels
• IHC: SMA+, Nuclear ẞ-catenin
• Relatively indolent with good
survival
Nasopharyngeal Angiofibroma
• Vascular tumor with variably sized
blood vessels set in fibrotic stroma.
• Stroma is myxoid to dense with
stellate fibroblasts.
• Almost exclusively young to
adolescent boys (“Juvenile
angiofibroma”)→ classically causes
epistaxis & obstruction
• Locally aggressive and can recur.
• Treat with embolization and
surgery.
Salivary Gland Tumors
Normal Salivary Gland
• Normal components:
• (1) Ducts: Interlobar, to
intercalated, and striated. Cuboidal
to columnar epithelium.
Surrounded by myoepithelial cells.
• (2) Acini: Serous (esp. in parotid,
with zymogen granules) to mucous
(esp. sublingual), surrounded by
myoepithelial cells.
• (3) Fat (esp. in parotid)
Oncocytic Tumors
Oncocytic hyperplasia
• aka Oncocytosis
• Non-neoplastic, mass-
forming proliferation
of oncocytes.
• Unencapsulated.
• Often multifocal,
admixed with normal
salivary tissue.
Oncocytoma
• Benign
• Circumscribed to
encapsulated proliferation
of oncocytes.
• Actually Biphasic
• Inner oncocytes
• Outer myoepithelial cells
• Usually in parotid
• No significant
pleomorphism, mitotic
activity, or invasive
growth.
Oncocytic Carcinoma
• Malignant
• Oncocytic lesion with
pleomorphism, mitoses,
and/or invasion.
• May or may not be
encapsulated.
Warthin Tumor
• Old name: Papillary cystadenoma
lyphomatosum
• Benign
• Key elements:
• Mature lymphoid tissue
• Bilayered oncocytic epithelium
• Cystic to papillary growth
• Strongly linked to smoking, can be bilateral
• Likely develops from transformation of salivary
gland tissue entrapped in a lymph node.
• Almost exclusively in parotid, usually at angle
of jaw.
• Aspirated fluid often thick, dark “motor oil.”
Warthin Tumor
Secretory Carcinoma
• Formerly: “Mammary Analogue
Secretory Carcinoma”
• Tubular, papillary and cystic
growth.
• Eosinophilic, granular to
vacuolated cytoplasm
• Sometimes has distinctive
eosinophilic secretions in lumina.
• Stains: Positive for S100 and
mammaglobin.
• Malignant, but relatively
indolent.
Basaloid Tumors
Basal Cell Adenoma / Monomorphic Adenoma
• Benign, well-circumscribed,
usually in parotid
• Solid, trabecular, or tubular
growth
• Perpendicular basal cells on
outside of nests
• Epithelial cells on inside of
nests
• No significant stroma, aside
from possibly a “membrane”
surrounding a nest
Basal Cell Adenocarcinoma
• Malignant
• Like a basal cell adenoma,
but with invasion,
necrosis, and numerous
mitotic figures.
Acinic Cell Carcinoma
• Composed of acinar cells with variable
cytoplasm and architecture.
• Cells are large, polygonal with
basophilic granular cytoplasm.
• Sometimes prominent lymphoid
infiltrate
• Usually in parotid.
• Can seen in children.
• Stains: Positive for DOG-1 and SOX-10
• Malignant, but generally not aggressive
Adenoid Cystic Carcinoma
• Cribriform, tubular or solid growth
• 2 cell types: 1) Myoepithelial and 2)
Ducts
• Low-grade: Mostly myoepithelial,
stain with p40 and SMA
• High-grade: Mostly ductal cells, stain
with CD117 and CK
• Grading: % Solid (ductal) component
• Infiltrative→ Extensive PNI→ Pain →
Paralysis
• Persistent local spread.
• 5yr survival, but poor long-term
survival.
Spindled Cells Tumors
Pleomorphic Adenoma
• aka Benign Mixed Tumor
• Benign, but can recur if not completely excised.
