Professional Documents
Culture Documents
Hsslive Xii Chemistry CH 9 Coordination Compounds by Sajeev
Hsslive Xii Chemistry CH 9 Coordination Compounds by Sajeev
Uploaded by
rasalgafoorrvgOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Hsslive Xii Chemistry CH 9 Coordination Compounds by Sajeev
Hsslive Xii Chemistry CH 9 Coordination Compounds by Sajeev
Uploaded by
rasalgafoorrvgCopyright:
Available Formats
IUPAC Naming of Complexes
[Co(NH3)5Cl]Cl2 Pentaammine chloro cobalt (III) Chloride
[Ni(CO)4] Tetra carbonyl nickel (0)
[Cr(NH3)4Cl2]Br Tetraammine dichloro chromium(III) bromide
K4[Fe(CN)6] Potassium hexacyao ferrate (II)
[Ag(NH3)2][Ag(CN)2] Diammine silver(I) dicyano argentite(I)
K3[Fe(C2O4)3] Potassium trioxalato ferrate(III)
[PtCl2(NH3)4][PtCl4] Tetraammine dichloro platinum (IV)
[Co(NH3)5SO4]Cl Pentaammine sulphato cobalt(III) chloride tetrachloro palatinate (II)
[Co(NH3)5Cl]SO4 Pentaammine chloro cobalt (III) sulphate 3+
[Cr(en)3] Tris ( ) Chromium (III) ion
Double salts : They dissociate completely in to component ions, when dissolved in water. Eg: Mohr Salt
Complex Salts : They do not dissociate completely.
Ligands : The neutral molecules or ions that are attached directly to the central atom or ion in a complex.
in
• Monodentate Ligands : It contains only one donor atom. Eg. Cl-, CN-, NH3
• Bidentate Ligands: Having two donor atoms. Eg : Oxalate (C2O4)2-, Ethylene Diamine (en)
• Polydentate Ligands: Have more than two donor atoms. eg : EDTA (Hexadentate Ligand)
• Chelating ligands: Ligands which can form ring structure with the metal. Eg: [Cu(en)2]2+
• Ambidentate ligands: Monodentate ligands which can link through two different donor atoms. Eg: CN- & NC-
.
• Homoleptic Complexes: Complexes having only one type of ligands are homoleptic complexes.
ve
• Heteroleptic complexes: Complexes having more than one type of ligands are heteroleptic.
Werner’s theory of complexes
i) In co-ordination compounds metals show two types of valencies - Primary valency & Secondary valency
ii) Primary valency (Ionisable, satisfied by negative ions)
iii) Secondary valency (Co-ordination number, fixed for a metal, non-ionisable, satisfied by neutral molecules or
negative ions).
iv) Secondary valency can give direction regarding the spatial arrangements of molecules and the geometry.
sli
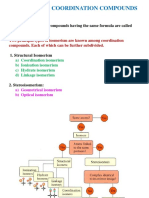
Isomersm in complexes
A. Structural isomerism (Same formula but different structural arrangement)
1. Ionisaion isomerism: Same formula but give different ions in solution. Eg:[Co(NH3)5Br] SO4 & [Co(NH3)5SO4]Br
2. Hydrate isomerism: Differ in No of water molecules inside and outside the sphere.
Eg: [Cr(H2O)6]Cl3 and [Cr(H2O)5Cl]Cl2H2O
3. Linkage isomerism: due to the presence of ambidentate ligands. Eg: [Co(NH3)3(CN)3] and [Co(NH3)3(NC)3]
Hs
4. Coordination isomerism: Due to inter change ligands between anion and cation.
Eg: [Cu ((NH3)4] [PtCl4] and [Pt (NH3)4][CuCl4]
B. Stereo isomerism (Same formula but different spatial arrangement)
1. Geometrical isomerism:- cis – trans isomerism
Cis isomer: identical ligands occupy adjacent position.
Trans isomer: identical ligands occupy opposite position.
! "
2. Optical isomerism:- Compound differ in optical rotation
Dextro: Compound rotates plane of polarised light to right.
Laevo: Compound rotates plane of polarised light to left.
Bonding in Complexes
Valence bond theory: According to this theory, the metal atom or ion und
underer the in_uence of ligands form inner
orbital and outer orbital complex. These hybridized orbitals are allowed to overlap with ligand orbitals that can
donate electron pairs for bonding.
