Professional Documents
Culture Documents
Syn Converter Nitriding
Syn Converter Nitriding
Uploaded by
Gaurav GuptaCopyright:
Available Formats
You might also like
- Stress Relaxation Cracking Failure in Heat Exchanger Connection Pipes in A Petrochemical PlantDocument1 pageStress Relaxation Cracking Failure in Heat Exchanger Connection Pipes in A Petrochemical PlantRitesh Kumar MallickNo ratings yet
- Cracking and Repair of Closing Welds in 2.25 Cr1 Mo Steel Vessels Operating in High Temperature Synthesis GasDocument9 pagesCracking and Repair of Closing Welds in 2.25 Cr1 Mo Steel Vessels Operating in High Temperature Synthesis Gasvaratharajan g rNo ratings yet
- Boiler Corrosion MagnetiteDocument14 pagesBoiler Corrosion MagnetiteJakeTheSnake69100% (1)
- Qpedia Oct14 Heat Transfer Calculations of ThermosyphonDocument5 pagesQpedia Oct14 Heat Transfer Calculations of ThermosyphonHMMSPNo ratings yet
- Reformer Tubes Tim HillDocument20 pagesReformer Tubes Tim HillProkopNo ratings yet
- Die CastingDocument6 pagesDie Castingkutik3bugerNo ratings yet
- Reformer Component Management After An Overheating Incident That Resulted in Tube FailuresDocument12 pagesReformer Component Management After An Overheating Incident That Resulted in Tube FailuresGrootNo ratings yet
- 1RF Tube Failure PDFDocument8 pages1RF Tube Failure PDFMd. Imran HossainNo ratings yet
- WJ - 1982 - R B Dooley Dissimilar Weld Boiler Tube FailureDocument5 pagesWJ - 1982 - R B Dooley Dissimilar Weld Boiler Tube FailureKuthuraikaranNo ratings yet
- Aiche-36-022Ammonia Converter-Ll Outlet LinesDocument16 pagesAiche-36-022Ammonia Converter-Ll Outlet LinesHsein WangNo ratings yet
- Weld Cracks in An Ammonia Converter: James D. CampbellDocument6 pagesWeld Cracks in An Ammonia Converter: James D. Campbellvaratharajan g rNo ratings yet
- Failure and Repair of The Liner of A High Pressure Carbamate CondenserDocument10 pagesFailure and Repair of The Liner of A High Pressure Carbamate CondenserGrootNo ratings yet
- Failure Analysis and Remaining Life Assessment of Service Exposed Primary Reformer Heater TubesDocument21 pagesFailure Analysis and Remaining Life Assessment of Service Exposed Primary Reformer Heater TubesOwais MalikNo ratings yet
- Steam Boiler Inspections Using Remote Field Testing: by Mynor Celis, P.Eng, Russell NDE SystemsDocument11 pagesSteam Boiler Inspections Using Remote Field Testing: by Mynor Celis, P.Eng, Russell NDE SystemsAnonymous lmCR3SkPrKNo ratings yet
- Six Sigma Methodology For Primary Reformer ReliabilityDocument10 pagesSix Sigma Methodology For Primary Reformer ReliabilityGrootNo ratings yet
- Experience With Maintaining A 40 Year Old Reformer: Ajay Joshi & Stan HeaneyDocument14 pagesExperience With Maintaining A 40 Year Old Reformer: Ajay Joshi & Stan Heaneyvaratharajan g rNo ratings yet
- Failure of 110 Bar WHB's Due To Poor Quality Boiler Feed WaterDocument9 pagesFailure of 110 Bar WHB's Due To Poor Quality Boiler Feed Watervaratharajan g rNo ratings yet
- CR39W (HPNB)Document2 pagesCR39W (HPNB)Jean-Noël LerouxNo ratings yet
- 5 IR Correction Program (CorrectIR)Document21 pages5 IR Correction Program (CorrectIR)barry nancoo100% (1)
- Cause of Damage and Repair of Reformed Gas Firetube Boiler: Zeng Zhong Quan Mark A. HoldermanDocument11 pagesCause of Damage and Repair of Reformed Gas Firetube Boiler: Zeng Zhong Quan Mark A. Holdermanvaratharajan g rNo ratings yet
- 2023 Paper 1BDocument11 pages2023 Paper 1BabubakarNo ratings yet
- Restlife Estimation of Creeping Components by Means of ReplicasDocument7 pagesRestlife Estimation of Creeping Components by Means of ReplicasRolando Nuñez MonrroyNo ratings yet
- Claus Waste Heat Boiler Economics Part 2: Mechanical ConsiderationsDocument6 pagesClaus Waste Heat Boiler Economics Part 2: Mechanical ConsiderationsAlexNo ratings yet
- Failure of PigtailsDocument32 pagesFailure of Pigtailsbarry nancoo100% (1)
- Defect Assessment of A Pressure Vessel Nozzle: Power & Pressure Systems Durability and Life Extension Jun-02Document27 pagesDefect Assessment of A Pressure Vessel Nozzle: Power & Pressure Systems Durability and Life Extension Jun-02venkatrangan2003No ratings yet
- Hfo1Document17 pagesHfo1dreamboy87No ratings yet
- Temperature MeasurementDocument9 pagesTemperature Measurementbarry nancooNo ratings yet
- Coke Drum Monitoring Inspection Assessment and Repair For Service Life Improvement Chadda Foster Wheeler DCU Rio de Janiero 2014Document31 pagesCoke Drum Monitoring Inspection Assessment and Repair For Service Life Improvement Chadda Foster Wheeler DCU Rio de Janiero 2014Piyush PrasadNo ratings yet
- A Thermodynamic Theory of Short-Term and Creep RuptureDocument6 pagesA Thermodynamic Theory of Short-Term and Creep Ruptureeid elsayedNo ratings yet
- Damage Analysis of Service Exposed Reformer Tubes in Petrochemical IndustriesDocument16 pagesDamage Analysis of Service Exposed Reformer Tubes in Petrochemical IndustriesAndrea CalderaNo ratings yet
