Professional Documents
Culture Documents
OQR (Oragnic Quick Revision) (ALIPHATIC) : Mcpba Conc. H SO D
OQR (Oragnic Quick Revision) (ALIPHATIC) : Mcpba Conc. H SO D
Uploaded by
manya9b3250%(2)50% found this document useful (2 votes)
346 views2 pages1. The document discusses various organic chemistry reactions involving aliphatic and aromatic compounds.
2. Specific reactions mentioned include ozonolysis, Clemmensen reduction, Wolf-Kishner reduction, Hunsdiecker reaction, iodoform reaction, aldol condensation, and Schiff base formation.
3. The reactions involve the use of reagents such as magnesium bromide, zinc-mercury amalgam, sulfuric acid, potassium hydroxide, and periodic acid to transform functional groups on organic molecules.
Original Description:
Original Title
17080_1694529510
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Document1. The document discusses various organic chemistry reactions involving aliphatic and aromatic compounds.
2. Specific reactions mentioned include ozonolysis, Clemmensen reduction, Wolf-Kishner reduction, Hunsdiecker reaction, iodoform reaction, aldol condensation, and Schiff base formation.
3. The reactions involve the use of reagents such as magnesium bromide, zinc-mercury amalgam, sulfuric acid, potassium hydroxide, and periodic acid to transform functional groups on organic molecules.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
50%(2)50% found this document useful (2 votes)
346 views2 pagesOQR (Oragnic Quick Revision) (ALIPHATIC) : Mcpba Conc. H SO D
OQR (Oragnic Quick Revision) (ALIPHATIC) : Mcpba Conc. H SO D
Uploaded by
manya9b321. The document discusses various organic chemistry reactions involving aliphatic and aromatic compounds.
2. Specific reactions mentioned include ozonolysis, Clemmensen reduction, Wolf-Kishner reduction, Hunsdiecker reaction, iodoform reaction, aldol condensation, and Schiff base formation.
3. The reactions involve the use of reagents such as magnesium bromide, zinc-mercury amalgam, sulfuric acid, potassium hydroxide, and periodic acid to transform functional groups on organic molecules.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 2
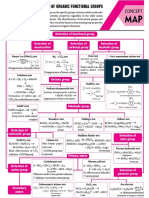
O3/Zn
(Ozonolysis)
CHO [O]
COOH OQR [Oragnic Quick Revision] (ALIPHATIC) (1) CH3MgBr OH
COOH (2) H2O
CHO
O Zn-Hg
Fe-tube Oxidation KOH OCH (Clemmenson reduction)
CHºCH C6H6 O Conc. HCl
500°C V2O5, 500°C O O OCH3
Benzene (Acetal) NH2-NH2
O CH3–C–C–H (Wolf-Kishner reduction)
OH OH CHCOOK KOH, D
H+|CH3OH (Hunsdiecker reaction)
CH3–CH2–CH2 CH3–CH–CH3 CHºCH OH I2 Å
(1) AgOH
Acetylene Kolbe's O KOH Å H CH3–Br
CHCOOK OCH3 CHl3+CH3CO2Na
C + H2 Electrolysis reaction D NaOH (2) Br2/CCl4
(Hemiacetal) (Iodoform reaction)
(Aldol-condensation)
O
Å CH3–CO2H
Br2/H NH2–CH3 CaO/D
N–CH2
(Cold.) CH2–Br 1° amine
CH2OH (Schiff-base) O
CH4 conc. dil. Alk. KMnO4 (pH=4-5)
Methane CH3–CH=CH2 CH2=CH2 I OH Al(OCHMe2)3
Å
H2SO4 (Baeyer's reagent) H P+I2 H O (CH3CO)2 Ca
–
Ethylene CH2OH