• Most common tumor of salivary glands
• Three components, encapsulated, well-circumscribed:
• (1) Ductular structures
• (2) Myoepithelial cells (can be spindled, epithelioid, plasmacytoid, etc…), intimately
admixed with stroma
• (3) Mesenchymal-like tissue (often myxoid stroma, but can be chondroid, etc…)
• Can see: tyrosine crystals, squamous metaplasia, cystic degeneration
• Cytogenetics: PLAG1-HMGA2 fusions
Pleomorphic Adenoma
Pleomorphic Adenoma
• Cytology: Prominent fibrillar, metachromatic stroma that on a Diff-quick stain looks
like “Troll Hair.”
• Also visible ductal cells and myoepithelial cells intimately admixed with the stroma
Squamoid Tumors
Squamous metaplasia
• In minor salivary gland often
called: “Necrotizing
Sialometaplasia”
• Classic Mimic of SCC!
• Lobular architecture is
maintained; smooth,
rounded contours
• Often associated
inflammation and reactive
changes
• Acinar coagulative necrosis
Mucoepidermoid Carcinoma
• Three components:
• (1) Mucinous cells (stain with PASD/mucicarmine)
• (2) Squamous cells
• (3) “Intermediate cells” (neither squamous nor mucinous, with scanter
cytoplasm)
• Most common malignant salivary cancer.
• Often in parotid, but can get anywhere.
• Broad age range, including children
• Cytogenetics: MAML2 gene fusions almost definitional now
Mucoepidermoid Carcinoma
Mucoepidermoid Carcinoma
High-Grade Salivary Gland Tumors
Salivary Duct Carcinoma
• Resembles breast ductal
carcinoma
• Large ducts with comedonecrosis
• Stains: Androgen receptor (AR)
and HER2 positive
• Often in parotid, sometimes
arising from a pleomorphic
adenoma
• Very Aggressive
Carcinoma ex-pleomorphic adenoma
• Carcinoma arising from a
Pleomorphic Adenoma
• Very pleomorphic, with lots of
mitoses, necrosis and
destructive growth.
• Cytogenetics: PLAG1-HMGA1
(in PA) and TP53 (in
carcinoma)
• Often long history of mass
(i.e., a PA), with rapid
enlargement.
Grading Salivary Gland Tumors
Milan System
Jaw Lesions
Developmental Cysts
Dentigerous Cyst
• aka Follicular Cyst
• Most common developmental cyst.
• Unilocular.
• Envelops the crown of an unerupted tooth (most common around
impacted teeth, especially mandibular third molars→ most common in
teenagers).
• Wall of fibrous tissue with regular squamoid epithelium (sometimes
glandular).
• Variable inflammation, cholesterol clefts, epidermal hyperplasia.
• Cured with excision.
Dentigerous Cyst
Odontogenic Keratocyst (OKC)
• Odontogenic cyst with thin,
regular lining of parakeratinized
squamous epithelium with basal
palisading hyperchromatic cells.
• Second-most common
odontogenic cyst.
• Occur over a broad age range,
majority in the mandible.
• Usually incidental painless
findings.
• Higher rate of recurrence (~25%).
Lateral Periodontal Cyst
• Odontogenic cyst lined by non-
keratinizing squamous
epithelium.
• Arises from the lateral aspect or
between the roots of erupted
teeth.
• Epithelium usually only 1-2 cells
thick with focal thicker whorled
areas.
• Uncommon.
• Usually adults in the mandible.
• Multicystic variant = Botryoid
odontogenic cyst
Gingival Cyst
• Odontogenic cysts in the alveolar
mucosa just below oral mucosa
surface.
• Lined by a thin layer of squamous
mucosa 1-2 cells thick with focal
thickening.
• Very common in infants (but often
not biopsied as often
spontaneously resolve).
Glandular Odontogenic Cyst
• Cyst with epithelial features that
simulate salivary gland
differentiation.
• Variable epithelium from 2-3 cells to
thicker stratified squamous
epithelium.
• Rare
• Associated with roots of multiple
teeth.
• High rate of recurrence.
Calcifying Odontogenic Cyst
• A simple cyst lined by
• (1) ameloblastoma-like
• (2) “ghost” cells
• Sometimes called
“Calcifying ghost cell
odontogenic cyst.”
• Rare.
• Recurrence is rare.