- Tube Damage Mechanism and Repair Techniques PDFDocument16 pagesTube Damage Mechanism and Repair Techniques PDFArjed Ali ShaikhNo ratings yet
- Failure Analysis of Reformer Tubes: Technicalarticle-Peer-ReviewedDocument6 pagesFailure Analysis of Reformer Tubes: Technicalarticle-Peer-ReviewedOwais MalikNo ratings yet
- Risk Based Assessment of A 25,000 Ton Ammonia Storage Tank: D. Daly, Gregory J. Deis, D. Mclntyre, and R. SmallwoodDocument8 pagesRisk Based Assessment of A 25,000 Ton Ammonia Storage Tank: D. Daly, Gregory J. Deis, D. Mclntyre, and R. Smallwoodvaratharajan g rNo ratings yet
- Primary: Battery SecondaryDocument31 pagesPrimary: Battery SecondaryAlamgir Kabir ShuvoNo ratings yet
- Repair of HP Mod AlloyDocument8 pagesRepair of HP Mod AlloyKarna2504No ratings yet
- Chapter6-Electrochemistry (Part 2)Document27 pagesChapter6-Electrochemistry (Part 2)BagusprPrasetyoNo ratings yet
- Corrosion of Water Walls and Superheaters in Wte MSW PlantsDocument114 pagesCorrosion of Water Walls and Superheaters in Wte MSW Plantsvijay_nani124No ratings yet
- DNFM 2009 OverviewDocument8 pagesDNFM 2009 OverviewJohn M. CavoteNo ratings yet
- Optimization of AUT - PA Inspection Techniques For Detection of (HIC & SOHIC) Using Ominiscan MX in Fixed Plant EquipmentsDocument11 pagesOptimization of AUT - PA Inspection Techniques For Detection of (HIC & SOHIC) Using Ominiscan MX in Fixed Plant EquipmentsRoderick Barrantes AlpuertoNo ratings yet
- Alloys and Phase RuleDocument12 pagesAlloys and Phase RuleViswa NathanNo ratings yet
- Sulfuric Acid and Hydrochloric Acid Dew-Point Corrosion-Resistant SteelDocument0 pagesSulfuric Acid and Hydrochloric Acid Dew-Point Corrosion-Resistant SteelMatt AgonyaNo ratings yet
- Heat Exchanger (New)Document38 pagesHeat Exchanger (New)Rochie DiezNo ratings yet
- Materials Challenges in Nuclear EnergyDocument24 pagesMaterials Challenges in Nuclear EnergyW.t. HanNo ratings yet
- High Emissivity CoatingDocument12 pagesHigh Emissivity CoatingĐoàn TrangNo ratings yet
- Modelling of Naphtha Cracking For Olefins Production - Joao MarcosDocument9 pagesModelling of Naphtha Cracking For Olefins Production - Joao MarcosBahar MeschiNo ratings yet
- Reformer Furnace 02Document8 pagesReformer Furnace 02Cesar Armando LanzNo ratings yet
- Creep Damage and Expected Creep LifeDocument9 pagesCreep Damage and Expected Creep LifeTrương Ngọc SơnNo ratings yet
- Case Study On Copper CorrosionDocument15 pagesCase Study On Copper CorrosionClaudia MmsNo ratings yet
- G 1Document3 pagesG 1choodeshNo ratings yet
- Reformer Tube FailureDocument9 pagesReformer Tube FailureAhmad Riaz KhanNo ratings yet
- Predict Amine20 OverviewDocument23 pagesPredict Amine20 Overviewqueno1No ratings yet
- Turbo CS 8 CO2 CorrosionDocument23 pagesTurbo CS 8 CO2 CorrosionRonald GeorgeNo ratings yet
- Residual StressDocument92 pagesResidual StressJay PadamaNo ratings yet
- 58 Weld Repairs - Creep PerformanceDocument21 pages58 Weld Repairs - Creep PerformanceLTE002No ratings yet
- Effect Casting Conditions Melt Quality JMat Proc Technol 2007 AluminioDocument7 pagesEffect Casting Conditions Melt Quality JMat Proc Technol 2007 Aluminiopaola montserrat flores moralesNo ratings yet
- Engineering Failure Analysis: Nam-Hyuck Lee, Sin Kim, Byung-Hak Choe, Kee-Bong Yoon, Dong-Il KwonDocument5 pagesEngineering Failure Analysis: Nam-Hyuck Lee, Sin Kim, Byung-Hak Choe, Kee-Bong Yoon, Dong-Il KwonAnand VarmaNo ratings yet
- Induction Furnace Testing of The Durability of Prototype Crucibles in A MoltenDocument7 pagesInduction Furnace Testing of The Durability of Prototype Crucibles in A MoltenOmar TahaNo ratings yet
- Reformer TubesDocument3 pagesReformer TubesTarun ChandraNo ratings yet
- Mechanical Properties of Biomedical Co-33Cr-5Mo-0.3N Alloy at Elevated TemperaturesDocument7 pagesMechanical Properties of Biomedical Co-33Cr-5Mo-0.3N Alloy at Elevated TemperaturesDeva RajNo ratings yet
- Cleaning of Cy-1,2,3Document2 pagesCleaning of Cy-1,2,3Gaurav GuptaNo ratings yet
- ChecklistsDocument12 pagesChecklistsGaurav GuptaNo ratings yet
- Vessel SizingDocument40 pagesVessel SizingGaurav GuptaNo ratings yet
- Asu 2Document1 pageAsu 2Gaurav GuptaNo ratings yet
- Sustainability in Energy Brochure Egypes 2024 12 FebDocument8 pagesSustainability in Energy Brochure Egypes 2024 12 FebGaurav GuptaNo ratings yet
- Size Reduction Questions With AnnoDocument33 pagesSize Reduction Questions With AnnoGaurav GuptaNo ratings yet
- AntifoamDocument15 pagesAntifoamGaurav GuptaNo ratings yet
- CT Per. Power SavingDocument5 pagesCT Per. Power SavingGaurav GuptaNo ratings yet
- Lec 1Document42 pagesLec 1Gaurav GuptaNo ratings yet
- CH 4 Raw Mill FeedingDocument3 pagesCH 4 Raw Mill FeedingGaurav GuptaNo ratings yet
- Casale Strategies For Complete Ammonia Plant Revamping, The Example of Al BayroniDocument8 pagesCasale Strategies For Complete Ammonia Plant Revamping, The Example of Al BayroniGaurav GuptaNo ratings yet
- ASU Electric HeaterDocument1 pageASU Electric HeaterGaurav GuptaNo ratings yet
- Air SepDocument3 pagesAir SepGaurav GuptaNo ratings yet
