Professional Documents
Culture Documents
Assignment - Phase Diagrams of Water and Carbon Dioxide (Hand Out 1.6.B)
Assignment - Phase Diagrams of Water and Carbon Dioxide (Hand Out 1.6.B)
Uploaded by
uaeali072Original Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Assignment - Phase Diagrams of Water and Carbon Dioxide (Hand Out 1.6.B)
Assignment - Phase Diagrams of Water and Carbon Dioxide (Hand Out 1.6.B)
Uploaded by
uaeali072Copyright:
Available Formats
Name: _____________ Esis: ___________________ Total (15 points)
HANDOUT
1.6.B
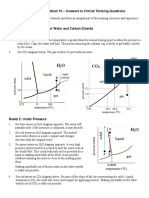
Phase Diagrams of Water and Carbon Dioxide
PHASE DIAGRAM OF WATER PHASE DIAGRAM OF CO2
D D
22,089 C 7,387
C
liquid
water
Pressure (kPa)
ice
Pressure (kPa)
101 517
B
solid
0.6 101 gas
B
water vapor
A A
(gas)
0 0.01 100 374 –78.5 –56.7 31
Temperature (°C) Temperature (°C)
A phase diagram tells you what phase a substance is in at various pressures and
temperatures. Phase diagrams are created based on the results of many observations of
a substance.
1. (a) Use the phase diagrams to determine the most stable phase of water and
carbon dioxide at room temperature (25oC) and atmospheric pressure
(101 kPa). (2 points)
(b) Do these predictions agree with your experience? (1 points)
2. Use the phase diagram of water to answer the following:
(a) At what temperature does ice melt at 101 kPa? (1 points)
(b) At what temperature does water boil at 101 kPa? (1 points)
Pre-AP Chemistry 32 Student Resource
© 2021 College Boa
(c) Albuquerque, New Mexico, is approximately 5,500 feet above sea level, which HANDOUT
1.6.B
means the normal atmospheric pressure there is less than 101 kPa.
i. In Albuquerque, will water freeze at a lower temperature or a higher
temperature than at 101 kPa? (1 points)
ii. In Albuquerque, will water boil at a higher or lower temperature than at
101 kPa? (1 points)
3. A weather balloon is similar to a helium party
balloon but is designed to rise high into the
atmosphere without breaking. As the balloon
rises, the air pressure drops below 101 kPa.
If it were to reach outer space, where there
is essentially no air, the air pressure would be
0 kPa, the lowest possible reading.
Suppose you attached a bucket of water at
25°C to a weather balloon and let it rise to
great heights. What would happen to the
phase of the water, assuming the water stayed
at 25°C? Draw an arrow to show on the below phase diagram of water to support
your answer. (2 points)
4. (a) When dry ice was placed in a sealed bag, the bag filled with gas. Based on
the phase diagram of carbon dioxide, what process is causing the bag to fill?
Draw an arrow to show on the below phase diagram of CO2 to support your
answer. (2 points)
© 2021 College Board
Student Resource 33
Lesson 1.6: What’s So Dry About Dry Ice?