Co ordination Number Hybridisation Geometry Example
4 sp3 Tetrahedral [Ni(CO)4] Diamagnetic, [NiCl4)]2- Paramagnetic
4 dsp2 Square Planar [Ni (CN)4]2- inner orbital complex diamagnetic
6 3 2
sp d Octahedral [CoF6]3- Outer orbital Complex diamagnetic
6 2 3
d sp Octahedral [Fe(CN)6]3- inner orbital Complex Paramagnetic
Eg: [Co(NH3)6]3+
For this complex action the 6 valance electrons of cobalt will occupy the 3d orbital, whereas the 6 ligands will
occupy 3d,4s and 4p orbitals in d2sp3 hybridisation.
d2sp3 hybrid orbitals of Co3+
3+
Co
in
The complex is diamagnetic and
low spin with octahedral geometry.
Magnetic properties of complexes:
Spin only Magnetic moment, µ = BM where n= No. of unpaired electrons.
Crystal field theory
.
The five d-orbitals
orbitals are split into lower and higher energy lev
level due to approach of ligands is known as crystal field
theory. The five d-orbitals in a gaseous
ous metal atom/ion have same energy.
ve
!
sli
" # $ %& $
Hs
Spectrochemical series: It is a series in which lignds are arranged in the increasing order of field strength.
Applications of co-ordinations
ordinations compounds: Chlorophyll - Complex of Mg
1. Detection and estimation of metals Haemoglobin - Complex of Fe
Vitamin B12 - Complex of Co
2. Estimation of hardness of H2O using EDTA
3. Industrial catalyst (Wilkinson’s catalyst, [RhCl(PP
[RhCl(PPh3)3] in the hydrogenation of alkenes. () ) * + ,
4. Chelating ligands are used for the treatment of met
metal poisoning
5. Cisplatin is used to inhibit the growth of tumours cis [PtCl2(NH3)2]
# $ % & # '&
You might also like
- Practical 22.1 Iron Wool Redox TitrationDocument6 pagesPractical 22.1 Iron Wool Redox TitrationDanielle CarterNo ratings yet
- Bendable Concrete PDFDocument14 pagesBendable Concrete PDFKunal Bathija100% (24)
- Unit Ix - CoordinationDocument18 pagesUnit Ix - CoordinationxyzNo ratings yet
- UNIT 9 Topic: Coordination CompoundsDocument9 pagesUNIT 9 Topic: Coordination CompoundsDeva RajNo ratings yet
- New Module-2 Inorganic and Organometallic Chem Fall-2023Document67 pagesNew Module-2 Inorganic and Organometallic Chem Fall-2023VICHUNo ratings yet
- Coordination Compounds 2Document48 pagesCoordination Compounds 2pavithra KumarNo ratings yet
- Chemistry Module 2 Part 2Document60 pagesChemistry Module 2 Part 2RiyazNo ratings yet
- Transition Metals and Coordination ChemistryDocument77 pagesTransition Metals and Coordination ChemistryAdistaNo ratings yet
- D-Block Elements: Short Answer QuestionsDocument11 pagesD-Block Elements: Short Answer QuestionsMahesh Babu100% (1)
- Chem NotesDocument17 pagesChem NotesCND PurPNo ratings yet
- Chapter 9 11Document15 pagesChapter 9 11Ritik KumarNo ratings yet
- Yvh Uh SB Ytv DI5 XBW Ma 8 DDocument6 pagesYvh Uh SB Ytv DI5 XBW Ma 8 DTushant RaoNo ratings yet
- Simplified Focus Area Notes Ii CorrDocument8 pagesSimplified Focus Area Notes Ii Corrwargod RAMZNo ratings yet
- Transition Elements PDFDocument18 pagesTransition Elements PDFArslanAliNo ratings yet
- Metal L Bonding Dr. Alka GuptaDocument39 pagesMetal L Bonding Dr. Alka GuptaVijay sahuNo ratings yet
- Coordination CompoundsDocument12 pagesCoordination Compoundsnadeemmessi30No ratings yet
- Coordination Compounds Class 12Document112 pagesCoordination Compounds Class 12nm.ananya2008No ratings yet
- Nomenclature of 6 - 2018 - 11 - 04!09 - 13 - 23 - PMDocument9 pagesNomenclature of 6 - 2018 - 11 - 04!09 - 13 - 23 - PMnoor ul ainNo ratings yet
- Dr. A. A. Akinsiku: Selected Topics in Chemistry For Chemical Engineering 1 BYDocument63 pagesDr. A. A. Akinsiku: Selected Topics in Chemistry For Chemical Engineering 1 BYIfiok UsoroNo ratings yet
- Revision Notes On Co-Ordination CompoundsDocument12 pagesRevision Notes On Co-Ordination CompoundsAnonymous vRpzQ2BLNo ratings yet
- 1CHEM261 Part1A WVZDocument30 pages1CHEM261 Part1A WVZndlovumpendulo281No ratings yet