- Failure of CT and Remedial StrategiesDocument14 pagesFailure of CT and Remedial StrategiesGaurav GuptaNo ratings yet
- LSS BB Project - Monohydrate Plant Capacity UtilizationDocument67 pagesLSS BB Project - Monohydrate Plant Capacity UtilizationGaurav GuptaNo ratings yet
- Desulphurisation Adsorbent Physical Properties Lab AnalysisDocument1 pageDesulphurisation Adsorbent Physical Properties Lab AnalysisGaurav GuptaNo ratings yet
- KAMDocument25 pagesKAMGaurav GuptaNo ratings yet
- Technical Handbook Giroform 4 2017Document64 pagesTechnical Handbook Giroform 4 2017Lý Phương NamNo ratings yet
- Organisation ChartDocument1 pageOrganisation ChartKaleem SamiNo ratings yet
- MYCSI-BSM Webinar Steel Framing.Document45 pagesMYCSI-BSM Webinar Steel Framing.Raymond WongNo ratings yet
- Rhine Adjustable FloorDocument4 pagesRhine Adjustable FloorhersonNo ratings yet
- RT&HT As Per IBRDocument2 pagesRT&HT As Per IBRSitaram JhaNo ratings yet
- HSE Presentation On Fire Prevention and ProtectionDocument20 pagesHSE Presentation On Fire Prevention and ProtectionMansoor BabbarNo ratings yet
- Daniel I.T Report 2021Document40 pagesDaniel I.T Report 2021Meza Paul AngelNo ratings yet
- General FormulaDocument53 pagesGeneral FormulaMiko AbiNo ratings yet
- Hardtop F15Document4 pagesHardtop F15Biju_PottayilNo ratings yet
- Gbaran PKG 1 Schedule FinalDocument2 pagesGbaran PKG 1 Schedule FinalOkeymanNo ratings yet
- Manual DanffosDocument12 pagesManual DanffosBORJANo ratings yet
- Welding Sequence To Avoid Distortion in Steel StructureDocument19 pagesWelding Sequence To Avoid Distortion in Steel StructureDavidNo ratings yet
- Product SIKA Gip AdhesiveDocument2 pagesProduct SIKA Gip AdhesiveArya PrimadikaNo ratings yet
- 10 11648 J Ajche 20170503 12Document6 pages10 11648 J Ajche 20170503 12Girmole WorkuNo ratings yet
- PART 92 Cu-Ni Welding - Part 1 Cu-Ni Material PropertiesDocument16 pagesPART 92 Cu-Ni Welding - Part 1 Cu-Ni Material Propertiesravindra_jivaniNo ratings yet
- Astm A860 - A860m - 22Document5 pagesAstm A860 - A860m - 22Mohd Fakhri Bin OmarNo ratings yet
- 3 Calciner Technology 105 MinutesDocument29 pages3 Calciner Technology 105 MinutesIrshad HussainNo ratings yet
- Question Bank For CAT IIDocument3 pagesQuestion Bank For CAT IIVicky GautamNo ratings yet
- Cswip 3.1 Question PaperDocument213 pagesCswip 3.1 Question Papersateesh50% (2)
- G N M CodesDocument2 pagesG N M Codes55511223344No ratings yet
- Question Paper Code: X10713: (10×2 20 Marks)Document3 pagesQuestion Paper Code: X10713: (10×2 20 Marks)Afsal Mohamed KaniNo ratings yet
- UB 400 User GuideDocument2 pagesUB 400 User GuideLuuThiThuyDuongNo ratings yet
- Cyclor - P ER 004 EN 1603 - v2Document2 pagesCyclor - P ER 004 EN 1603 - v2Tho HoangNo ratings yet
- 2019 Rolleri Bending Hammerle Bystronic IntDocument19 pages2019 Rolleri Bending Hammerle Bystronic IntSladjana TrninicNo ratings yet
- Linen and LaundryDocument7 pagesLinen and LaundryAngelica CasullaNo ratings yet
- Din 00976-1 2002 (En)Document7 pagesDin 00976-1 2002 (En)angelbeatNo ratings yet
- Study The Effect of Stress Relief Annealing On The Tensil Properties Hardness and Microstructure of The Medium Carbon SteelDocument13 pagesStudy The Effect of Stress Relief Annealing On The Tensil Properties Hardness and Microstructure of The Medium Carbon SteelZaid TariqNo ratings yet
- Inorganic Fuel GasesDocument13 pagesInorganic Fuel GasesjantskieNo ratings yet
- 6 Grinding & Other Abrasive ProcessesDocument82 pages6 Grinding & Other Abrasive Processessakali ali100% (1)
Syn Converter Nitriding
Syn Converter Nitriding
Uploaded by
Gaurav GuptaOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Syn Converter Nitriding
Syn Converter Nitriding
Uploaded by
Gaurav GuptaCopyright:
Available Formats
High temperature Nitriding
corrosion in
Ammonia converters
This paper provides case histories of failures in ammonia converters and their exchangers, different failures
occurred in tubes, tubesheets, basket, thermowell and thermal barrier, two failed samples were as brittle as a
glass. Investigation on samples which were in services between 3 -25 years, showed that the main reason of
failures was Nitridation due to presence of high pressure, high temperature and nitrogen environment.
By increasing the Nickel content in the steel, the Nitriding rate decrease but doesn't stop. By the way, the
Nitrated layer increased as the plant aged, subsequently ductility decreased sharply.
A. Hassan Faraji
Razi Petrochemical Co.-Iran
Introduction
R azi Petrochemical Complex (RPC) is one The complex comprise of three gas treating units,
of the largest fertilizer plant in Iran three sulfur recovery units, two sulfuric plants, a
was formerly a Subsidiary of National phosphoric plant, two D.A.P. plants, a urea plant
Petrochemical Company (NPC), it is located on and three ammonia plants.
the northern coast of the Persian Gulf. RPC was
privatized in February 2008 and a consortium of The ammonia plants were designed by M.W.