(Syn) CH3CH(OH)CH3 NH2OH OH
Glycol N D
D Conc. H2SO4 Acetone (pH=4-5)
CH3Br OH + CH2OH O
mCPBA H3O OH l–CH2–CO2H +CaCO3
CH3–CH–CH3 CH3–CH2 O CH2OH CH3–C–H OH O
CH3CH3 (Anti) Glycol Å
O CH2N2
O H
(Diazotization) HNO2 OSO3H Ethane Cyclic ketal
PCl5 O
Cl OH O
CH3–CH2–CH2–NH2 Cl D2O/DO
CH3–CH F
CH3CH3 Propanamine Cl + SO2 + HCl CD3–C–CD3
Ethane D CH3–CH2Br Prolonged
Zn/D MCPBA
Ethyl bromide heating
H2/Ni LiAlH4 CH3–CH=CH–CH3 PhCHO, KOH O H O
Br2/CCl4 HBr/H2O2 Ph Et
Br Cross aldol
CH3–CH2Br KCN CH2–CH2CN Br2/CCl4 O
Ethyl bromide Propane nitrile CH2=CH2 CH2BrCH2Br (Anti-markonikov rule) OH Å
OH
Br HCN H3O
Ethylene CN CO2H
CH3–CH–CH–CH3 Cyanohydrin D
Br (Anti add)
CH2CNCH2CN Cl2+Ca(OH)2 O
Alc KOH CHCl3+(CH3COO)2Ca
SOCl2 Ethylene Cyanide Br O–C
CH3CH2OH CH2Br–CH2Br HBr Ag heat D
Ethyl alcohol CH3–CºC–CH3 C–O
CH3CH2COOH Ethylene bromide
(Markonikov rule) HCºCH O O
Propanoic acid +CaCO3 (Lactide)
O
CH2COOH D
CH3CH2–Cl
O Red, Orange CHO CH2OH
CH3–CHO CH2COOH Conc. - KOH (i) SOCl2 (ii) CH2N2 (iii) Ag2O/D (iv) H3O
+
CH2=CHBr or yellow ppt CH3CH2COOH
Acetaldehyde Succinic acid O Intramolecular CO2K
Å
Arndt Eistert reaction
Vinyl bromide Succinic CHO
CH3CH2COCl Cannizaro O
Propanoyl chloride anhydride reaction H2
CH3–C–Cl CH3CHO
Pd-BaSO4
CHBrCOOH AgOH CHOHCOOH (Rossenmund reduction) PCC or
2NaNH2
CH3–COOH CHºCH OH SeO2/D Cu/573
CH3CH2CONH2 2CH3l
CHBrCOOH
Acetic acid Acetylene CHOHCOOH
Propanamide Dibromo Dihydroxy succinic
Å
NaHSO3 Ag2O (Tollen's reagent) LiAlH4
H3CH–SO3Na CH3CHO CH3–CO2H CH3–CH2–OH
Succinic acid acid or (Silver mirror test)
Tartaric acid Ag (i) C2H5OH Na
CH3CºCCH3
(ii) DIBAL-H CH3CH2ONa
CH3COONa Red ppt SN2
Sodium acetate (Cu2O) CH3–CHO
CH3CH2NH2
HI
Ethyl amine CH3–CH2–O–CH3 CH3–CH2–OH + CH3I
CH3CHO [Zeisel method] HI
Ethanal
Br2 CH3CH2I
cis-2-butene trans-2-butene Meso EtOEt
OH OH CH3–CH–CH3
CCl4 OH OH
CH4 140°C HCHO HO
conc. CH3–CH2–O–CH2–CH3 OH– H
Methane CH3CH2N = C Continues ether synthesis OH CH3–CH–O–CH2–CH3
CH3CH2OH HO
Ethylisonitrile Ethyl alcohol H2SO4 170° CH2=CH2 OH O O–OH Ether peroxide
OQR [Oragnic Quick Revision] (Aromatic)
SO2NH–CH3
NH2 CH3–NH2
SO2Cl KOH
(1) HNO3/H2SO4 OH Soluble OH OH OH
Å Br Br Br Br2/CS2
(2) H SO2N–(CH3)2 2
NO2 (CH3)2–NH H2O 273K
O NH2 Hinsberg KOH
O (1) Br2/CH3COOH NO2 reagent Insoluble Br2 (White ppt) Br
CH3–C–Cl NH–C–CH2 (CH3)3–N
(Acetanilide) (2) OH No reaction Br2/H2O (loso)
Pyridine Br
(Acetylation reaction) (D.D.T.) Å
OH O(COCH2)
H+ NH3 ONa CO 2/high press
SO3Na dil. HCl
COOH CH3COCl COOH
NH2 NH2 NH2
Br2/CS2 Koble's Schmidt
Br Br Acetyl Salicylic acid
Cl Sod. Phenolate
reaction Salicylic acid
or NO2 SO3 or Aspirin (drug)
Aniline Br2 /H2O Br Selective red. Zwitter ion Soda lime Heat
Tribromo Anline Na HO N O
NO2
OH Indophenol
LiAlH4 O
Ph–CH2–NH2 SO3H NH2 O
CH2Cl
NO2 Cl
Cl Phenol
Benzene
CONH2 CHO Benzene Acetophenone
Benzyl chloride NO2 SO3H
POCl3
Ph–CºN m-Dinitro Benzene Sulphanilic acid
or P2O5
Benzamides or PCl Ph–N=N–NH–Ph
5 Cl
(N–N coupling)
Ph–C–CH3
Na (birch red.)
