Professional Documents
Culture Documents
CH30 - Tablet and Compaction
CH30 - Tablet and Compaction
Uploaded by
Thorn MailOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
CH30 - Tablet and Compaction
CH30 - Tablet and Compaction
Uploaded by
Thorn MailCopyright:
Available Formats
CHAPTER 30: TABLET AND COMPACTION
3rd Shift,
PHA 6124 LECTURE 1st Sem
JAVIER, D.C.
OUTLINE JOURNEY OF A PILL
I. Tablets 1. After you swallow a pill, it makes it way to the stomach
A. General Tablet Types 2. It’s broken down in the stomach, small intestine and liver
B. How do Tablets Work? 3. Then circulates through the bloodstream
C. Advantages of Tablets 4. Until it reaches its target and goes to work
D. Quality Attributes of Tablets
II. Tablet Manufacturing ADVANTAGES OF TABLETS
A. Powder Compression ● Oral route is convenient and safe way of drug
B. Compaction administration
1. Compaction Cycle ● Stable as compared to liquid dosage forms (less microbial
C. Tablet Toolings contamination)
III. Mechanisms Of Tablet Compression ● Accurate drug dosing
A. Rumpf’s Bond Classification ● Convenience in handling (blisters vs glass bottles)
IV. Powder/Granule Properties (Impact To Compatibility) ● Cheaper mass production (less packaging materials &
V. Technical Problems shipping costs)
A. During Compression
B. Properties To Be Controlled QUALITY ATTRIBUTES OF TABLETS
C. During Compression Unit Operations For Tablet ● Should have the correct dose of the drug/API
Manufacturing (Wet Vs Dry Granulation) ● Should have a consistent appearance (size, color, weight)
VI. Tablet Excipients ○ Tablets are prone to aesthetic issues (mottling: prevent
Solid Dosage Forms: by coating with opaque, sugar, or enteric coat)
● Dry Mixing → Granulation → Dry: Slugging & Roller ● The drug should be released from the tablet in a controlled
Compaction and reproducible way
● Drying (if wet granulation) ● Should be biocompatible (does not contain harmful
○ Moist heat oven: when solvent is alcoholic ingredients)
● Packaging ● Should have a sufficient mechanical strength to withstand
rigors/fracture/erosion/handling (hardness & friability)
TABLETS ● Should be formulated into a product acceptable to patient
● ORAL ROUTE – most common way of administering drugs (within the patient’s taste to promote adherence)
● Latin: tabuletta
○ small disc-like cylindrical specimen TABLET MANUFACTURING
● Latin: compressi
○ due to the manufacturing principle of compression
POWDER COMPRESSION
● 1843 – first patent for a hand-operated device was granted
to form tablets ● the dominating technique of forming tablets characterized
● Solid preparations each containing a single dose of one or by forcing particles into close proximity by confined
more active substances, European Pharmacopeia compression
Commission (2016 definition) ● defined as the reduction in volume of a powder owing to
● Obtained by compression of uniform volumes of force application
particles/suitable granules ● causes particle bonding which provides coherence to the
compressed powder (thus the term compact)
● This is necessary to occupy less space and bind the
GENERAL TABLET TYPES
particles together
● ex. plain compressed tablet
Swallowed whole
● coated tablet
COMPACTION
● tablets without disintegrant
Chewed ● The formation of a solid specimen of defined geometry by
● ex. chewable tablet
powder compression
Dissolved/Dispersed ● ex. effervescent tablet
● Compaction cycle: die filling → tablet formation → tablet
in water
ejection
Retained in the ● ex. sublingual tablet (under tongue),
mouth/oral cavity ● buccal tablet (bet. cheeks)
COMPACTION CYCLE
Die Filling
HOW DO TABLETS WORK? ● Accomplished by gravitational flow of the powder into
● Tablets are mainly used for systemic drug delivery 1 the die table. The die is closed by the lower punch
● Drug must be released/liberated from the tablet ● Die is the holder/compartment of the powder or the
(disintegration → dissolution → absorption) cavities(holes)
● Absorption into the systemic circulation follows from 2 Tablet Formation
lining of intestinal mucosa
● Excipients will also be absorbed
SURNAMES | 3FPH - 1ST SEMESTER, 3RD SHIFTING 1
PHARMACEUTICAL MANUFACTURING (WITH QUALITY ASSURANCE AND CGMP) PHA 6124 LEC | S.Y. 2023 - 2024
● Upper punch descends and compacts the powder in ○ There is no elasticity in powder, unlike a sponge for
the die (compression phase) example,
● After force application, the upper punch ascends and ○ Although there are particular powders that are elastic.