Turkish companies acquired RPC in an Kellogg and Ammonia Casale and commissioned
international tender. New shareholders are Gubre in 1969, 1975 and 2008 respectively. The
Fabrikalari Turk Anonim sirketi – Asya gas complex was out of service during Iran –Iraq war
energy – Tabosan -Shghayegh Sazeh Rangin and activities for 8 years (1980-1988).
personnel.
Discussion
The plant was commissioned in 1969. Design
capacity is 3.4 MMTPY (Million Metric Ton Per This paper discusses eight case studies of
Day) produces ammonia, prill and granular urea, Nitriding attack that have occurred with stainless
granular sulphur, Phosphoric and sulphuric acids steel in converters of Ammonia plants over long
and D.A.P. periods.
2010 183 AMMONIA TECHNICAL MANUAL
High temperature corrosion:
Most popular modes of high temperature
corrosion are: Oxidation – Carburization –Metal
dusting –Sulfidation –Nitridation. The process of
high temperature normally comes with high
pressure as well. Carburization and Nitridation are
similar but they are different to other modes of
high temperature corrosion.
Nitriding
The Nitriding process was found about a century
ago. This process is used for improving
mechanical properties (for instance case
hardening) of low alloy steels at high temperature Figure 1- Iron- Nitrogen equilibrium diagram
(about 932 °F/500°C) by presence of ammonia
environment.
Nitriding mechanism
Nitriding is a chemical process that results from
diffusion of Nitrogen into the metal. When the This phenomena consists of:
concentration of nitrogen exceeds solubility limit,
precipitation of nitrate in the metal occurs in the • Adsorption of ammonia in the metal surface.
matrix as well as at grain boundaries. • Ammonia decomposition to Nitrogen and
Hydrogen.
Based on equilibrium phase diagram of Nitrogen- • Diffusion of N into the steel then formation
Iron (Fig. 1) because of partial solubility of of FeN, Fe3N, Fe4N (in steel).
Nitrogen in Iron (up to 6%), gamma prime (γ’) • Diffusion of N into the stainless steel then
with composition of Fe4N is formed. The Gamma formation of CrN, Cr2N, FeN, Fe3N, Fe4N (in
prime layer is very hard and brittle (white layer). stainless steel).
The external layer is consisting of Gamma- Fe2-
3N, Gamma prime (γ’- Fe4N) and Chromium If Carbon/Carbon compound exists the Nitriding
Nitrate (CrN). Two types of Chromium Nitrates rate increases.
were formed which were in the external layer
(CrN) and in internal layer (Cr2N). There are two parameters that influence
When Nitrogen content in the steel is more than Nitriding:
8%, Epsilon compound (Fe3N) is formed (Fig. 1).
The Nitrided layer causes the austenitic stainless • Process parameters (Temperature-Ammonia
steel to be magnetic. partial pressure-Thermal shocks)
• Material parameters (Al-Cr-V-Mo are
beneficial and Ni- Si- Mn- are detrimental)
Case studies - Description of damage
Ammonia plant no.1 and 2 which were designed
by M.W.Kellogg were faced with Nitriding attack
in their converters. Ammonia no. 1 was
commissioned on 1969 but after about three years
AMMONIA TECHNICAL MANUAL 184 2010
major problem occurred in the converter and it
was completely replaced by a new one. Sample A
& B refer to this converter (Fig. 2).
In October 1992 during internal inspection of
converter (Ammonia no. 1) which was opened for
catalyst replacement, we encountered major
Nitridation on thermal barrier bellow 4th bed.
In April 2004 ammonia plant no.2 had a
turnaround for rehabilitation of the converter
basket. Samples C&E refer to this converter (Fig.
2). Formerly flow direction was axial and now is
combination of axial and radial (Ammonia
Casale).
In April 2005 the converter internals of ammonia
plant no. 1 were replaced with the same type of
converter internals as ammonia plant no 2.
Samples D, F, G, H and I refer to this converter
(Fig. 2).
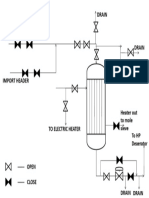
Figure 2- General view of ammonia converter.
Description converter process (MW Kellogg)
The synthesis gas entered through the bottom of
the converter (Fig. 2). Gas direction is upward
between the HP shell and the basket, to the outer
interchanger shell. Then the gas returns downward
in the shell side of the heat interchanger, and it is
preheated against hot converter effluent inside the
interchanger tubes. The flow passes downward
through four catalyst beds in a "catalyst basket."
There is a quench ring in each bed for controlling
the temperature. Then the gas returns upward
through a central pipe and enters the interchanger
(950 °F/510 °C) and leaves the interchanger (550
°F/288° C) from top of the converter.
2010 185 AMMONIA TECHNICAL MANUAL
Case study A & B:
As already described, ammonia converter (plant
no. 1) was replaced due to major problems after
three years service. One tube was removed from
interchanger for metallurgical investigation (Fig.
2 and Fig. 5). Two samples were selected from
different heights of the tube. Sample A (Fig.3-left)
was on top of the tube (tubeside outlet) where the
temperature is about 500°F/260°C and sample B
(Fig.3-Middle) was from bottom tube (tubeside
inlet) where the temperature is about 900°F/482°C Figure 5 - Cross section of tube sample B.
as shown in Fig. 2. Tube material was AISI 304 -
1/2" -7M length-BWG 18 (1.25mm). Sample A Case study C&E:
was nonmagnetic but sample B was magnetic as
shown in Fig. 3. Figure 4 shows a cross section of Sample C was removed from the ammonia
tube (Sample A). No sign of Nitriding layer. converter (plant no.2) interchanger (bottom
section) in April 2004 after 21 years as shown in
Fig. 2. Tube material was AISI 316 -1/2 " -7M
length-BWG 18 (1.25mm) based on chemical
analysis. The sample was magnetic as shown in
fig. 3 (Right). A cross section of the tube is
shown in Fig. 6. Nitriding layer is about 0.33mm
thick.
Figure 3-samples of interchanger's tubes
Figure 6- Cross section of tube sample C. Nitriding
layer is visible on inside the tube
This sample is the bottom tubesheet of the
Figure 4- cross section of tube sample A. interchanger on ammonia no. 2 (Fig. 2 & Fig. 7).
