Professional Documents
Culture Documents
Organic Chemistry Class 12 Cbse Notes
Organic Chemistry Class 12 Cbse Notes
Uploaded by
eashwarsiddha0 ratings0% found this document useful (0 votes)
1 views23 pagesThis document contains organic chemistry revision notes for the third exam of the 2023-2024 school year provided by Mr. S. Jerome Prakash Pravin of Velammal International School, Panchetti. The notes cover topics including carboxylic acids, amines, diazonium salts, and IUPAC nomenclature. Reactions, properties, mechanisms, uses and the order of basicity of different functional groups are discussed over multiple pages of detailed content.
Original Description:
Original Title
Organic Chemistry class 12 cbse notes
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document contains organic chemistry revision notes for the third exam of the 2023-2024 school year provided by Mr. S. Jerome Prakash Pravin of Velammal International School, Panchetti. The notes cover topics including carboxylic acids, amines, diazonium salts, and IUPAC nomenclature. Reactions, properties, mechanisms, uses and the order of basicity of different functional groups are discussed over multiple pages of detailed content.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
1 views23 pagesOrganic Chemistry Class 12 Cbse Notes
Organic Chemistry Class 12 Cbse Notes
Uploaded by
eashwarsiddhaThis document contains organic chemistry revision notes for the third exam of the 2023-2024 school year provided by Mr. S. Jerome Prakash Pravin of Velammal International School, Panchetti. The notes cover topics including carboxylic acids, amines, diazonium salts, and IUPAC nomenclature. Reactions, properties, mechanisms, uses and the order of basicity of different functional groups are discussed over multiple pages of detailed content.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 23
THE VELAMMAL INTERNATIONAL SCHOOL, PANCHETTI
ORGANIC CHEMISTRY REVISION NOTES FOR 2023 – 24 ( THIRD 33 % EXAM )
MR S JEROME PRAKASH PRAVIN Page 1
MR S JEROME PRAKASH PRAVIN Page 2
MR S JEROME PRAKASH PRAVIN Page 3
MR S JEROME PRAKASH PRAVIN Page 4
MR S JEROME PRAKASH PRAVIN Page 5
Iodoform reaction with sodium hypoiodite is also used for detection of CH3CO group or CH3CH(OH) group which
produces CH3CO group on oxidation.
MR S JEROME PRAKASH PRAVIN Page 6
MR S JEROME PRAKASH PRAVIN Page 7
MR S JEROME PRAKASH PRAVIN Page 8
MR S JEROME PRAKASH PRAVIN Page 9
MR S JEROME PRAKASH PRAVIN Page 10
MR S JEROME PRAKASH PRAVIN Page 11
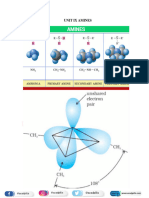
IUPAC NOMENCLATURE
MR S JEROME PRAKASH PRAVIN Page 12
• Carboxylic acids are weaker than mineral acids, but they are stronger acids than alcohols and many
simple phenols.
• Higher members of aliphatic carboxylic acids (C12 – C18) known as fatty acids.
MR S JEROME PRAKASH PRAVIN Page 13
MECHANISM
Uses of Carboxylic acid
1. Methanoic acid is used in rubber, textile, dyeing, leather and electroplating industries.
2. Ethanoic acid is used as solvent and as vinegar in food industry.
3. Hexanedioic acid is used in the manufacture of nylon-6, 6.
4. Esters of benzoic acid are used in perfumery.
5. Sodium benzoate is used as a food preservative.
6. Higher fatty acids are used for the manufacture of soaps and detergents.
MR S JEROME PRAKASH PRAVIN Page 14
MR S JEROME PRAKASH PRAVIN Page 15
MR S JEROME PRAKASH PRAVIN Page 16
Therefore, the order of boiling points of isomeric amines is as follows:
MR S JEROME PRAKASH PRAVIN Page 17
MR S JEROME PRAKASH PRAVIN Page 18
\
MR S JEROME PRAKASH PRAVIN Page 19
Quantitative evolution of nitrogen is used in estimation of amino acids and proteins.