Orthokeratinized Odontogenic Cyst
• Odontogenic cyst lined by orthokeratinized squamous epithelium with a
prominent granular layer.
Reactive/Inflammatory Conditions
Radicular (Periapical) Cyst
• Inflammatory odontogenic cyst associated with nonvital teeth.
• Most common cysts of the mandible.
• Usually in maxilla at apex of tooth root.
• Formed from proliferating tooth root sheath after pulp necrosis (usually
due to dental caries)
• Wall of inflamed fibrous/granulation tissue with nonkeratinizing
hyperplastic
• “arcading” squamous epithelium.
• Often foamy macrophages and cholesterol clefts and eosinophilic
“Rushton”
• bodies (nonspecific)
Radicular (Periapical) Cyst
Epithelial Odontogenic Tumors
Ameloblastoma
• Benign, intraosseous tumor.
• Most often in mandible.
• Most common odontogenic tumor.
• Variable appearance of solid to cystic.
• Most common is follicular type:
Columnar/cuboidal palisading cells
with hyperchromatic nuclei and
reverse polarity.
Calcifying Epithelial Odontogenic Tumor
• aka “Pindborg Tumor”
• Benign. 2
• Relatively rare.
• Components:
• (1) Polygonal cells with abundant
cytoplasm and intercellular bridges.
• (2) Abundant pink amyloid
• (3) Calcifications
• Infiltrate bone, but less
aggressive than ameloblastoma.
• Treat with excision.
Ameloblastic Carcinoma
• Malignant counterpart to
ameloblastoma.
• Rare.
• Ameloblastoma histology
(peripheral palisading, etc…) but
with cytologically malignant cells.
• Often see: nuclear pleomorphism,
mitoses, hyperchromasia,
vascular/perineural invasion,
and/or necrosis (the usual findings
of malignancy).
Neck Lesions
Thyroglossal Duct Cyst
• Persistence of the thyroglossal duct.
• Midline, often attached to hyoid
bone.
• Usually lined by respiratory
epithelium (sometimes squamous).
• May have thyroid in tissue in cyst
wall.
• Treatment is resection (Sistrunk
procedure)
Branchial Cleft Cyst
• Congenital malformations of
branchial apparatus.
• Any age, but often young adults
• Found in lateral neck.
• Often near anterior SCM.
• Usually lined by bland squamous
epithelium and the wall has abundant
lymphoid tissue, often with germinal
centers.
Thymic Cyst
• Wall contains thymic tissue (often
easiest to see is Hassall's
corpuscles)→ Lined by cuboidal,
columnar, or squamous epithelium.
• Usually children.
• Usually anterior cervical triangle.
• May see associated parathyroid.
Bronchogenic Cyst
• Lined by respiratory-type
epithelium
• Cyst wall contains
mucoserous glands,
cartilage, smooth muscle,
and scant lymphoid tissue.
• Usually children.
• Midline, near sternum.
Thyroid Gland
Non-Neoplastic Thyroid Lesions
Chronic Lymphocytic Thyroiditis
• aka “Hashimoto Thyroiditis”
• Most common autoimmune
thyroiditis.
• Hypothyroidism frequent.
• More common in women.
• Diffuse infiltration by
lymphocytes, often with
germinal centers.
Subacute Granulomatous Thyroiditis
• aka “de Quervain disease”
• Self-limited inflammation.
• More common in women, present
with prodrome → painful thyroid
gland
• Can occur after viral infection.
• Asymmetric, uneven inflammation
• Early: Acute inflammation
(hyperthyroid)
• Later: Epithelioid histiocytes,
multinucleated giant cells, chronic
inflammation, and fibrosis
(hypothyroid)
Riedel Thyroiditis
• aka “Invasive Fibrous Thyroiditis”
• Hard “wooden” thyroid.
• Fibrosclerosing inflammation of
the thyroid and adjacent soft
tissues
• Destruction/replacement of gland
by: dense collagen with keloidal-
like bands.
• Increased plasma cells, and
phlebitis.
• Very rare.
Graves Disease
• Thyroid stimulating antibodies →
stimulates thyroid hormone
synthesis → diffuse
proliferation→ hyperthyroid
• More common in women.