- SCH 1201 - Inorganic Chemistry Ii - Transition Metal ChemistryDocument305 pagesSCH 1201 - Inorganic Chemistry Ii - Transition Metal ChemistrysanelisofuturemoyoNo ratings yet
- Module-2-Dr RKDocument55 pagesModule-2-Dr RKIshaan SawantNo ratings yet
- Chem261 Part 1-A and BDocument33 pagesChem261 Part 1-A and BAriana NaidooNo ratings yet
- Lecture 2Document24 pagesLecture 2mallikapathakNo ratings yet
- Xii - Cbse Coordination Chemistry Material (23.11.2022)Document15 pagesXii - Cbse Coordination Chemistry Material (23.11.2022)Sanjana MohanNo ratings yet
- CFT and Chelate Effect-IDocument65 pagesCFT and Chelate Effect-IHitesh vadherNo ratings yet
- Coordination ChemistryDocument19 pagesCoordination ChemistryPrityyyNo ratings yet
- Chemistry Module 2 Application If Metal ComplexesDocument56 pagesChemistry Module 2 Application If Metal ComplexesRiyazNo ratings yet
- D-Block Metal Chemistry: General ConsiderationsDocument23 pagesD-Block Metal Chemistry: General ConsiderationsPrativa BeheraNo ratings yet
- Inorganic Chapter19Document23 pagesInorganic Chapter19barkatullah0% (1)
- Co-Ordination CompoundsDocument11 pagesCo-Ordination CompoundsShashank AgarwalNo ratings yet
- Xii - CH9 - Coordination CompoundsDocument8 pagesXii - CH9 - Coordination CompoundsYash RajNo ratings yet
- Coordination CompoundsDocument51 pagesCoordination CompoundsasdfNo ratings yet
- Coordinaton Compounds Short NotesDocument7 pagesCoordinaton Compounds Short NotesAlokNo ratings yet
- 12 Chemistry Impq CH07 The P Block Elements 03Document15 pages12 Chemistry Impq CH07 The P Block Elements 03Ricky SharmaNo ratings yet
- AC Coordination ComplexesDocument17 pagesAC Coordination ComplexesDipesh PanditNo ratings yet
- Activity 22: Monodentate One With Two Such Atoms Is Bidentate, One With Three Such Atoms Is Tridentate, EtcDocument8 pagesActivity 22: Monodentate One With Two Such Atoms Is Bidentate, One With Three Such Atoms Is Tridentate, EtcAhmed MahmoudNo ratings yet
- Coordination Chem 1 PDFDocument10 pagesCoordination Chem 1 PDFSundar SinghNo ratings yet
- VBT - Theory and IsomerismDocument25 pagesVBT - Theory and Isomerismzuneid-adamoNo ratings yet
- Crystal Field TheoryDocument58 pagesCrystal Field TheoryAakash GuptaNo ratings yet
- Ms ChauhanDocument4 pagesMs ChauhanNikhil VarshneyNo ratings yet
- Coordination CompoundsDocument97 pagesCoordination CompoundsAnant SharmaNo ratings yet
- Chapter 8 - Kimia-KompleksDocument28 pagesChapter 8 - Kimia-KompleksKhariya ArthannaNo ratings yet
- Chap 9part1Document69 pagesChap 9part1Marie Kris NogaNo ratings yet
- Coordination CompoundDocument6 pagesCoordination CompoundniyojetNo ratings yet
- Co-Ordination ChemistryDocument27 pagesCo-Ordination Chemistrymdsaadr856No ratings yet
- XIICoordination Module 4Document7 pagesXIICoordination Module 4Arpit KumarNo ratings yet
- Module 2 - Metal Complexes and OrganometallicsDocument55 pagesModule 2 - Metal Complexes and Organometallicstaara022006No ratings yet
- CH 9Document13 pagesCH 9Tr Mazhar PunjabiNo ratings yet
- Transition Metals Transition Metals: Chapter 22Document15 pagesTransition Metals Transition Metals: Chapter 22Mago_KroNnoXsNo ratings yet
- Coordination ChemistryDocument25 pagesCoordination Chemistrypatel_346879839No ratings yet
- Coordination ChemistryDocument25 pagesCoordination Chemistryggwp21No ratings yet
- Class 12 - Chemistry - Coordination CompoundsDocument39 pagesClass 12 - Chemistry - Coordination CompoundsRaja PRNo ratings yet
- Coordination NumberDocument11 pagesCoordination NumberSyed Qasim Shah100% (1)
- Coordination ChemistryDocument33 pagesCoordination ChemistryGOVIND RANJAN80% (5)
- Coordchem1j PDFDocument90 pagesCoordchem1j PDFVedanti BangarNo ratings yet
- Chem Chap 5 Coordination CompoundsDocument71 pagesChem Chap 5 Coordination Compoundsissacpaul382No ratings yet