Material is AISI 316, 62.5mm thickness.
Figure 5 shows a cross section of tube (Sample
B). Nitriding layer is 0.18 mm.
AMMONIA TECHNICAL MANUAL 186 2010
Figure 9 - Cross section of tube sample D.
Nitrated layer is shown on tube ID.
Figure 7- Bottom tubesheet of interchanger plant no. 2
Sample F was a distributor which was installed
below the quench ring. Chemical analysis showed
The tubesheet was magnetic and hardness was that the material was AISI 310-Thk. 6.00mm and
about 500 BHN and it was as brittle as glass (Fig. it didn't absorb the magnet (Fig. 10). Hardness of
8). this sample was 150 BHN.
Figure 8 - Tubesheet hardness about 500 BHN
Figure 10 - Cross section of sample F.
Distributor ring
Case studies D, F, G, H, I:
Sample G is first bed bottom (Fig.11). Material
In April 2005, all internals of ammonia converter was AISI 316, 25mm thickness. Hardness
(plant no.1) and interchanger were removed for measurement was done on surfaces and middle
rehabilitation (after 25 years). Samples D, F cross section, 220 and 130 BHN respectively. The
through I were selected (Fig. 2). sample was magnetic.
Sample D was removed from the (bottom section
of tube) as shown in Fig. 2. Tube material was
AISI 316 -1/2 ' -7M length-BWG 18 (1.25mm)
based on chemical analysis. This sample was
magnetic. A cross section of tube is shown in Fig.
9. Nitriding layer is 0.33mm.
Figure 11 - Cross section of sample G, a piece of
1st bed bottom, ammonia plant no. 1
2010 187 AMMONIA TECHNICAL MANUAL
Sample H is a thermowell which protect
thermocouple in the ammonia converter. Material
is AISI 321 - 6mm thickness. Outside Diameter
hardness is about 290 BHN and it absorbs magnet
but the hardness of inside diameter is about 140
BHN and nonmagnetic as cast.
Figure 12- Cross section of sample H,
thermowell
Sample I was thermal barrier which was installed
below the 4th bed. (Fig 2). Material was AISI 316
– 1 mm thickness. This sample was as brittle as
glass and numerous cracks are visible in Fig. 13.
It was magnetic and completely nitraded.
Figure 13 - Cross section of sample I,
numerous cracks are visible on surfaces
AMMONIA TECHNICAL MANUAL 188 2010
Investigations the temperature on this location was low (Tube
side outlet temperature is about 500 F°)
Table 1 is a summary of all case studies which
were removed from three ammonia converters Rate of Nitriding for AISI 304 (Sample B) is 0.06
(M.W Kellogg) and their interchangers for further mm/year. Nitridation rate for AISI 316 (Sample
investigations. Sample A was not magnetic and C) is 0.012 mm/year and the rate for sample D is
had no Nitriding layer. The main reason was that 0.01mm/year.
Depth of Thickness of
Exposure Hardness Magnetic
Sample Material nitrated layer material
Time (Year) BHN properties
(mm) (mm)
AISI
A. Tube 3 I.M. Nil 1.25 Non magnetic
304
AISI slightly
B. Tube 3 100 0.18 1.25
304 Magnetic
AISI
C. Tube 21 I.M. 0.33 1.25 Magnetic
316
AISI
D. Tube 25 I.M. 0.33 1.25 Magnetic
316
E. AISI
21 500 I.M. 62.5 Magnetic
Tubesheet 316
F. AISI
25 150 Nil 6.00 Non magnetic
Distributor 310
G. AISI Surface:220 Magnetic
25 0.18 25.00
Bottom bed 316 Core:130 Nonmagnetic
H. 290 O.D. Magnetic
AISI 321 25 0.13 8.00
Thermowell I.D. 140 Nonmagnetic
I. Thermal Completely Magnetic
AISI 316 25 1.00 1.00
barrier nitrated 100%
Table 1 - Nitriding attack of various materials in ammonia converters (M.W.Kellogg)
Removing a piece on sample E (tubesheet) was Figure 15 shows microstructure of thermowell
impractical, therefore we couldn't measure the (sample H). A thin Nitriding layer is visible.
Nitriding layer but the hardness was about 500 Depth of Nitriding on this material (AISI 321) is
BHN and the surface was as brittle as glass. 0.13mm.
The distributor (sample F) didn't absorb the
magnet and the hardness was same as original and The last sample (I) was thermal barrier which was
no Nitrated layer was seen on the surface. The completely Nitrited and it was as brittle as glass
main reason for this phenomenon was material due to low thickness and vulnerable material AISI
AISI 310 with higher Ni content. 316.
Both surfaces on sample G were Nitrited but
inside was as cast. Fig. 14 shows a cross section
and Nitriding layer which are very thin on the
surfaces. Depth of Nitriding is about 0.18mm.
2010 189 AMMONIA TECHNICAL MANUAL
• Based on the results of this paper, suitable
material for converter are AISI, 310, 321, 316
and 304 by priority.
• Thermal shock: During long periods
influenced the Nitriding rate.
• The Nitriding rate depends on:
1. Ni content - an increase in content in the
alloy, decreases the rate of Nitriding.
2. Temperature - The higher temperature
causes higher Nitriding rate.
3. Pressure - effect of pressure is
unremarkable compare to temperature, but
Figure 14 - Cross section of sample G, by increasing ammonia or nitrogen partial
cross section of 1st bed bottom pressure/concentration, Nitriding rate
increased.
Acknowledgment
Thanks and appreciation to my best friend Mr.
Giel Notten- NTT consultancy and my colleagues
in Inspection department of RPC (Mr.
Ebrahimzadeh, and Ms. Ashna) and Mr. Soltani
for co-operations and their advices in preparation
of this paper. Special thanks to RPC’s
management, Mr. Dashti and Mr. Bardideh, for
their advice and support.