leaves the powder compacted (decompression phase) ● the degree of deformation is related to the applied stress
Tablet Ejection and not the time of loading.
3 ● Lower punch rises and the formed tablet is ○ relative to force, kahit mabilis mo man icompress, it will
removed/ejected from the die stay that way.
TYPES OF TABLET PRESS ● Reduction is
● Tablet press is also called compression machine or done to occupy
tabletting machine LESS (not more)
● Manesty is a household name for industrial tablet press space
● Die Filling -
Single-Punch Rotary Press Computerized Press
operates through
(Eccentric Press) (Multistation Press) (Compaction Simulator) the free-flowing
principle due to
gravity
● (hindi kinakalog,
hindi vinavibrate;
mali ang pag
manufacture
kapag inalog)
● UPPER punch
compresses
● Multiple set of ● Controlled process ● LOWER punch
● One die and one
punches and die with inputted ejects
pair of punches parameters and
with output of
(upper and approx. 10,000
specification control
lower)(one ● Output of more than
tablets/minute 10,000
station)with ● Stations range tablets/minutes -
output of between 3 – 60 (pinakamaraming
approx. 200 stations (usually narerelease)
36) ● *board depth - how
tablets/minute deep ang die
● *blind station -
(controlled para
sira ang punch, kasya sa banig)
so tinatakpan ● Weak force → soft
ang die para ‘di ● Too strong force →
na magamit hard tablet
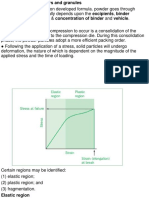
TABLET TOOLINGS Fig. 30.24 Particle deformation, elastic and plastic, during
● Fabricated using stainless steel compression. Adapted from Armstrong, 1982, with permission
● May be damaged and worn out due to high forces applied
during the compression phase RUMPF’S BOND CLASSIFICATION
● May be coated by a thin layer of another metal called MECHANISM OF PARTICLE COMPACTION
chrome to modify its surface properties
● Toolings must be handled and stored with care
● Usually stored in a controlled room environment with
controlled temperature and humidity (toolings have their
own rooms)
MECHANISMS OF PARTICLE COMPRESSION
● Board Exam Question for Compression - compressibility
of a powder is defined as its propensity, when held within a SOLID BRIDGES
confined space, to reduce in volume when subjected to a ● Also known as the diffusion theory of bonding
load.
● Formation of continuous solid phase
○ it becomes solid because of compaction and
● Vander Waals are weak bondings
compression
● WET granulation is considered as
■ liquid compaction is held by liquid fluid
strong.
■ solid compaction is held by pressure
● Particles, either all or some, can change their shape
temporarily by elastic deformation and permanently by BONDING BY LIQUIDS
plastic deformation ● Capillary and surface tension forces
○ when a tablet is formed from die filling, will it return to ● additional linkages
powder form? NO, it is already compacted (elastic
deformed; cannot go back to original shape once
compacted)
SURNAMES | 3FPH - 1ST SEMESTER, 3RD SHIFTING 2
PHARMACEUTICAL MANUFACTURING (WITH QUALITY ASSURANCE AND CGMP) PHA 6124 LEC | S.Y. 2023 - 2024
mg, it is crucial to follow a thorough validation procedure.
BINDER BRIDGES However, it's important to note that the dosing at this stage
● Viscous binders and adsorption layers is PURELY THEORETICAL.