- Coordination CompoundsDocument11 pagesCoordination CompoundsTharun TSNo ratings yet
- Endohedral Metallofullerenes: Fullerenes with Metal InsideFrom EverandEndohedral Metallofullerenes: Fullerenes with Metal InsideNo ratings yet
- Chemistry: a QuickStudy Laminated Reference GuideFrom EverandChemistry: a QuickStudy Laminated Reference GuideRating: 4.5 out of 5 stars4.5/5 (2)
- Main Group Metal Coordination Polymers: Structures and NanostructuresFrom EverandMain Group Metal Coordination Polymers: Structures and NanostructuresNo ratings yet
- Analysis of Helical Coil Heat Exchangers: by V.Swapna Priya Guide R S MauryaDocument23 pagesAnalysis of Helical Coil Heat Exchangers: by V.Swapna Priya Guide R S MauryaSwapna Priya VattemNo ratings yet
- Chemistry Midterm 1 Practice With AnswersDocument5 pagesChemistry Midterm 1 Practice With Answerskashaf93No ratings yet
- Chapter 21 - Electrode PotentialsDocument19 pagesChapter 21 - Electrode PotentialsFaix Hussain100% (1)
- A General Multiscale Pair Interaction Potential ModelDocument25 pagesA General Multiscale Pair Interaction Potential ModelHugo HernandezNo ratings yet
- 8.6 Copper Plating and ElectrolysisDocument12 pages8.6 Copper Plating and ElectrolysisADITYA RAHMANNo ratings yet
- BladderDocument18 pagesBladderpsNo ratings yet
- Terminal Velocity Lab: ObjectivesDocument4 pagesTerminal Velocity Lab: ObjectivesMIzan NursiadiNo ratings yet
- AODDocument8 pagesAODPrakash Mishra100% (1)
- SIGMADocument6 pagesSIGMAterlenka2No ratings yet
- Segmental Arch MechanicsDocument64 pagesSegmental Arch MechanicsSarvraj KohliNo ratings yet
- Envisci Lesson 7 Air Quality SupervisionDocument14 pagesEnvisci Lesson 7 Air Quality SupervisionJohn Carlo De Guzman OcampoNo ratings yet
- Marcet BoilerDocument10 pagesMarcet BoilerDhia EmpayarNo ratings yet
- Chem40 Section 1Document6 pagesChem40 Section 1Sam HagenNo ratings yet
- Feryforgues Are Fluorescence Quantum Yields So Tricky To Measure PDFDocument5 pagesFeryforgues Are Fluorescence Quantum Yields So Tricky To Measure PDFNadia WilsonNo ratings yet
- Physical Chemistry: Mole ConceptDocument18 pagesPhysical Chemistry: Mole ConceptambcvcsNo ratings yet
- s.3 & 4 o Level Chemistry Practicals Text BookDocument157 pagess.3 & 4 o Level Chemistry Practicals Text BookWANDEGA DENNISNo ratings yet
- Temper EmbrittlementDocument5 pagesTemper EmbrittlementClaudia Patricia Magaña RabanalesNo ratings yet
- Problem StatementDocument5 pagesProblem StatementShubhendra Nath SahaNo ratings yet
- The Recovery and Recycling of Mercury From Fluorescent Lamps Using Photocatalytic TechniquesDocument7 pagesThe Recovery and Recycling of Mercury From Fluorescent Lamps Using Photocatalytic TechniquesIna WhiteNo ratings yet
- A Review On Effect of Preheating and orDocument3 pagesA Review On Effect of Preheating and orKing SabiNo ratings yet
- Ore Geology - 3Document50 pagesOre Geology - 3Atul SinghNo ratings yet
- CV1013 - Concrete - 5 Mix Design - S2 19-20Document31 pagesCV1013 - Concrete - 5 Mix Design - S2 19-20Ash KongNo ratings yet
- Komar University of Science and Technology: Pharmacy Orientation and Calculation IIDocument25 pagesKomar University of Science and Technology: Pharmacy Orientation and Calculation IIAsma GhazyNo ratings yet
- Finding The Surface Area of Rectangular PrismDocument32 pagesFinding The Surface Area of Rectangular PrismHrrym RamirezNo ratings yet
- 8 Energetics Notes PDFDocument27 pages8 Energetics Notes PDFMustufa Feroz100% (1)
- Hyluma F2k-Lab Script Electron Charge Mass RatioDocument2 pagesHyluma F2k-Lab Script Electron Charge Mass RatioDobocan IoanaNo ratings yet
- AF1 Cultura Inglesa - Ingenierias - 2000116Document3 pagesAF1 Cultura Inglesa - Ingenierias - 2000116EdgarLopezFloresNo ratings yet
- Electroplating-Objectives, Operations and ProcessesDocument17 pagesElectroplating-Objectives, Operations and ProcessesVishalMehtreNo ratings yet