• Diffuse follicular epithelium
hyperplasia→ non-branching
papillary projections
• Scalloping of colloid.
• Often tall, pink cells.
• Frequent lymphocytic infiltration.
Adenomatous Hyperplasia
• “Goiter”
• Multinodular thyroid gland
enlargement due to follicular
epithelial hyperplasia.
• Very common.
• More common in females.
• Mostly unencapsulated nodules
with pushing borders
• Variably sized nodules mostly
contain abundant colloid
• Total thyroidectomy for
symptomatic disease
Thyroid Tumors
Papillary Thyroid Carcinoma
• Malignant tumor with
follicular epithelial cell
differentiation and distinct
nuclear features.
• Most common form of
thyroid cancer in both adults
and children.
• More common in women.
• Often presents with a
painless thyroid mass.
• IHC: (+)TTF-1, PAX8,
Thyroglobulin, CK7
Variants
• Conventional (classic) PTC:
• Papillary architecture or may have mixed
architectures like follicles.
• Frequent psammoma bodies.
• Densely eosinophilic colloid.
• Papillary microcarcinoma:
• Tumor variant ≤ 1 cm.
• Malignant, but excellent prognosis.
• Encapsulated variant:
• Totally surrounded by a fibrous capsule.
• Excellent prognosis.
Variants
• Follicular variant:
• Exclusively follicular architecture.
• Can be infiltrative or encapsulated with invasion.
• Tall Cell variant:
• Cells 2-3x as tall as they are wide with abundant
eosinophilic cytoplasm.
• Must account for ≥30% of tumor.
• More aggressive behavior.
• Columnar cell variant:
• Rare.
• Columnar cells with prominent pseudostratification.
• Lack conventional nuclear features.
• Resembles endometrioid/intestinal adenocarcinoma
morphologically.
Variants
• Diffuse sclerosing variant:
• Rare.
• Diffuse involvement with sclerosis and
solid nests of tumor cells.
• Also background lymphocytic
inflammation and psammoma bodies.
• Cribriform-morular variant:
• Mixture of cribriform, follicular, papillary,
trabecular, and solid growth with round
squamoid structures (morules).
• Frequent vascular invasion.
• Almost exclusively in females.
• IHC: LEF-1 positive.
Follicular Adenoma
• Benign
• Completely surrounded by a
fibrous capsule
• Cells are cuboidal with round,
basally located nuclei, smooth
nuclear contours and uniform
chromatin.
• ABSENT: capsular/vascular
invasion, PTC-like nuclei
Follicular Carcinoma
• Malignant.
• Nuclear features of PTC are absent.
• Often present with painless mass.
• Requires either capsular or vascular
invasion.
• Subclassified into 3 groups:
• 1) Minimally invasive → capsular invasion
only → excellent prognosis
• 2) Encapsulated angioinvasive→ risk of
hematogenous metastasis (often
bone/lung)
• 3) Widely invasive → extensive
involvement of thyroid and soft tissues,
often with prominent vascular invasion
Hürthle (Oncocytic) Cell Tumors
• Neoplasms composed of oncocytic cells with
abundant eosinophilic granular cytoplasm.
• Hürthle cell adenoma
• Essentially a follicular adenoma composed of
Hürthle cells.
• Encapsulated.
• Benign.
• Hürthle cell carcinoma
• Contains vascular and/or capsular invasion
(essentially a follicular carcinoma with Hürthle cells)
• Most use term only if “majority” (greater than 75%)
of tumor has this morphology (otherwise use term
“Hürthle cell features”)
Non-invasive Follicular Thyroid Neoplasm with
Papillary-like Nuclear Features (“NIFTP”)
• Diagnostic Requirements:
• Encapsulated or Clear demarcation
• Follicular pattern of growth with:
• - No true papillae
• - No psammoma bodies
• - <30% solid, trabecular, or insular growth pattern
• Nuclear features of papillary carcinoma
• No lymphovascular or capsular invasion
• No tumor necrosis
• No significant mitotic activity (<3 mitoses/10 HPF)
Anaplastic Thyroid Carcinoma
• Highly aggressive thyroid malignancy
composed of undifferentiated follicular
epithelial cells.