● Binders are powder materials that are mixed with the ● When calculating the tablet weight
granulating fluid, like Povidone for example ● 1 tab 10 mg API, for 10 tablets the Acceptance Criteria is
● if granulating fluid is water + the addition binder = 58.95 - 62.95 mg - weight
PRINCIPLES: bonding by liquid and binder bridges ● To calculate how much is the tablet weight in comparison
● mechanism is recommended: if tablet is prone to CHIPPING to the label claim…
59.60
T1 = 59.60 mg → 60 𝑚𝑔 = 98. 7%
INTERMOLECULAR AND ELECTROSTATIC FORCES
● also known as adsorption bonding CAPPING
● intimate contact and are therefore adsorbed to each other ● Capping occurs due to a deficiency in bonds, force, or
● binding by means of charges binding.
● The direction of compression (pababa) causes the
MECHANICAL INTERLOCKING detachment at the top rather than the sides or elsewhere.
● Inter-particulate hooking
LAMINATION
● This phenomenon usually requires that ● Lamination occurs when cracks form along the sides due to
the particles have an atypical shape, a misalignment in the compression direction.
such as needle-shaped, or highly
irregular and rough particles.
TECHNICAL PROPERTIES TO BE CONTROLLED
● Poor API distribution will cause
Homogeneity and underdose and overdose
POWDER/GRANULE PROPERTIES Segregation ● Poor segregation tendency will cause
(IMPACT TO COMPACTABILITY) Tendency underdose, overdose, and variation of
tablet weight
● Poor flowability will cause the powder
Flowability
to stick / remain on hopper
Compression ● Very dry powders will tend to be
Properties and crumbled
Compactability
● Powders with high moisture content
Friction And
tend to be sticky and become a hard
Adhesion
tablet
Properties
○ Cement formation is a good example
● Rat Holing - problem: part of the hopper
● tendency of the granule to create space in the middle; hindi
bumababa sa gravity. inaalog siya which is wrong
This diagram shows all of the characteristics of your granule ● Bridging - lata ng nido, kinukutsyara, yung side nag sstuck
● wrong characteristics, will not form as a tablet ● Consequence of both - no step 1 of compaction cycle, no
● important: batch and lot number, material itself die filling
● If the concept is interlocking but the shapes of granules
are circles (hindi tugma) TECHNICAL PROBLEMS DURING COMPRESSION UNIT
OPERATIONS FOR TABLET MANUFACTURING
TECHNICAL PROBLEMS DURING COMPRESSION (WET VS DRY GRANULATION)
● High weight and dose
● variation of the tablets
● Low mechanical strength (friable tablets)
● Capping and lamination
● Sticking of powders on the punch tips (chipping)
○ sticks because of moisture due to excess granulating
dose; drying is not complete.
○ during dry granulation, there are two main reasons why
powders still tend to stick despite the absence of the
granulating fluid:
■ high relative humidity, moisture on punches allows
granules to stick to the surface
■ powder is hygroscopic
● High friction during tablet ejection
○ There should be seamless ejection, wala friction
EXAMPLE:
● How do you determine the specifications when Fig. 30.5 Overview of the sequence of unit operations used in the
manufacturing 1 million tablets from a 10,000 kg batch? For production of tablets with precompaction treatment by granulation
instance, in determining the dosage for a single die at 10
SURNAMES | 3FPH - 1ST SEMESTER, 3RD SHIFTING 3
PHARMACEUTICAL MANUFACTURING (WITH QUALITY ASSURANCE AND CGMP) PHA 6124 LEC | S.Y. 2023 - 2024
● Added to drug formulation to ensure that granules and
tablets can be formed with the required mechanical
strength (improves compactability)
● starch, sucrose (not always a sweetener!