Cl
COONH4 COOH CH3CH3Cl in presence liq. NH3 NO2 NH2 N2Cl CN COOH
Sandmeyer's
NH3 [O] of AlCl3 HNO3+H2SO4 Sn + HCl Diazo reaction reaction dil. HCl
alk. KMnO4 Friedal Craft's reduction 0°-5°C CuCN/HCN H 2O
50°C
Ammonium Benzoic acid Toluene or reaction Nitro Benzene Aniline NaNO2/HCl Benzene Cyno Benzene Benzoic acid
Benzoate Methyl Benzene Ph–CºCH
Diazoniumchloride [Benzonitile]
R–Cl/AlCl3
Cl No reaction
Cl Cl
3Cl2/hv
BENZENE Cl
COCl
COONa O
Cl Cl
Cl O O
Benzene Hexachloride OH Chloro Benzene
O Benzoyl chloride
(BHC) or Sod. Benzoate HO N=N Cl
Gammaxine maleic
(C6H6Cl6 or 666)
insecticide anhydride (C-N coupling)
CH3
Cl O2N NO2 OH O Chloro Benzene
Cl Cl 6Cl2 CHO (1) O2
+ OH
AlCl3 NO2 (2) H2O
+
CHO
Cl Cl Cumene CH2
Cl Trinitro Toluence
Benzene Benzaldehyde (Industrial preparation of Phenol)
Hexachloro benzene (Explosive) Phenol
O NH2 Benzaldehyde
(HCB) (TNT) O (Wittig reaction)
(C6Cl6) Ph–C–Cl / Benzoylation
Ph–NH–C–Ph
3H2/Ni Schotton baumann Rxn
Aniline
high temp/pressure
OH NaHCO3 O
Cyclo Hexane PhCOONa
dil HNO3 Conc.HNO3 Br
O
OH OH CH3
O NO2 CH=CHCOOH COOH C–O–Et O CHO
O2N NO2 OH OH EtOH
O2N NO2 KMnO4/D (Benzoquinone)
+ Conc.HNO3 (Better method for preparation +
H Salicylaldehyde Toluene
O NO2 of Picric acid) Cinnamic acid Benzoic acid (Esterification)
(Major) Picric acid Phenol NO2 (Clemmenson reduction)
reaction
Picric acid
(i) Conc. H2SO4 (ii) HNO3
You might also like
- Environmental Systems and Processes - Principles, Modeling, and Design - Walter J. Weber JRDocument567 pagesEnvironmental Systems and Processes - Principles, Modeling, and Design - Walter J. Weber JRDuygu Akkoç67% (3)
- Zinc FinalDocument12 pagesZinc Finalamanuel tafeseNo ratings yet
- Coatings Solutions GuideDocument88 pagesCoatings Solutions Guidekhiemnguyen8668No ratings yet
- Functional Group Interconversion Scheme PDFDocument1 pageFunctional Group Interconversion Scheme PDFBilal AhmadNo ratings yet
- Team Bootcamp - Reaction Summary Sheet PDFDocument30 pagesTeam Bootcamp - Reaction Summary Sheet PDFDagne PovedaNo ratings yet
- CH 19Document68 pagesCH 19Britany DyerNo ratings yet
- Chapter 10-Structure and Synthesis of AlcoholsDocument22 pagesChapter 10-Structure and Synthesis of Alcohols張湧浩No ratings yet
- Aliphatic and Aromatic FlowchartDocument4 pagesAliphatic and Aromatic FlowchartapaperclipNo ratings yet
- AIEEE Chemistry Quick ReviewDocument1 pageAIEEE Chemistry Quick ReviewYashwanth KalyanNo ratings yet
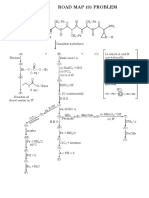
- Roadmap Problem - 9Document1 pageRoadmap Problem - 9abhyudaipathwayNo ratings yet
- Aldol Reaction - ChemistryDocument7 pagesAldol Reaction - ChemistryGamer HelperNo ratings yet
- 20 HaloalkanesDocument7 pages20 HaloalkanesizabelNo ratings yet
- Reaction of Ketone CompleteDocument1 pageReaction of Ketone CompleteJoko SusiloNo ratings yet
- 6carboxylic AcidsDocument1 page6carboxylic AcidssharmimiameerasanadyNo ratings yet