• A very important class of compounds used for synthesis of a variety of aromatic compounds.
• Secondary and tertiary amines react with nitrous acid in a different manner.
In the reaction with secondary amine, N,N-diethylbenzenesulphonamide is formed.
MR S JEROME PRAKASH PRAVIN Page 20
Since N, N-diethylbenzene sulphonamide does not contain any hydrogen atom attached to nitrogen atom, it
is not acidic and hence insoluble in alkali.
Tertiary amines do not react with benzenesulphonyl chloride.
1. This property of amines reacting with benzenesulphonyl chloride in a different manner is used for
the distinction of primary, secondary and tertiary amines and also for the separation of a mixture of
amines.
2. However, these days benzenesulphonyl chloride is replaced by p-toluenesulphonyl chloride.
MR S JEROME PRAKASH PRAVIN Page 21
MR S JEROME PRAKASH PRAVIN Page 22
The order of basicity of amines in the gaseous phase
tertiary amine > secondary amine > primary amine > NH3
The order of basic strength in case of methyl substituted amines and ethyl substituted amines in aqueous solution
• (C2H5 ) 2 NH > (C2H5 ) 3 N > C2H5 NH2 > NH3
• (CH3 ) 2NH > CH3NH2 > (CH3 ) 3N > NH3
Importance of Diazonium Salts in Synthesis of Aromatic Compounds
• Diazonium salts are very good intermediates for the introduction of –F, –Cl, –Br, –I, –CN, –OH, –NO2 groups
into the aromatic ring.
• Aryl fluorides and iodides cannot be prepared by direct halogenation.
• The cyano group cannot be introduced by nucleophilic substitution of chlorine in chlorobenzene but
cyanobenzene can be easily obtained from diazonium salt.
• The replacement of diazo group by other groups is helpful in preparing those substituted aromatic
compounds which cannot be prepared by direct substitution in benzene or substituted benzene.
IUPAC NOMENCLATURE
MR S JEROME PRAKASH PRAVIN Page 23
You might also like
- TM 5-2410-241-24PDocument640 pagesTM 5-2410-241-24P"Rufus"100% (2)
- Advanced Pharmaceutical analysisFrom EverandAdvanced Pharmaceutical analysisRating: 4.5 out of 5 stars4.5/5 (2)
- Tensile Test Report - COMPLETEDocument9 pagesTensile Test Report - COMPLETEJasanta Jinor50% (2)
- Azo DyesDocument55 pagesAzo Dyessmit18950% (2)
- Chemistry Lab ReportDocument9 pagesChemistry Lab ReportEuwan Tyrone PriasNo ratings yet
- Amines S-3Document8 pagesAmines S-3sciencewing rbiNo ratings yet
- Chemistry IDocument21 pagesChemistry INyam Yi MeiNo ratings yet
- Strategies For Peptide Synthesis: An Overview: Peptide Coupling Reagent - H ODocument43 pagesStrategies For Peptide Synthesis: An Overview: Peptide Coupling Reagent - H Oalen19819072100% (1)
- I. Nitrogen Containing Functional Groups: B.Sc. Semester-IV Core Course-IX (CC-IX) Organic Chemistry-IIIDocument21 pagesI. Nitrogen Containing Functional Groups: B.Sc. Semester-IV Core Course-IX (CC-IX) Organic Chemistry-IIIadityanarayanrout10No ratings yet
- ShrawanivvDocument22 pagesShrawanivvtrustedtrader.099No ratings yet
- New Diazonium SaltDocument5 pagesNew Diazonium SaltCiber AreaNo ratings yet
- L 1 Crude Oils Chemistry and CompositionDocument13 pagesL 1 Crude Oils Chemistry and CompositionSaroo BastkyNo ratings yet