Figure 15 - Cross section of sample H,
cross section of thermowell References
• Metal Handbook 9TH Vol. 11. Failure analysis
and prevention (ASM)
• High temperature corrosion of engineering alloy
Conclusion (ASM). By: George Y. Lai
• Behavior of Stainless Steel in hot ammonia
• Investigations on samples A & B which were atmosphere – CORROSION NACE (April 1961)
selected from top and bottom of one tube • On the corrosion behavior of Stainless Steel in
proved that high temperature has a strong ammonia synthesis atmosphere Vol. 20 -1960)
negative effect in the Nitriding of stainless • Investigation of Nitriding mechanism at transition
steels. metal surface: NH3 adsorption and decomposition
• The Nitrited layer is hard and brittle, visible on Fe, Ni, Cr. By: Hansong Chang, David B.
and mainly consists of Chromium Nitride Reister - Journal of physic.
which is simply distinguished from base • Ammonia, the chemical assignment2.
metals. Environmental and Industrial Chemistry Submitted
by: Jenny Stafford, Angela Stafford, Greta Kyllo-
• When designing, be careful of low thickness
Mayville State University
material, additional corrosion allowance to be • Choose material for H.T. environments By: Peter
added for Nitridation. Elliot - CEP Feb2001
AMMONIA TECHNICAL MANUAL 190 2010
• Ammonia Casale S.A papers – 1995
• Practical Nitriding and Ferritic Niticaborizing –
ASM 2003
• Lifetime assessment of NH3 plants –paper no. 1C
–AIChE 2001
• www.heattreatmentnews.com
• Ammonia gas Nitriding of Fe-18Cr-8Ni alloy at
lower than 823 K.
• Journal of Material science 25 (1990).
• Corrosion Engineering Guide - KCI Publishing.
Giel Notten-NTT consultancy
2010 191 AMMONIA TECHNICAL MANUAL
AMMONIA TECHNICAL MANUAL 192 2010
You might also like
- Stress Relaxation Cracking Failure in Heat Exchanger Connection Pipes in A Petrochemical PlantDocument1 pageStress Relaxation Cracking Failure in Heat Exchanger Connection Pipes in A Petrochemical PlantRitesh Kumar MallickNo ratings yet
- Cracking and Repair of Closing Welds in 2.25 Cr1 Mo Steel Vessels Operating in High Temperature Synthesis GasDocument9 pagesCracking and Repair of Closing Welds in 2.25 Cr1 Mo Steel Vessels Operating in High Temperature Synthesis Gasvaratharajan g rNo ratings yet
- Boiler Corrosion MagnetiteDocument14 pagesBoiler Corrosion MagnetiteJakeTheSnake69100% (1)
- Qpedia Oct14 Heat Transfer Calculations of ThermosyphonDocument5 pagesQpedia Oct14 Heat Transfer Calculations of ThermosyphonHMMSPNo ratings yet
- Reformer Tubes Tim HillDocument20 pagesReformer Tubes Tim HillProkopNo ratings yet
- Die CastingDocument6 pagesDie Castingkutik3bugerNo ratings yet
- Reformer Component Management After An Overheating Incident That Resulted in Tube FailuresDocument12 pagesReformer Component Management After An Overheating Incident That Resulted in Tube FailuresGrootNo ratings yet
- 1RF Tube Failure PDFDocument8 pages1RF Tube Failure PDFMd. Imran HossainNo ratings yet
- WJ - 1982 - R B Dooley Dissimilar Weld Boiler Tube FailureDocument5 pagesWJ - 1982 - R B Dooley Dissimilar Weld Boiler Tube FailureKuthuraikaranNo ratings yet
- Aiche-36-022Ammonia Converter-Ll Outlet LinesDocument16 pagesAiche-36-022Ammonia Converter-Ll Outlet LinesHsein WangNo ratings yet
- Weld Cracks in An Ammonia Converter: James D. CampbellDocument6 pagesWeld Cracks in An Ammonia Converter: James D. Campbellvaratharajan g rNo ratings yet
- Failure and Repair of The Liner of A High Pressure Carbamate CondenserDocument10 pagesFailure and Repair of The Liner of A High Pressure Carbamate CondenserGrootNo ratings yet
- Failure Analysis and Remaining Life Assessment of Service Exposed Primary Reformer Heater TubesDocument21 pagesFailure Analysis and Remaining Life Assessment of Service Exposed Primary Reformer Heater TubesOwais MalikNo ratings yet
- Steam Boiler Inspections Using Remote Field Testing: by Mynor Celis, P.Eng, Russell NDE SystemsDocument11 pagesSteam Boiler Inspections Using Remote Field Testing: by Mynor Celis, P.Eng, Russell NDE SystemsAnonymous lmCR3SkPrKNo ratings yet
- Six Sigma Methodology For Primary Reformer ReliabilityDocument10 pagesSix Sigma Methodology For Primary Reformer ReliabilityGrootNo ratings yet
- Experience With Maintaining A 40 Year Old Reformer: Ajay Joshi & Stan HeaneyDocument14 pagesExperience With Maintaining A 40 Year Old Reformer: Ajay Joshi & Stan Heaneyvaratharajan g rNo ratings yet
- Failure of 110 Bar WHB's Due To Poor Quality Boiler Feed WaterDocument9 pagesFailure of 110 Bar WHB's Due To Poor Quality Boiler Feed Watervaratharajan g rNo ratings yet
- CR39W (HPNB)Document2 pagesCR39W (HPNB)Jean-Noël LerouxNo ratings yet
- 5 IR Correction Program (CorrectIR)Document21 pages5 IR Correction Program (CorrectIR)barry nancoo100% (1)
- Cause of Damage and Repair of Reformed Gas Firetube Boiler: Zeng Zhong Quan Mark A. HoldermanDocument11 pagesCause of Damage and Repair of Reformed Gas Firetube Boiler: Zeng Zhong Quan Mark A. Holdermanvaratharajan g rNo ratings yet
- 2023 Paper 1BDocument11 pages2023 Paper 1BabubakarNo ratings yet