- Organic Reagents: 1. Alcoholic KOH 2. Aluminium EthoxideDocument9 pagesOrganic Reagents: 1. Alcoholic KOH 2. Aluminium EthoxideAarya Nandal100% (1)
- Alcohol EtherDocument39 pagesAlcohol Etheraryamandas321100% (1)
- Conversion Chart Organic ChemistryDocument1 pageConversion Chart Organic ChemistryVishakha0% (1)
- X UV Light or Heat: Reactions in Topic XIDocument3 pagesX UV Light or Heat: Reactions in Topic XImichelsonyip100% (1)
- All Organic Reagent PDFDocument6 pagesAll Organic Reagent PDFreaper1371293No ratings yet
- Haloalkanes and Haloarenes1Document15 pagesHaloalkanes and Haloarenes1Poorni RenuNo ratings yet
- Organic Short NotesDocument64 pagesOrganic Short NotesRehan SayyedNo ratings yet
- Carbonyl CompoundsDocument45 pagesCarbonyl CompoundsYogesh BansalNo ratings yet
- Organic Chemistry ReactionDocument3 pagesOrganic Chemistry ReactionGAMEPORIUMNo ratings yet
- 7 Coordination CompoundsDocument329 pages7 Coordination CompoundsArka100% (1)
- G R Reduction AlkaneDocument43 pagesG R Reduction AlkaneManthan HaritashNo ratings yet
- Detection of Organic Functional GroupDocument1 pageDetection of Organic Functional Groupchandan3biswasNo ratings yet
- Functional Group Reactions: C Synthesis Strategies, Chem 315/316 / Beauchamp 1Document19 pagesFunctional Group Reactions: C Synthesis Strategies, Chem 315/316 / Beauchamp 1Zia urRehman100% (1)
- 11 Alcohols Phenols and EthersDocument2 pages11 Alcohols Phenols and EthersVarun Sankpal100% (1)
- Grignard Reagent Q.B.Document12 pagesGrignard Reagent Q.B.Aariya KumariNo ratings yet
- CH CH CHCH CH H CH CH CH CH CH CH H CH: Byvineet Khatri SirDocument13 pagesCH CH CHCH CH H CH CH CH CH CH CH H CH: Byvineet Khatri Sirsarvesh goyalNo ratings yet
- Alkyl Halides Aryl Halides and Aromatic CompoundsTheoryDocument58 pagesAlkyl Halides Aryl Halides and Aromatic CompoundsTheoryTausif AhmadNo ratings yet
- JC2 WORKSHEET Reaction BenzeneDocument3 pagesJC2 WORKSHEET Reaction BenzeneHarvani SumawijayaNo ratings yet
- Chemistry Advanced Level Problem Solving (ALPS-5) - SolutionDocument12 pagesChemistry Advanced Level Problem Solving (ALPS-5) - SolutionAnanmay Chauhan100% (1)
- Micro - OC - DPP Without Answer (110-116)Document24 pagesMicro - OC - DPP Without Answer (110-116)Ayush JaiswalNo ratings yet
- Reactions of Aromatic CompoundsDocument43 pagesReactions of Aromatic Compoundsnathasya100% (1)
- 1 Roh Carboxylic Acids: H CroDocument15 pages1 Roh Carboxylic Acids: H CroandrewwrobleNo ratings yet
- Heck - 3 20 17Document11 pagesHeck - 3 20 17Preeti YadavNo ratings yet
- Chem 212 Alkyl Halide Problems 4Document1 pageChem 212 Alkyl Halide Problems 4kevinamyNo ratings yet
- Hydrocarbons Jumbo Sheet by MKA SirDocument44 pagesHydrocarbons Jumbo Sheet by MKA SirRahul SinghNo ratings yet
- Chemistry Form 6 Sem 3 07Document65 pagesChemistry Form 6 Sem 3 07Ng Swee Loong StevenNo ratings yet
- Carboxylic Acid Jeemain - GuruDocument26 pagesCarboxylic Acid Jeemain - GuruKumar KumarNo ratings yet