• Variable morphology with 3 main patterns:
• Sarcomatoid→ spindled cells resembling
pleomorphic sarcoma,
• Giant cell→ highly pleomorphic cells some of
which have multiple nuclei,
• Epithelial→ Squamoid nests
• Common findings: Necrosis, mitoses, invasive
growth.
• Prognosis: Very aggressive with often < 1year
survival
Medullary Thyroid Carcinoma
• Malignant tumor of the thyroid with
parafollicular C-cells differentiation.
• Frequent LN metastases at presentation with
elevated serum calcitonin.
• Common patterns of growth include: solid,
lobular, trabecular, and/or insular.
• Tumor cells can appear: round, polygonal,
plasmacytoid, or spindled.
• Nuclei are “Neuroendocrine” (round, speckled
“salt and pepper”) with occasional
pseudoinclusions.
• Cytoplasm is eosinophilic to amphophilic and
granular.
• Frequent stromal amyloid.
Parathyroid Tumors
Normal Parathyroid
• Three main components:
• (1) Chief cells: main cell type, round
central nucleus, clear to amphophilic
cytoplasm
• (2) Oxyphil cells: large cells with
abundant pink cytoplasm
• (3) Fat (and fibrous tissue): dividing
cells into lobules
Parathyroid Adenoma
• Benign parathyroid neoplasm.
• Relatively common.
• Can arise in any of the 4 glands, or be
ectopic.
• Well-circumscribed, often encapsulated
• Composed of chief cells (most
common), oncocytes, or a mixture.
• Cells have round, central nuclei with
dense chromatin.
• Typically NO FAT
Parathyroid Carcinoma
• Rare.
• Malignant neoplasm derived from parathyroid
cells.
• Usually presents with hyperparathyroidism.
• Requires evidence of one of the following:
• Invasive growth involving adjacent structures
• Invasion of vessels in capsule or beyond
• Metastases
• Usually subdivided by broad fibrous bands.
• Variable pleomorphism/mitoses.
Ear Lesions
Otic Polyp
• Reactive response to
longstanding Otitis media.
• Granulation tissue with
dense chronic inflammation.
• May have entrapped surface
epithelium, cholesterol
clefts, or calcifications.
Chondrodermatitis Nodularis Helicis
(1) Surface hyperplasia
surrounding ulceration
with keratin plug
(2) Dermal fibrinoid necrosis
(3) Necrotic Cartilage (usually)
Helix or anti-helix of ear
• Clinically mistaken for SCC.
Cholesteatoma
• Keratinizing cyst in middle ear→ destroys ossicular
chain → conductive hearing loss and foul-smelling
discharge
• Three required components
• (1) Stratified squamous epithelium with a granular layer
• (2) Keratinaceous debris
• (3) Inflamed fibrous stroma
• Frequently associated cholesterol clefts and foreign
body giant cell reaction (cholesterol granuloma)
• Can be secondary to chronic otitis media or
congenital.
• Can be locally destructive and recur.
Otosclerosis
• Bone overgrowth→ fixation
of ossicular chain →
conductive hearing loss.
• Unclear etiology.
• Immature trabecular bone
and vascular stroma.
• Usually bilateral and
symmetrical.