- Restlife Estimation of Creeping Components by Means of ReplicasDocument7 pagesRestlife Estimation of Creeping Components by Means of ReplicasRolando Nuñez MonrroyNo ratings yet
- Claus Waste Heat Boiler Economics Part 2: Mechanical ConsiderationsDocument6 pagesClaus Waste Heat Boiler Economics Part 2: Mechanical ConsiderationsAlexNo ratings yet
- Failure of PigtailsDocument32 pagesFailure of Pigtailsbarry nancoo100% (1)
- Defect Assessment of A Pressure Vessel Nozzle: Power & Pressure Systems Durability and Life Extension Jun-02Document27 pagesDefect Assessment of A Pressure Vessel Nozzle: Power & Pressure Systems Durability and Life Extension Jun-02venkatrangan2003No ratings yet
- Hfo1Document17 pagesHfo1dreamboy87No ratings yet
- Temperature MeasurementDocument9 pagesTemperature Measurementbarry nancooNo ratings yet
- Coke Drum Monitoring Inspection Assessment and Repair For Service Life Improvement Chadda Foster Wheeler DCU Rio de Janiero 2014Document31 pagesCoke Drum Monitoring Inspection Assessment and Repair For Service Life Improvement Chadda Foster Wheeler DCU Rio de Janiero 2014Piyush PrasadNo ratings yet
- A Thermodynamic Theory of Short-Term and Creep RuptureDocument6 pagesA Thermodynamic Theory of Short-Term and Creep Ruptureeid elsayedNo ratings yet
- Damage Analysis of Service Exposed Reformer Tubes in Petrochemical IndustriesDocument16 pagesDamage Analysis of Service Exposed Reformer Tubes in Petrochemical IndustriesAndrea CalderaNo ratings yet
- Tube Damage Mechanism and Repair Techniques PDFDocument16 pagesTube Damage Mechanism and Repair Techniques PDFArjed Ali ShaikhNo ratings yet
- Failure Analysis of Reformer Tubes: Technicalarticle-Peer-ReviewedDocument6 pagesFailure Analysis of Reformer Tubes: Technicalarticle-Peer-ReviewedOwais MalikNo ratings yet
- Risk Based Assessment of A 25,000 Ton Ammonia Storage Tank: D. Daly, Gregory J. Deis, D. Mclntyre, and R. SmallwoodDocument8 pagesRisk Based Assessment of A 25,000 Ton Ammonia Storage Tank: D. Daly, Gregory J. Deis, D. Mclntyre, and R. Smallwoodvaratharajan g rNo ratings yet
- Primary: Battery SecondaryDocument31 pagesPrimary: Battery SecondaryAlamgir Kabir ShuvoNo ratings yet
- Repair of HP Mod AlloyDocument8 pagesRepair of HP Mod AlloyKarna2504No ratings yet
- Chapter6-Electrochemistry (Part 2)Document27 pagesChapter6-Electrochemistry (Part 2)BagusprPrasetyoNo ratings yet
- Corrosion of Water Walls and Superheaters in Wte MSW PlantsDocument114 pagesCorrosion of Water Walls and Superheaters in Wte MSW Plantsvijay_nani124No ratings yet
- DNFM 2009 OverviewDocument8 pagesDNFM 2009 OverviewJohn M. CavoteNo ratings yet
- Optimization of AUT - PA Inspection Techniques For Detection of (HIC & SOHIC) Using Ominiscan MX in Fixed Plant EquipmentsDocument11 pagesOptimization of AUT - PA Inspection Techniques For Detection of (HIC & SOHIC) Using Ominiscan MX in Fixed Plant EquipmentsRoderick Barrantes AlpuertoNo ratings yet
- Alloys and Phase RuleDocument12 pagesAlloys and Phase RuleViswa NathanNo ratings yet
- Sulfuric Acid and Hydrochloric Acid Dew-Point Corrosion-Resistant SteelDocument0 pagesSulfuric Acid and Hydrochloric Acid Dew-Point Corrosion-Resistant SteelMatt AgonyaNo ratings yet
- Heat Exchanger (New)Document38 pagesHeat Exchanger (New)Rochie DiezNo ratings yet
- Materials Challenges in Nuclear EnergyDocument24 pagesMaterials Challenges in Nuclear EnergyW.t. HanNo ratings yet
- High Emissivity CoatingDocument12 pagesHigh Emissivity CoatingĐoàn TrangNo ratings yet
- Modelling of Naphtha Cracking For Olefins Production - Joao MarcosDocument9 pagesModelling of Naphtha Cracking For Olefins Production - Joao MarcosBahar MeschiNo ratings yet
- Reformer Furnace 02Document8 pagesReformer Furnace 02Cesar Armando LanzNo ratings yet
- Creep Damage and Expected Creep LifeDocument9 pagesCreep Damage and Expected Creep LifeTrương Ngọc SơnNo ratings yet
- Case Study On Copper CorrosionDocument15 pagesCase Study On Copper CorrosionClaudia MmsNo ratings yet
- G 1Document3 pagesG 1choodeshNo ratings yet
- Reformer Tube FailureDocument9 pagesReformer Tube FailureAhmad Riaz KhanNo ratings yet
- Predict Amine20 OverviewDocument23 pagesPredict Amine20 Overviewqueno1No ratings yet
- Turbo CS 8 CO2 CorrosionDocument23 pagesTurbo CS 8 CO2 CorrosionRonald GeorgeNo ratings yet
- Residual StressDocument92 pagesResidual StressJay PadamaNo ratings yet
- 58 Weld Repairs - Creep PerformanceDocument21 pages58 Weld Repairs - Creep PerformanceLTE002No ratings yet
- Effect Casting Conditions Melt Quality JMat Proc Technol 2007 AluminioDocument7 pagesEffect Casting Conditions Melt Quality JMat Proc Technol 2007 Aluminiopaola montserrat flores moralesNo ratings yet
- Engineering Failure Analysis: Nam-Hyuck Lee, Sin Kim, Byung-Hak Choe, Kee-Bong Yoon, Dong-Il KwonDocument5 pagesEngineering Failure Analysis: Nam-Hyuck Lee, Sin Kim, Byung-Hak Choe, Kee-Bong Yoon, Dong-Il KwonAnand VarmaNo ratings yet
- Induction Furnace Testing of The Durability of Prototype Crucibles in A MoltenDocument7 pagesInduction Furnace Testing of The Durability of Prototype Crucibles in A MoltenOmar TahaNo ratings yet