- Isomerism - Handwritten NotesDocument7 pagesIsomerism - Handwritten Notesgovind_galamNo ratings yet
- Diazonium Salts, Azo DyesDocument8 pagesDiazonium Salts, Azo DyesDotsha Raheem100% (4)
- Alkyl Aryl Halides PDFDocument21 pagesAlkyl Aryl Halides PDFLakshit SanghrajkaNo ratings yet
- Name Reactions of Organic ChemistryDocument7 pagesName Reactions of Organic ChemistryNaynam SharmaNo ratings yet
- Organic Conversion A & B Revise Before NEETDocument2 pagesOrganic Conversion A & B Revise Before NEETAquib JavedNo ratings yet
- Alkyl Halides and Aryl Halides - QBDocument23 pagesAlkyl Halides and Aryl Halides - QBNETHAKANI SUJATHA100% (1)
- Benzoin CondensationDocument3 pagesBenzoin Condensationprivatesanket710No ratings yet
- 22-Sharpless Asymmetric Epoxidation ReactionDocument5 pages22-Sharpless Asymmetric Epoxidation ReactionSankar AdhikariNo ratings yet
- Chemoselectivity Regioselectivity Stereoselectivity: (Which) (Where) (How)Document18 pagesChemoselectivity Regioselectivity Stereoselectivity: (Which) (Where) (How)Akhil PratapNo ratings yet
- Phenol SynthDocument1 pagePhenol Synthapi-465421809No ratings yet
- Alkenes Alkynes Oxidation PDFDocument32 pagesAlkenes Alkynes Oxidation PDFRamuNo ratings yet
- Chem 212 Alkyl Halide Problems 2Document1 pageChem 212 Alkyl Halide Problems 2kevinamy100% (1)
- Enol Dan EnolatDocument40 pagesEnol Dan EnolatRiyan KateeNo ratings yet
- Raj7 PDFDocument22 pagesRaj7 PDFAvdhoot GautamNo ratings yet
- BOC Complete 1 To 5 DPPDocument5 pagesBOC Complete 1 To 5 DPPBhawna SharmaNo ratings yet
- Pdf-Haloalkanes and HaloarenesDocument159 pagesPdf-Haloalkanes and HaloarenesOmkar Singh Shekhawat100% (2)
- Coordination Chemistry JEE AdvancedDocument44 pagesCoordination Chemistry JEE AdvancedKartikey SharmaNo ratings yet
- Road Map (3) Problem: Aq. KOHDocument1 pageRoad Map (3) Problem: Aq. KOHShubham RajNo ratings yet
- Carbonyl Compound WorksheetDocument25 pagesCarbonyl Compound WorksheetOmendra SinghNo ratings yet
- Allen Organic QUICK RevisionDocument2 pagesAllen Organic QUICK RevisionChetna Ahlawat100% (2)
- Allen Organic Quic RivisionDocument2 pagesAllen Organic Quic Rivisionsaisupreeth0913No ratings yet
- Biodiesel ReportDocument26 pagesBiodiesel ReportItsura AnnetteNo ratings yet
- Table of Histopath Stains # 1Document3 pagesTable of Histopath Stains # 1Lou Vernadel ApolloNo ratings yet
- CompotecLine HosesDocument2 pagesCompotecLine HosesIchsan RosidinNo ratings yet
- 2021 Nexus Pharma Injection Price List FinalDocument5 pages2021 Nexus Pharma Injection Price List FinalRyu SanurNo ratings yet
- 650 Question Raileay Exam Science Part 1Document50 pages650 Question Raileay Exam Science Part 1sreenuNo ratings yet
- Carboxylic Acids & Derivatives: Chapter Practice ProblemsDocument3 pagesCarboxylic Acids & Derivatives: Chapter Practice ProblemsSushank MishraNo ratings yet