• end
You might also like
- Day 1 - Well Bore PositioningV2Document64 pagesDay 1 - Well Bore PositioningV2wwwNo ratings yet
- Nasal TumorDocument20 pagesNasal TumorMahmoud ElsherbenyNo ratings yet
- Lecture, 9Document38 pagesLecture, 9محمد ربيعيNo ratings yet
- 10 - Oralcavity TumorsDocument28 pages10 - Oralcavity Tumorsibrahim100% (1)
- Oral Cavity & OesophagousDocument44 pagesOral Cavity & OesophagousrashmiNo ratings yet
- BenignDocument63 pagesBenignAFREEN SADAFNo ratings yet
- 29 5 24vulvaDocument47 pages29 5 24vulvaEmaan NoorNo ratings yet
- Chapter 16 - Head and NeckDocument8 pagesChapter 16 - Head and NeckAgnieszka WisniewskaNo ratings yet
- Malignant Tumors of Oral CavityDocument144 pagesMalignant Tumors of Oral CavitySivakumar LakshminarayananNo ratings yet
- Benign TumorsDocument78 pagesBenign TumorsLavanya KalapalaNo ratings yet
- Benign and Malignant Tumor of The Oral CavityDocument82 pagesBenign and Malignant Tumor of The Oral CavitymelNo ratings yet
- P2 Breast Pathology (Patho Surg) PDFDocument120 pagesP2 Breast Pathology (Patho Surg) PDFThakoon TtsNo ratings yet
- FGT 2 Uterus To TrophoblasticDocument52 pagesFGT 2 Uterus To TrophoblasticPavan chowdaryNo ratings yet
- Chapter 21 - The Lower Urinary Tract and Male Genital SystemDocument38 pagesChapter 21 - The Lower Urinary Tract and Male Genital SystemAgnieszka WisniewskaNo ratings yet
- Oral TumorsDocument69 pagesOral TumorsOlga SolomonNo ratings yet
- Oral Neoplasma IDocument31 pagesOral Neoplasma INandika 'ndek' DewaraNo ratings yet
- Bengin SGT 2023Document11 pagesBengin SGT 2023Hazem MouradNo ratings yet
- Lecture 9 GitDocument42 pagesLecture 9 GitWaqar Ul HaqNo ratings yet
- Respiratorio 2Document22 pagesRespiratorio 2Lola PNo ratings yet
- 11 Hematological MalignanciesDocument34 pages11 Hematological MalignanciesalitahawarbarintNo ratings yet
- NonMelanocytic Cutaneous Neoplasm 2021Document101 pagesNonMelanocytic Cutaneous Neoplasm 2021karimahihdaNo ratings yet
- Lumps and Ulcer, Sebaceous Cyst, Lipoma, Dermoid CystDocument88 pagesLumps and Ulcer, Sebaceous Cyst, Lipoma, Dermoid CystAbdulsalam DostNo ratings yet
- Epithelial Tumours - SreejaDocument184 pagesEpithelial Tumours - SreejaaakiNo ratings yet
- Learning Unit 8 - Neoplasia of The Female Genital Tract - Part2Document36 pagesLearning Unit 8 - Neoplasia of The Female Genital Tract - Part2Marelize ErasmusNo ratings yet
- 11 - SurgpathDocument22 pages11 - Surgpathemely p. tangoNo ratings yet
- Bronchogenic CarcinomaDocument86 pagesBronchogenic CarcinomaAkshita Amit AgarwalNo ratings yet
- Cancerous Lesion in Oral: DRG Rina Kartika Sari SPPM Departemen Ilmu Penyakit Mulut FKG UnissulaDocument50 pagesCancerous Lesion in Oral: DRG Rina Kartika Sari SPPM Departemen Ilmu Penyakit Mulut FKG UnissulamufiNo ratings yet
- Salv GLND Histopath Slides 051116Document64 pagesSalv GLND Histopath Slides 051116Amesz Itzu Tsang SynysterNo ratings yet
- Intraoral Benign TumorsDocument4 pagesIntraoral Benign TumorsSaman SadeghiNo ratings yet
- Salivary Glands TumoursDocument102 pagesSalivary Glands TumoursSokna SyNo ratings yet
- Head and Nexck - PPTXDocument80 pagesHead and Nexck - PPTXVijay SudhaNo ratings yet
- Neoplasia: Introductory Surgery ClassDocument37 pagesNeoplasia: Introductory Surgery ClassPrincewill SeiyefaNo ratings yet
- Neoplastic Disorders of The Conjunctiva Tumors of Epithelial OriginDocument13 pagesNeoplastic Disorders of The Conjunctiva Tumors of Epithelial OriginInTan RamliNo ratings yet