- CH - 13 AMINES NotesDocument21 pagesCH - 13 AMINES NotesKESHAV KUMARNo ratings yet
- NPK FertilizerDocument11 pagesNPK FertilizerShivaniNo ratings yet
- Protein Structure and FunctionDocument176 pagesProtein Structure and FunctionbanyacskijuliusNo ratings yet
- NPK FertilizerDocument11 pagesNPK FertilizermajidNo ratings yet
- Organic 2 Chapter 1&2Document94 pagesOrganic 2 Chapter 1&2bahru demekeNo ratings yet
- Derivatives of Dehydroabietic Acid As Polymer AdditivesDocument24 pagesDerivatives of Dehydroabietic Acid As Polymer AdditivesHimanshu PanchalNo ratings yet
- Desproporcionacion de ColofoniaDocument8 pagesDesproporcionacion de Colofoniacarlos_bautista_55No ratings yet
- Clear Zinc Pyrithione Preparations: Terry Gerstein, M.S.Document16 pagesClear Zinc Pyrithione Preparations: Terry Gerstein, M.S.mazahir razaNo ratings yet
- Xii - CH13 - Organic Compounds Containing NitrogenDocument5 pagesXii - CH13 - Organic Compounds Containing NitrogenYash RajNo ratings yet
- Tetrahedron Report Number 373: Alexander Mekillop A and William R Sanderson BDocument22 pagesTetrahedron Report Number 373: Alexander Mekillop A and William R Sanderson BÁn GelaNo ratings yet
- Practicala Viva Question - 2023Document1 pagePracticala Viva Question - 2023Durgesh PatilNo ratings yet
- Amines Amino Acids ProteinsDocument13 pagesAmines Amino Acids ProteinsClifford Dwight RicanorNo ratings yet
- Detective Tests For Amino Acids: A Report Submitted To The Department of Dentistry University of DuhokDocument18 pagesDetective Tests For Amino Acids: A Report Submitted To The Department of Dentistry University of DuhokKistan MuhsinNo ratings yet
- AA和4VP交替Document6 pagesAA和4VP交替Li WangNo ratings yet
- PetroleumDocument54 pagesPetroleumJthan ReyesNo ratings yet
- Cyclocondensation Reactions of 5 AminopyDocument15 pagesCyclocondensation Reactions of 5 AminopymanishaNo ratings yet
- 2018 JC2 H2 Nitrogen Compounds Notes (Upload For Students)Document26 pages2018 JC2 H2 Nitrogen Compounds Notes (Upload For Students)Amelia WongNo ratings yet
- Volumetric AnalysisDocument3 pagesVolumetric AnalysisSubhash DhungelNo ratings yet
- Chap 2 - AMINO ACIDS - SeDocument56 pagesChap 2 - AMINO ACIDS - SeMPP ALIF FSGNo ratings yet
- Ribonucleotide: StructureDocument7 pagesRibonucleotide: StructureJames FranklinNo ratings yet
- BL NurBio Activity 6 - Proteins Color Reactions (Revised 6.22.20)Document8 pagesBL NurBio Activity 6 - Proteins Color Reactions (Revised 6.22.20)Niño PadacaNo ratings yet
- 15 - Amines (New) PDFDocument25 pages15 - Amines (New) PDFthinkiitNo ratings yet
- An Improved Sodium Fusion Procedure and A New TestDocument2 pagesAn Improved Sodium Fusion Procedure and A New TestJorge MartinezNo ratings yet
- 1.3 Acids, Bases and Indicators-1-1Document11 pages1.3 Acids, Bases and Indicators-1-1Festus NanokNo ratings yet
- Posible Analisis AA en PapotaDocument3 pagesPosible Analisis AA en PapotaGerman CarleNo ratings yet
- Activity 5 Laboratory ProcedureDocument8 pagesActivity 5 Laboratory Procedure3amabelle arevaloNo ratings yet
- AminesDocument13 pagesAminesSohamNo ratings yet
- Post Lab Discussion ReviewerDocument3 pagesPost Lab Discussion ReviewerAngelica Camille B. AbaoNo ratings yet