- Questions and Answers What Is The Name of The Heteropolysaccharides in The Basement Membrane?Document6 pagesQuestions and Answers What Is The Name of The Heteropolysaccharides in The Basement Membrane?Solomon D FatormaNo ratings yet
- Biomolecules: Dr. Vishwajeet S. GhorpadeDocument49 pagesBiomolecules: Dr. Vishwajeet S. GhorpadeVishwajeet GhorpadeNo ratings yet
- United States Patent (10) Patent No.: US 7,806,945 B2: The To In... TDocument21 pagesUnited States Patent (10) Patent No.: US 7,806,945 B2: The To In... TesiNo ratings yet
- Presentation On Lexan (Co-Polymer)Document26 pagesPresentation On Lexan (Co-Polymer)Saleha SohailNo ratings yet
- SFDA Products Classification GuidanceDocument42 pagesSFDA Products Classification GuidanceJameel AhmedNo ratings yet
- 3rd-Temadur 20Document2 pages3rd-Temadur 20Erikas KulpinasNo ratings yet
- Converting Rubber Seals To TPE by Gemini Plastics Inc GPIDocument11 pagesConverting Rubber Seals To TPE by Gemini Plastics Inc GPIRui TsaiNo ratings yet
- 1H NMR Spectroscopy in Organic Chemistry - MCQDocument18 pages1H NMR Spectroscopy in Organic Chemistry - MCQShunmugasundaram Arunachalam0% (1)
- Chem Question BankDocument71 pagesChem Question BankSai SriramNo ratings yet
- g6 Paper FinalDocument37 pagesg6 Paper FinalmondayspicyzNo ratings yet
- Interaksi Mineral - Vitamin-LipidaDocument30 pagesInteraksi Mineral - Vitamin-LipidafayzaNo ratings yet
- How To Layer The Ordinary: Deciem Black Friday 23% Off EverythingDocument3 pagesHow To Layer The Ordinary: Deciem Black Friday 23% Off EverythingSaray OjNo ratings yet
- D0006NFDocument8 pagesD0006NFDinoNo ratings yet
- Paint (Dzikri)Document14 pagesPaint (Dzikri)Dzikri Khoirul UmamNo ratings yet
- Journal of Environmental Management: Research ArticleDocument9 pagesJournal of Environmental Management: Research ArticleIsmail RahimNo ratings yet
- Biodegradable Plastic of Jicama Starch (Pachyrhizus Erosus) With Precipitate Calcium Carbonate As A FillerDocument5 pagesBiodegradable Plastic of Jicama Starch (Pachyrhizus Erosus) With Precipitate Calcium Carbonate As A FillerMonaliza MandacNo ratings yet
- SparktechDocument15 pagesSparktechSameer AliNo ratings yet
- Shrisharvad Chemistry ProjectDocument11 pagesShrisharvad Chemistry ProjectATTITUDE PLAYERSNo ratings yet
- General Organic and Biological Chemistry 3Rd Edition Frost Test Bank Full Chapter PDFDocument36 pagesGeneral Organic and Biological Chemistry 3Rd Edition Frost Test Bank Full Chapter PDFallison.young656100% (23)
- The Effect of Lactulose On The Composition of The Intestinal Microbiota and Short Chain Fatty Acid Production in Human Volunteers and A ComputerDocument13 pagesThe Effect of Lactulose On The Composition of The Intestinal Microbiota and Short Chain Fatty Acid Production in Human Volunteers and A ComputerDianaNo ratings yet
- Current Trends in Biopolymers For Food Packaging ADocument20 pagesCurrent Trends in Biopolymers For Food Packaging A23mtpfe004No ratings yet
- BIO-Chapter 20. BiotechDocument27 pagesBIO-Chapter 20. Biotechkathleen hoNo ratings yet