- Benign and Malignant Lesions of JawDocument15 pagesBenign and Malignant Lesions of JawThaer ZabenNo ratings yet
- Sinonasal/Nasopharyngeal Tumors: BenignDocument9 pagesSinonasal/Nasopharyngeal Tumors: BenignIsa EnacheNo ratings yet
- Oral Cavity - Pre Malig Lesions & SCCDocument25 pagesOral Cavity - Pre Malig Lesions & SCCDeepika LamichhaneNo ratings yet
- Malignant Conditions of Oral CavityDocument64 pagesMalignant Conditions of Oral CavityYug KathuriaNo ratings yet
- AdenomaDocument3 pagesAdenomalovelyc95No ratings yet
- Blok Spesial Sense NewDocument34 pagesBlok Spesial Sense NewYola SurbaktiNo ratings yet
- Neoplasia and Iflammation General ConceptDocument61 pagesNeoplasia and Iflammation General ConceptKimberly AnnNo ratings yet
- Lecture 3 Ovarian PathologiesDocument5 pagesLecture 3 Ovarian Pathologiesslmsmn101No ratings yet
- SBRC Male Genital Tract 1Document50 pagesSBRC Male Genital Tract 1Erin HillNo ratings yet
- Non-Odontogenic Tumors of The JawsDocument5 pagesNon-Odontogenic Tumors of The JawsSaman SadeghiNo ratings yet
- Benign Salivary Gland Tumors - Dr. Nermine El Bahey (2019-2020)Document13 pagesBenign Salivary Gland Tumors - Dr. Nermine El Bahey (2019-2020)MOHAMED AMINNo ratings yet
- Salivary Gland NeoplasmsDocument63 pagesSalivary Gland Neoplasmsyashwanth bNo ratings yet
- SkinDocument142 pagesSkinp6hccq6jd7No ratings yet
- Epithelium IIDocument44 pagesEpithelium IIRanjit DanielNo ratings yet
- Oculars TumorDocument109 pagesOculars TumorNovita EmyNo ratings yet
- Skin MalignanciesDocument84 pagesSkin MalignanciesDrbarathirajaNo ratings yet
- Malignant Tumors: Dr.N.Govindrajkumar Reader Dept - Oral &maxillo Facial PathologyDocument50 pagesMalignant Tumors: Dr.N.Govindrajkumar Reader Dept - Oral &maxillo Facial PathologypriyaNo ratings yet
- Oral Path 2 FinalsDocument6 pagesOral Path 2 FinalsJasmin JimenezNo ratings yet
- 3 Male Genital and KidneyDocument119 pages3 Male Genital and KidneyFaerusNo ratings yet
- Neoplasia Patho - 1Document53 pagesNeoplasia Patho - 1Alishba MushtaqNo ratings yet
- Presentation 1Document21 pagesPresentation 1RiyaduNo ratings yet
- Neoplasms of The KidneyDocument5 pagesNeoplasms of The KidneyEMILY N CRUZ-VARGASNo ratings yet
- Squamous Cell CarcinomaDocument53 pagesSquamous Cell CarcinomaWacky BlankNo ratings yet
- NeoplasiaDocument99 pagesNeoplasiaNazma AkterNo ratings yet
- Salivary Gland TumorDocument67 pagesSalivary Gland TumorMeri NovitaNo ratings yet
- Malignant Salivary Gland Tumors - Dr. Nermine El Bahey (2019-2020)Document11 pagesMalignant Salivary Gland Tumors - Dr. Nermine El Bahey (2019-2020)MOHAMED AMINNo ratings yet
- Penile CaDocument76 pagesPenile CaUsmle GuyNo ratings yet
- The Perfect Multiple Myeloma Diet Cookbook:The Complete Nutrition Guide To Naturally Combat Multiple Myeloma For Blood Cancer Prevention With Delectable And Nourishing RecipesFrom EverandThe Perfect Multiple Myeloma Diet Cookbook:The Complete Nutrition Guide To Naturally Combat Multiple Myeloma For Blood Cancer Prevention With Delectable And Nourishing RecipesNo ratings yet
- Project Management Plan Template ProcessDocument7 pagesProject Management Plan Template ProcessShanekia Lawson-WellsNo ratings yet
- R19M TECHThermalEnggISemDocument32 pagesR19M TECHThermalEnggISemSuchiNo ratings yet
- Challenges Facing GraduateDocument95 pagesChallenges Facing GraduateGetu DeleluNo ratings yet