- CHEMISTRYDocument17 pagesCHEMISTRYyadavpreetika72No ratings yet
- Amino Acid & ProteinDocument9 pagesAmino Acid & ProteinMaryGraceVelascoFuentesNo ratings yet
- AnilinDocument18 pagesAnilinM Septian PrasetyoNo ratings yet
- Nuclear Organizer RegionsDocument18 pagesNuclear Organizer RegionsMugomba JuliusNo ratings yet
- CNP 4 Amino AcidsDocument37 pagesCNP 4 Amino AcidsShakir AliyiNo ratings yet
- New Strategies For The Hofmann ReactionDocument7 pagesNew Strategies For The Hofmann ReactionCasper JorckNo ratings yet
- Introduction To Crude Products and Test MethodsDocument90 pagesIntroduction To Crude Products and Test MethodsGayathri GanesanNo ratings yet
- Heterocyclic CompoundsDocument38 pagesHeterocyclic CompoundsMuhammad NaqiNo ratings yet
- Chemical Characterization of Ribonucleic AcidDocument3 pagesChemical Characterization of Ribonucleic AcidAngeloMuñozNo ratings yet
- Journal Pre-Proofs: Tetrahedron LettersDocument10 pagesJournal Pre-Proofs: Tetrahedron LettersMuhammad Syahrir PratamaNo ratings yet
- Notes For Chemistry Class 12 Chapter 13 AminesDocument22 pagesNotes For Chemistry Class 12 Chapter 13 AminesVanshika JainNo ratings yet
- AminesDocument9 pagesAminesTr Mazhar PunjabiNo ratings yet
- Reduce Salt Corrosion Rates With Stronger Base AminesDocument4 pagesReduce Salt Corrosion Rates With Stronger Base AminesPaolo VisentinNo ratings yet
- Ubc - 1973 - A1 S65Document241 pagesUbc - 1973 - A1 S65joebug34No ratings yet
- B Amino AcidsDocument55 pagesB Amino AcidsYujin AhnNo ratings yet
- Ácido SulfónicoDocument4 pagesÁcido SulfónicoLeo Silver RomeroNo ratings yet
- Xanthoproteic Acid Test Is A Chemical Test For Specific Functional Groups in AminoDocument3 pagesXanthoproteic Acid Test Is A Chemical Test For Specific Functional Groups in AminoyapyapvinxNo ratings yet
- Class - 12 Chemistry - AminesDocument34 pagesClass - 12 Chemistry - AminesAnonymous RuslwNZZlNo ratings yet
- Chemistry Notes For Class 12 Chapter 13 Amines PDFDocument16 pagesChemistry Notes For Class 12 Chapter 13 Amines PDFmanju priyaNo ratings yet
- Iridium Complexes in Organic SynthesisFrom EverandIridium Complexes in Organic SynthesisLuis A. OroNo ratings yet
- Glycochemical Synthesis: Strategies and ApplicationsFrom EverandGlycochemical Synthesis: Strategies and ApplicationsShang-Cheng HungNo ratings yet
- Golf Fourse WhereDocument2 pagesGolf Fourse WhereeashwarsiddhaNo ratings yet
- Chat - GPT Being My BaeDocument2 pagesChat - GPT Being My BaeeashwarsiddhaNo ratings yet
- Ibm 101Document2 pagesIbm 101eashwarsiddhaNo ratings yet
- Introduction Presentation EashwarDocument6 pagesIntroduction Presentation EashwareashwarsiddhaNo ratings yet
- Ceratainity PrincipleDocument2 pagesCeratainity PrincipleeashwarsiddhaNo ratings yet
- An Essay About MyselfDocument2 pagesAn Essay About MyselfeashwarsiddhaNo ratings yet
- EashwarDocument1 pageEashwareashwarsiddhaNo ratings yet
- University ShortlistDocument2 pagesUniversity ShortlisteashwarsiddhaNo ratings yet
- FaceLib PresentationDocument8 pagesFaceLib PresentationeashwarsiddhaNo ratings yet
- Food Order Source CodeDocument13 pagesFood Order Source CodeeashwarsiddhaNo ratings yet