Unit 1: Structure and Properties of Matter
HANDOUT (b) At room temperature, at what pressure would you expect dry ice to melt
1.6.B
instead of sublime? (1 points)
5. Why do you think solid carbon dioxide is called dry ice? (1 points)
6. Based on the phase diagrams, which particles do you think are more strongly
attracted to one another, those in water or those in carbon dioxide? (2 points)
Pre-AP Chemistry 34 Student Resource
© 2021 College Board
You might also like
- BoschEUI EUPRepairInstructionsDocument56 pagesBoschEUI EUPRepairInstructionsYeltsin Ore100% (8)
- List Asme A112Document2 pagesList Asme A112Hung Cheng0% (1)
- AAPA Guide To The Handling Storage Manufacture of PMB's Final Version Jan 2013 PDFDocument15 pagesAAPA Guide To The Handling Storage Manufacture of PMB's Final Version Jan 2013 PDFhongleukNo ratings yet
- AP Chemistry MC WorkshopDocument6 pagesAP Chemistry MC WorkshopSNIGDHA PATLOLANo ratings yet
- NK C SI R: Exercise-02 MCQ (One or More Choice Correct)Document13 pagesNK C SI R: Exercise-02 MCQ (One or More Choice Correct)Vivek SharmaNo ratings yet
- HW3 SKKK2123 1516-1Document3 pagesHW3 SKKK2123 1516-1tamil vaananNo ratings yet
- Chem 10171 Fall 2017 Problem Set 8: Due November 3, 2017Document1 pageChem 10171 Fall 2017 Problem Set 8: Due November 3, 2017william fuNo ratings yet
- © 2013 Marshall Cavendish International (Singapore) Private Limited 1Document5 pages© 2013 Marshall Cavendish International (Singapore) Private Limited 1Kaung Myat SanNo ratings yet
- Apch16 ps1 06Document2 pagesApch16 ps1 06aoiwefoweiNo ratings yet
- © 2013 Marshall Cavendish International (Singapore) Private Limited 1Document6 pages© 2013 Marshall Cavendish International (Singapore) Private Limited 1Kaung Myat SanNo ratings yet
- CM1131 - L25 - Phase Diagrams of Carbon Dioxide and Water - IVLEDocument13 pagesCM1131 - L25 - Phase Diagrams of Carbon Dioxide and Water - IVLENafeesahbmiNo ratings yet
- CF Ph-1 Practice Paper CMM-3Document3 pagesCF Ph-1 Practice Paper CMM-3Divyansh Jain KingNo ratings yet
- Aguide To Dure Success Sure SuccessDocument5 pagesAguide To Dure Success Sure SuccessRAYYAN AHMADNo ratings yet
- Class 9 Science 1Document5 pagesClass 9 Science 1chandralok_kumarNo ratings yet
- 9 Science Exemplar Chapter 1 PDFDocument5 pages9 Science Exemplar Chapter 1 PDFRudraNo ratings yet
- Physical Sciences Physics NSC P2 QP Sept 2022 EngDocument22 pagesPhysical Sciences Physics NSC P2 QP Sept 2022 EngZwavhudiNo ratings yet
- Equilibrium TestDocument3 pagesEquilibrium Testarunganesh691No ratings yet
- Solutions Game Changer 24 DecemberDocument98 pagesSolutions Game Changer 24 Decemberdk004266No ratings yet
- Sheet 5 PDFDocument3 pagesSheet 5 PDFEng-Mohamed AbdelkhalekNo ratings yet
- MCQ Cbse 9 Chapter-1 ScienceDocument5 pagesMCQ Cbse 9 Chapter-1 ScienceAgam VermaNo ratings yet
- Physics Revision SheetDocument92 pagesPhysics Revision Sheetadvaitkshirasgar786No ratings yet
- ch1 1Document9 pagesch1 1vrndrnirmalkar11No ratings yet
- CBSE Class 9 DPPs-75-76Document2 pagesCBSE Class 9 DPPs-75-76Mehul MayankNo ratings yet
- MECH5204 Assn2Document1 pageMECH5204 Assn2joshoidwNo ratings yet
- Problem Set 2 20161 4Document3 pagesProblem Set 2 20161 4qoo30115No ratings yet
- Matter in Our Surroundings - Daily Home Assignment 03 - (Neev 2024)Document3 pagesMatter in Our Surroundings - Daily Home Assignment 03 - (Neev 2024)Wooh gamerZNo ratings yet
- CB IX Sci Ch1 Matter in Our Surroundings MCQDocument3 pagesCB IX Sci Ch1 Matter in Our Surroundings MCQSachi PatelNo ratings yet