- Miss Gee by AudenDocument5 pagesMiss Gee by AudenVandana100% (1)
- An Empirical Equation Relating Fatigue Limit and Mean Stress - NASA PDFDocument31 pagesAn Empirical Equation Relating Fatigue Limit and Mean Stress - NASA PDFjohnyboyNo ratings yet
- Variable Unit-Linked Training Manual: Vul/Ulp Licensing Manual (December 2014) I 1Document57 pagesVariable Unit-Linked Training Manual: Vul/Ulp Licensing Manual (December 2014) I 1Gus Delfin100% (1)
- Sales Territory Design & ManagementDocument20 pagesSales Territory Design & Managementsrijit vermaNo ratings yet
- EatStreet Delivery r3prfDocument4 pagesEatStreet Delivery r3prfCatalin Cimpanu [ZDNet]No ratings yet
- Script TemplateDocument8 pagesScript TemplateamberkaplanNo ratings yet
- Kaos GnosticismDocument1 pageKaos Gnosticismanton eNo ratings yet
- Quarter 2 - Module 2: The Arts of Renaissance and Baroque PeriodDocument16 pagesQuarter 2 - Module 2: The Arts of Renaissance and Baroque PeriodFrancisco ManabatNo ratings yet
- Mindanao State University: College of Health Sciences Marawi CityDocument2 pagesMindanao State University: College of Health Sciences Marawi CityYanumy limb100% (1)
- Concerto in C Minor - Johann Christian Bach - CelloDocument24 pagesConcerto in C Minor - Johann Christian Bach - CellojosianeNo ratings yet
- Senior High School 2010Document123 pagesSenior High School 2010John EltNo ratings yet
- Abbotsford Law Court Provincial AdvanceDocument108 pagesAbbotsford Law Court Provincial Advancesampv90No ratings yet
- Three-Dimensional Incompressible FlowDocument12 pagesThree-Dimensional Incompressible FlowrevandifitroNo ratings yet
- September - December 2023 Predicted IELTS Speaking QuestionsDocument14 pagesSeptember - December 2023 Predicted IELTS Speaking QuestionsShiela Getubig-RojoNo ratings yet
- Treating Straw For Animal FeedingDocument36 pagesTreating Straw For Animal FeedingHybrid BurtonNo ratings yet
- (Lesson2) Cultural, Social, and Political Institutions: Kinship, Marriage, and The HouseholdDocument5 pages(Lesson2) Cultural, Social, and Political Institutions: Kinship, Marriage, and The HouseholdPlat JusticeNo ratings yet
- (123doc) - De-Dap-An-Chuyen-Anh-Tphcm-2008-2009Document14 pages(123doc) - De-Dap-An-Chuyen-Anh-Tphcm-2008-2009Đình HảiNo ratings yet
- IntrotovedicmathDocument3 pagesIntrotovedicmathmathandmultimediaNo ratings yet
- AJODO-2013 Angelieri 144 5 759Document11 pagesAJODO-2013 Angelieri 144 5 759osama-alali100% (1)
- THE BIOLOGICAL METAPHOR Skin-Facade Analogy and Biomimetic ArchitectureDocument13 pagesTHE BIOLOGICAL METAPHOR Skin-Facade Analogy and Biomimetic Architecture3-32-Mohamed Hossam sidekNo ratings yet
- Building High Performing Teams: Facilitated by Marion StoneDocument34 pagesBuilding High Performing Teams: Facilitated by Marion Stoneumar.daha6484No ratings yet
- World's Famous NewspapersDocument8 pagesWorld's Famous NewspapersgopiganjaNo ratings yet
- Eco Map 3Document1 pageEco Map 3Ferent Diana-roxanaNo ratings yet
- KofiAgawu 2016 4TheRhythmicImaginati TheAfricanImaginationDocument40 pagesKofiAgawu 2016 4TheRhythmicImaginati TheAfricanImaginationMitia D'Acol100% (1)
- Public-Key Cryptography: Data and Network Security 1Document15 pagesPublic-Key Cryptography: Data and Network Security 1asadNo ratings yet
- 3.1.3.2 Batu Saluran KemihDocument64 pages3.1.3.2 Batu Saluran Kemihwinda musliraNo ratings yet