Traditional
sticky when melted), gelatin
● Specialized compared to Traditional
Most Common ● Polyvinylpyrrolidone (PVP), hydroxypropyl
methylcellulose (HPMC)
Binders are Usually Added in Several Different Ways:
● As dry powder mixed with the powders before
Fig. 30.6 Overview of the sequence of unit operations used in the agglomeration (wet granulation); POWDER TO POWDER
production of tablets by direct compaction. ● As dry powder mixed with the powder before compaction –
also called dry binder (dry granulation) BEFORE PLACING
TABLET EXCIPIENTS INSIDE THE HOPPER
● Filler (or diluent), Disintegrant, Solution binder, Dry binder, ● As a solution forming the granulating solution
Glidant, Lubricant, Antiadherent
SOLUTION BINDER EXAMPLES DRY BINDER EXAMPLE
FILLER - pampuno ● Gelatin ● Cellulose
● Aids to form tablets of a target size suitable for handling ● Polyvinylpyrrolidone ● Methylcellulose
● Increases bulk volume and tablet size
● Cellulose derivative (eg ● polyvinylpyrrolidone
○ You will not add more API to increase the tablet size,
hydroxypropyl methylcellulose)
you add fillers.
● Potent drugs which would only require a very small amount ● Polyethylene glycol ● Polyethylene glycol
of the API in a tablet would rely on fillers to form a suitable ● Sucrose starch
tablet form
● Ideal characteristics: inert, non- hygroscopic, GLIDANT
biocompatible, water soluble, acceptable taste ● Improves flowability of powders helps eradicate rat holing
● MOST COMMON: Lactose (but limited to those without and bridging.
lactose intolerance) ● Used usually for direct compaction.
EXAMPLES MOST COMMON:
Lactose Sucrose Glucose Mannitol ● Talc (1-2% by weight)
Sorbitol Dicalcium phosphate Calcium Carbonate Cellulose ● Colloidal silica (0.2% by weight)
● Magnesium stearate (<0.1% by weight)
DISINTEGRANT - not found in chewable tablets
LUBRICANT
● disintegrants swell inside the stomach, it will increase size
there. Due to the increase in size, it will result to breaking. ● Ensures tablet formation and ejection can occur with low
● Aids the breaking up of the tablet into fragments upon friction between the solid and the die wall
contact with water (swelling mechanism) ● Improper ejection can automatically stop the compression
● MOST COMMON: Starch machine
Disintegration Process has 2 Steps 2 Mechanisms of Lubrication
1. liquid wets and penetrates the pores of the tablet ● Fluid lubrication – layer of fluid is located between and
2. the tablet breaks up into smaller fragments separates the moving surface reducing the friction (liquid
paraffin)
● Boundary lubrication – very thin film of lubricant reduces
3 Types of Disintegrants
the friction (most commonly used)
1. Water-uptake facilitator (liquid transport into pores)
○ Water from outside is attracted to the surface of tablet
EXAMPLES
2.Tablet-rupture facilitator (swelling during water sorption)
● Magnesium stearate ● Sodium lauryl sulfate
liberation (makes bubbles 🫧
3.Gas producer (a disintegrant will cause carbon dioxide
) by carbonate/bicarbonate
decomposition with acidic water) – used only for
● Stearic acid ● Sodium stearyl fumarate
● Polyethylene glycol ● Liquid paraffin
effervescent tablets
ANTIADHERENT
EXAMPLES ● If glidant is not enough, add this to increase lubrication
● Starch ● Sodium starch glycolate ● Reduces adhesion between the powder and punch faces
● Cellulose ● Sodium carboxymethylcellulose and thus prevents particle sticking to the punches
● Cross-linked polyvinylpyrrolidone ● Tablets with marking and symbols are usually more prone
to picking or sticking
BINDER ● kabag embossing mo sa word na Biogesic
● Most common: Magnesium stearate
● Also called adhesive (usually 2-10% by weight in the