- Reformer TubesDocument3 pagesReformer TubesTarun ChandraNo ratings yet
- Mechanical Properties of Biomedical Co-33Cr-5Mo-0.3N Alloy at Elevated TemperaturesDocument7 pagesMechanical Properties of Biomedical Co-33Cr-5Mo-0.3N Alloy at Elevated TemperaturesDeva RajNo ratings yet
- Cleaning of Cy-1,2,3Document2 pagesCleaning of Cy-1,2,3Gaurav GuptaNo ratings yet
- ChecklistsDocument12 pagesChecklistsGaurav GuptaNo ratings yet
- Vessel SizingDocument40 pagesVessel SizingGaurav GuptaNo ratings yet
- Asu 2Document1 pageAsu 2Gaurav GuptaNo ratings yet
- Sustainability in Energy Brochure Egypes 2024 12 FebDocument8 pagesSustainability in Energy Brochure Egypes 2024 12 FebGaurav GuptaNo ratings yet
- Size Reduction Questions With AnnoDocument33 pagesSize Reduction Questions With AnnoGaurav GuptaNo ratings yet
- AntifoamDocument15 pagesAntifoamGaurav GuptaNo ratings yet
- CT Per. Power SavingDocument5 pagesCT Per. Power SavingGaurav GuptaNo ratings yet
- Lec 1Document42 pagesLec 1Gaurav GuptaNo ratings yet
- CH 4 Raw Mill FeedingDocument3 pagesCH 4 Raw Mill FeedingGaurav GuptaNo ratings yet
- Casale Strategies For Complete Ammonia Plant Revamping, The Example of Al BayroniDocument8 pagesCasale Strategies For Complete Ammonia Plant Revamping, The Example of Al BayroniGaurav GuptaNo ratings yet
- ASU Electric HeaterDocument1 pageASU Electric HeaterGaurav GuptaNo ratings yet
- Air SepDocument3 pagesAir SepGaurav GuptaNo ratings yet
- Failure of CT and Remedial StrategiesDocument14 pagesFailure of CT and Remedial StrategiesGaurav GuptaNo ratings yet
- LSS BB Project - Monohydrate Plant Capacity UtilizationDocument67 pagesLSS BB Project - Monohydrate Plant Capacity UtilizationGaurav GuptaNo ratings yet
- Desulphurisation Adsorbent Physical Properties Lab AnalysisDocument1 pageDesulphurisation Adsorbent Physical Properties Lab AnalysisGaurav GuptaNo ratings yet
- KAMDocument25 pagesKAMGaurav GuptaNo ratings yet
- Technical Handbook Giroform 4 2017Document64 pagesTechnical Handbook Giroform 4 2017Lý Phương NamNo ratings yet
- Organisation ChartDocument1 pageOrganisation ChartKaleem SamiNo ratings yet
- MYCSI-BSM Webinar Steel Framing.Document45 pagesMYCSI-BSM Webinar Steel Framing.Raymond WongNo ratings yet
- Rhine Adjustable FloorDocument4 pagesRhine Adjustable FloorhersonNo ratings yet
- RT&HT As Per IBRDocument2 pagesRT&HT As Per IBRSitaram JhaNo ratings yet
- HSE Presentation On Fire Prevention and ProtectionDocument20 pagesHSE Presentation On Fire Prevention and ProtectionMansoor BabbarNo ratings yet
- Daniel I.T Report 2021Document40 pagesDaniel I.T Report 2021Meza Paul AngelNo ratings yet
- General FormulaDocument53 pagesGeneral FormulaMiko AbiNo ratings yet
- Hardtop F15Document4 pagesHardtop F15Biju_PottayilNo ratings yet
- Gbaran PKG 1 Schedule FinalDocument2 pagesGbaran PKG 1 Schedule FinalOkeymanNo ratings yet
- Manual DanffosDocument12 pagesManual DanffosBORJANo ratings yet
- Welding Sequence To Avoid Distortion in Steel StructureDocument19 pagesWelding Sequence To Avoid Distortion in Steel StructureDavidNo ratings yet
- Product SIKA Gip AdhesiveDocument2 pagesProduct SIKA Gip AdhesiveArya PrimadikaNo ratings yet
- 10 11648 J Ajche 20170503 12Document6 pages10 11648 J Ajche 20170503 12Girmole WorkuNo ratings yet
- PART 92 Cu-Ni Welding - Part 1 Cu-Ni Material PropertiesDocument16 pagesPART 92 Cu-Ni Welding - Part 1 Cu-Ni Material Propertiesravindra_jivaniNo ratings yet
- Astm A860 - A860m - 22Document5 pagesAstm A860 - A860m - 22Mohd Fakhri Bin OmarNo ratings yet
- 3 Calciner Technology 105 MinutesDocument29 pages3 Calciner Technology 105 MinutesIrshad HussainNo ratings yet
- Question Bank For CAT IIDocument3 pagesQuestion Bank For CAT IIVicky GautamNo ratings yet
- Cswip 3.1 Question PaperDocument213 pagesCswip 3.1 Question Papersateesh50% (2)
- G N M CodesDocument2 pagesG N M Codes55511223344No ratings yet
- Question Paper Code: X10713: (10×2 20 Marks)Document3 pagesQuestion Paper Code: X10713: (10×2 20 Marks)Afsal Mohamed KaniNo ratings yet
- UB 400 User GuideDocument2 pagesUB 400 User GuideLuuThiThuyDuongNo ratings yet
- Cyclor - P ER 004 EN 1603 - v2Document2 pagesCyclor - P ER 004 EN 1603 - v2Tho HoangNo ratings yet
- 2019 Rolleri Bending Hammerle Bystronic IntDocument19 pages2019 Rolleri Bending Hammerle Bystronic IntSladjana TrninicNo ratings yet
- Linen and LaundryDocument7 pagesLinen and LaundryAngelica CasullaNo ratings yet
- Din 00976-1 2002 (En)Document7 pagesDin 00976-1 2002 (En)angelbeatNo ratings yet
- Study The Effect of Stress Relief Annealing On The Tensil Properties Hardness and Microstructure of The Medium Carbon SteelDocument13 pagesStudy The Effect of Stress Relief Annealing On The Tensil Properties Hardness and Microstructure of The Medium Carbon SteelZaid TariqNo ratings yet
- Inorganic Fuel GasesDocument13 pagesInorganic Fuel GasesjantskieNo ratings yet
- 6 Grinding & Other Abrasive ProcessesDocument82 pages6 Grinding & Other Abrasive Processessakali ali100% (1)