- Xii-Board 3RD 33% Physics QP - 18.11.2023Document7 pagesXii-Board 3RD 33% Physics QP - 18.11.2023eashwarsiddhaNo ratings yet
- Development of Methods For Determining Water Content in Oil UsingDocument120 pagesDevelopment of Methods For Determining Water Content in Oil Usinggilar herliana putraNo ratings yet
- CMOS Implemented VDTA Based Colpitt OscillatorDocument4 pagesCMOS Implemented VDTA Based Colpitt OscillatorijsretNo ratings yet
- Bonna Sabla Concrete PipesDocument19 pagesBonna Sabla Concrete Pipesst_calvoNo ratings yet
- IMG - 0213 PSME Code 2008 201Document1 pageIMG - 0213 PSME Code 2008 201Hnqr584hNo ratings yet
- Properties of SoundDocument13 pagesProperties of SoundAnjhana KumarNo ratings yet
- DehydrationDocument13 pagesDehydrationSaa D ShamimNo ratings yet
- DP5900Document2 pagesDP5900Jorge SanchezNo ratings yet
- My Add Maths NotesDocument21 pagesMy Add Maths NotesliveelaughloveNo ratings yet
- Java Questions1-By TarekDocument78 pagesJava Questions1-By TarektotitarekNo ratings yet
- 1 s2.0 S0003682X12002496 Main PDFDocument9 pages1 s2.0 S0003682X12002496 Main PDFDusan VeljkovicNo ratings yet
- Terapi Seft Spiritual Emotional Freedom TechniqueDocument7 pagesTerapi Seft Spiritual Emotional Freedom TechniqueWiwik AristianiNo ratings yet
- Section 5 - Neehal Islam Dastagir - 18204069 - BUS302 Term Project ReportDocument7 pagesSection 5 - Neehal Islam Dastagir - 18204069 - BUS302 Term Project ReportNeehal Dastagir100% (1)
- International Journal of Industrial Ergonomics: Sangeun Jin, Gary A. MirkaDocument5 pagesInternational Journal of Industrial Ergonomics: Sangeun Jin, Gary A. MirkaAlexiNo ratings yet
- 1 PBDocument20 pages1 PBYou IfkirnNo ratings yet
- Analysing Batch Reactor DataDocument3 pagesAnalysing Batch Reactor DatautpalmtbiNo ratings yet
- Air SeparationDocument18 pagesAir Separationrahul67% (3)
- "Master Organic Chemistry": Anti-Markovnikoff BH H Syn AdditionDocument1 page"Master Organic Chemistry": Anti-Markovnikoff BH H Syn AdditionMoncef AbbesNo ratings yet
- ISOVER Integra AB SolidBlackDocument9 pagesISOVER Integra AB SolidBlackDiaconu FlorinNo ratings yet
- MFF218 TPM Slides MidsemDocument225 pagesMFF218 TPM Slides MidsemJeet SinghaniaNo ratings yet
- The Spectrogram As A Tool For Analysis of Nonlinear StructuresDocument108 pagesThe Spectrogram As A Tool For Analysis of Nonlinear Structuresorestis willemenNo ratings yet
- PV ProgramDocument26 pagesPV ProgramArvind SharmaNo ratings yet
- Sciadv Abd0957Document17 pagesSciadv Abd0957Shivaprakash Jagalur MuttNo ratings yet
- Binnas o Somabesh (Permmutation and Combination)Document17 pagesBinnas o Somabesh (Permmutation and Combination)Dhiman Nath0% (1)
- What Keq PT3Document12 pagesWhat Keq PT3katherine corveraNo ratings yet
- 18AIC101J-FDA-CT-3: PART-B Answer All Questions (15 X 2 30)Document9 pages18AIC101J-FDA-CT-3: PART-B Answer All Questions (15 X 2 30)Tanmay Agrawal (RA2011047010093)No ratings yet
- Enhanced Oil Recovery From Depleted Reservoirs UsiDocument9 pagesEnhanced Oil Recovery From Depleted Reservoirs UsiAli MahmoudNo ratings yet
- 5070 w11 QP 12Document12 pages5070 w11 QP 12mstudy123456No ratings yet
- tl02 DatasheetDocument3 pagestl02 DatasheetadryanfahriNo ratings yet