- 0.properties of Pure SubstancesDocument36 pages0.properties of Pure SubstancessteyvohmannaNo ratings yet
- CH 1 (C) MCQ For PracticeDocument2 pagesCH 1 (C) MCQ For PracticeLakhwinder GrewalNo ratings yet
- 8 Chapter Chemical Equilibrium Text Book ExerciseDocument18 pages8 Chapter Chemical Equilibrium Text Book ExerciseSajid Azeem0% (1)
- Problem 12-4 Gas Cap ExpansionDocument2 pagesProblem 12-4 Gas Cap Expansionmhuf89No ratings yet
- Class 9 Matter in Our Surroundings McqsDocument3 pagesClass 9 Matter in Our Surroundings McqsHariharan VIIA1No ratings yet
- Answers T-12 Test-10 (Set-C) XI Evening 01.11.2023Document2 pagesAnswers T-12 Test-10 (Set-C) XI Evening 01.11.2023Ojasva TabletNo ratings yet
- Admin2 Chemistry 4 Clutch Chemistry Clutch 63 CH 14 Chemical Equilibrium 6839Document17 pagesAdmin2 Chemistry 4 Clutch Chemistry Clutch 63 CH 14 Chemical Equilibrium 6839KarthikNo ratings yet
- Unit 2 SolutionsDocument5 pagesUnit 2 SolutionsArchana KumariNo ratings yet
- Question Bank Cbse Previous YearsDocument62 pagesQuestion Bank Cbse Previous YearsGayatriNo ratings yet
- Old Exams For Chemistry by Topic - 142 - 181Document82 pagesOld Exams For Chemistry by Topic - 142 - 181ct3hNo ratings yet
- Chemistry: Important Questions Before Half Yearly ExamDocument14 pagesChemistry: Important Questions Before Half Yearly ExamSanjay GuptaNo ratings yet
- MULTIPLE CHOICE. Choose The One Alternative That Best Completes The Statement or Answers The QuestionDocument5 pagesMULTIPLE CHOICE. Choose The One Alternative That Best Completes The Statement or Answers The QuestionJanine Bagarinao CerillaNo ratings yet
- Lesson 1 Assign 2Document4 pagesLesson 1 Assign 2Aman SaxenaNo ratings yet
- Physical Sciences P2 May-June 2022 EngDocument20 pagesPhysical Sciences P2 May-June 2022 EngSimphiwe MpanzaNo ratings yet
- Part I 2019 Updated QBDocument7 pagesPart I 2019 Updated QBteresa tsoiNo ratings yet
- Exercise Phase DiagramDocument13 pagesExercise Phase DiagramfadhlichNo ratings yet
- CT - Gaseous State - Gaseous State Sheets - 21012021 - Gaseous State - Sheet 1 To 5Document26 pagesCT - Gaseous State - Gaseous State Sheets - 21012021 - Gaseous State - Sheet 1 To 5Anita Akhilesh YadavNo ratings yet
- Heat & Thermodynamics - Test - pdf-1Document10 pagesHeat & Thermodynamics - Test - pdf-1VishalNo ratings yet
- Science Exemplar Complete Book PDFDocument173 pagesScience Exemplar Complete Book PDFMaahi ChhabraNo ratings yet
- Exemplar Science 9thDocument86 pagesExemplar Science 9thDaksh PatilNo ratings yet
- Gen Chem QuizDocument18 pagesGen Chem QuizNoime Labayog AgravanteNo ratings yet
- H O CO: CHEM1102 Worksheet 10 - Answers To Critical Thinking QuestionsDocument4 pagesH O CO: CHEM1102 Worksheet 10 - Answers To Critical Thinking QuestionsJohn-dred BautistaNo ratings yet
- Chem Sample PaperDocument4 pagesChem Sample PaperridahNo ratings yet
- 4102581936815909Document6 pages4102581936815909ytxtron414No ratings yet
- Physical Sciences: Paper Ii: Please Turn OverDocument14 pagesPhysical Sciences: Paper Ii: Please Turn OverBonga DubeNo ratings yet
- PhyChem2 FinalsDocument2 pagesPhyChem2 FinalsKrystel LahomNo ratings yet
- Roll NoDocument2 pagesRoll Nojagga daakuNo ratings yet
- DPP Class Ix MatterDocument2 pagesDPP Class Ix MatterMehul Mayank100% (1)
- 08-Liquid Solution - EngDocument5 pages08-Liquid Solution - EngMr XNo ratings yet
- Worked Solutions 7Document8 pagesWorked Solutions 7赵倞No ratings yet
- CHEMISTRY Chapter 1 Assignment Class 9 CBSEDocument4 pagesCHEMISTRY Chapter 1 Assignment Class 9 CBSEgurdeepsarora8738No ratings yet
- Form 4 Easter Exam 2022Document7 pagesForm 4 Easter Exam 2022TechnixFNNo ratings yet
- C2 Topic 12 - Applications of Free EnergyDocument49 pagesC2 Topic 12 - Applications of Free EnergyMonilNo ratings yet
- Cutting-Edge Technology for Carbon Capture, Utilization, and StorageFrom EverandCutting-Edge Technology for Carbon Capture, Utilization, and StorageKarine Ballerat-BusserollesNo ratings yet
- Metl-Span CF Urethane Insulated Panels Weight, PSFDocument1 pageMetl-Span CF Urethane Insulated Panels Weight, PSFKennyNo ratings yet
- Dryer DesignDocument9 pagesDryer DesignGohar AbbasNo ratings yet
- b16 24Document24 pagesb16 24Gustavo FamaNo ratings yet
- Tugas 2 Bahasa Inggris Lanjut "Lithium (Li) "Document3 pagesTugas 2 Bahasa Inggris Lanjut "Lithium (Li) "AkanegakuboMomoNo ratings yet
- Water TankDocument40 pagesWater TankAdil MansuriNo ratings yet
- Tvss White PaperDocument4 pagesTvss White PaperZIPDASHNo ratings yet
- Brass and BronzeDocument7 pagesBrass and BronzeH A RanaNo ratings yet
- Specification For Trolly Mounted DCPDocument12 pagesSpecification For Trolly Mounted DCPAshok KumarNo ratings yet
- Manual Gratis Chery Tig GoDocument16 pagesManual Gratis Chery Tig GoPaulo Arráiz100% (1)
- High Performance Butterfly Valve HP 111Document4 pagesHigh Performance Butterfly Valve HP 111JOHNNo ratings yet
- Use of Quarry Dust As Fine Aggregate in ConcreteDocument8 pagesUse of Quarry Dust As Fine Aggregate in ConcreteOngeriJNo ratings yet
- Jar Test Lab Report Environmental EngineeringDocument7 pagesJar Test Lab Report Environmental EngineeringNur Hazimah100% (1)
- 2005 Miosha Part 18 StandardsDocument16 pages2005 Miosha Part 18 StandardsAlejandro CarosiNo ratings yet
- Compressive Strength of Self-Compacting Concrete During High-Temperature ExposureDocument7 pagesCompressive Strength of Self-Compacting Concrete During High-Temperature ExposureDonaldo CVNo ratings yet
- Evergreen: High-Efficiency Hermetic Centrifugal Liquid ChillersDocument20 pagesEvergreen: High-Efficiency Hermetic Centrifugal Liquid ChillersTariq AngelNo ratings yet
- European Standard Norme Europeenne Europaische NormDocument51 pagesEuropean Standard Norme Europeenne Europaische Normpaai1989_679523114No ratings yet
- Refrigeration 2Document25 pagesRefrigeration 2krazylionNo ratings yet
- RFA & RFI For 400KV Gantry Verticality CheckingDocument3 pagesRFA & RFI For 400KV Gantry Verticality Checkingsathya2040No ratings yet
- 7 Design of Deep Flexural MemberDocument56 pages7 Design of Deep Flexural MemberSarah SpearsNo ratings yet
- As 2551-1982 Steel Sheet and Strip - Cold-Rolled Electrolytic Zinc-CoatedDocument6 pagesAs 2551-1982 Steel Sheet and Strip - Cold-Rolled Electrolytic Zinc-CoatedSAI Global - APACNo ratings yet
- Assignment 1 APPAREL MANUFACTURING INDUSTRYDocument3 pagesAssignment 1 APPAREL MANUFACTURING INDUSTRYdimuthu jayawardeneNo ratings yet
- A General Review of The Causes and Acceptance of Shape ImperfectionsDocument6 pagesA General Review of The Causes and Acceptance of Shape ImperfectionsAnonymous vU7CuPNo ratings yet
- Brick BondsDocument42 pagesBrick BondsNaveen PahalNo ratings yet
- Meter Measuring ToolsDocument4 pagesMeter Measuring ToolsMuhammad LukmanNo ratings yet
- HookupDocument161 pagesHookupKumar Kote100% (2)
- Lincoln LN25PRODocument6 pagesLincoln LN25PROmartinNo ratings yet
- Kota Stone Flooring: Group Members-Nishtha Dhamija Ankita Pilaniya Shivnashi Aggarwal SimranDocument10 pagesKota Stone Flooring: Group Members-Nishtha Dhamija Ankita Pilaniya Shivnashi Aggarwal SimranAnkitaNo ratings yet