formulation) EXAMPLES: Magnesium stearate, Talc, Starch, Cellulose
○ MORE BINDER = HARDER TABLETS
SURNAMES | 3FPH - 1ST SEMESTER, 3RD SHIFTING 4
You might also like
- Astm D513-16Document9 pagesAstm D513-16Sebastian SanChezNo ratings yet
- Tableting: Compression & Compaction in Tablets FormationDocument25 pagesTableting: Compression & Compaction in Tablets FormationVon Valentine MhuteNo ratings yet
- Ind 9Document22 pagesInd 9osama2010bNo ratings yet
- Tablte Lecture Note EditedDocument39 pagesTablte Lecture Note EditedSolomonNo ratings yet
- Tablet Manufacturing ProcessDocument8 pagesTablet Manufacturing ProcessNaveen KingNo ratings yet
- Anaswara Ashok Modern PharmaceuticsDocument76 pagesAnaswara Ashok Modern PharmaceuticsrgofennpvotbcislrkNo ratings yet
- Drug DefectsDocument5 pagesDrug DefectsKaye OlidNo ratings yet
- 5 TabletDocument19 pages5 Tablet8ghy7y2nrgNo ratings yet
- Tablet Compression MethodsDocument7 pagesTablet Compression MethodsSORBAX PHARMACEUTICALSNo ratings yet
- HHV 5014 Nutraceutical Formulation Technology Unit 5: Quality Control Requirement (5.3: Formulation of Solid Dosage Form (Tablets & Capsules) )Document15 pagesHHV 5014 Nutraceutical Formulation Technology Unit 5: Quality Control Requirement (5.3: Formulation of Solid Dosage Form (Tablets & Capsules) )Priyanka PawarNo ratings yet
- Pmqa Lab AcetaminophenDocument7 pagesPmqa Lab AcetaminophencalliemozartNo ratings yet
- TabletsDocument72 pagesTabletsMuhammad NaqiNo ratings yet
- TabletsDocument14 pagesTabletsMuhammad WaleedNo ratings yet
- Compressed TabletsDocument39 pagesCompressed TabletsAnaliza Kitongan LantayanNo ratings yet
- Preparation #3 Paracetamol TabletsDocument17 pagesPreparation #3 Paracetamol TabletsIvy Rose OrozcoNo ratings yet
- Compaction and Compression of PowderDocument23 pagesCompaction and Compression of Powderjabed sarkar100% (1)
- 01.tablets (-I-)Document25 pages01.tablets (-I-)Subha ShankareeNo ratings yet
- Types of Tablets, Manufacture and DispensingDocument33 pagesTypes of Tablets, Manufacture and DispensingRICHARD RODERICK OLMEDONo ratings yet
- Pharm IV Lab ManualDocument125 pagesPharm IV Lab ManualVargheseNo ratings yet
- Research ArticleDocument11 pagesResearch ArticleFatima AmeenNo ratings yet
- ReportDocument10 pagesReportPemal KaurNo ratings yet
- Prep #31 - Captopril SuspensionDocument3 pagesPrep #31 - Captopril SuspensionKirsten Shayne ManingasNo ratings yet
- MicroDocument17 pagesMicroBola Bahgat Megalaa'No ratings yet
- Lecture 7 - Pharmaceutics IIIDocument30 pagesLecture 7 - Pharmaceutics IIIahmed sharafNo ratings yet
- DDS LEC - Tablets Part 2Document5 pagesDDS LEC - Tablets Part 2kapeNo ratings yet
- Drug Delivery SystemDocument15 pagesDrug Delivery SystemC-Net WorksNo ratings yet
- Tablet and Capsule ManufacturingDocument28 pagesTablet and Capsule ManufacturingShibaprasad DandapatNo ratings yet
- Solid Dosage Forms: Tablets: Abhay ML Verma (Pharmaceutics)Document5 pagesSolid Dosage Forms: Tablets: Abhay ML Verma (Pharmaceutics)meet2abhayNo ratings yet
- Presentation On GEA Courtoy: Presented by Presenter: Sangram KendreDocument79 pagesPresentation On GEA Courtoy: Presented by Presenter: Sangram KendreSangram KendreNo ratings yet
- Oral Solid Dosage FormsDocument26 pagesOral Solid Dosage FormsAl-Homam SalahNo ratings yet
- Tablets: TypesDocument23 pagesTablets: TypesdadadNo ratings yet
- Types of Tablets and Their CharecteristicsDocument49 pagesTypes of Tablets and Their CharecteristicsRohit IrkalNo ratings yet
- International Research Journal of Pharmacy: Processing Technologies For Pharmaceutical Tablets: A ReviewDocument4 pagesInternational Research Journal of Pharmacy: Processing Technologies For Pharmaceutical Tablets: A ReviewBella Puspita HapsariNo ratings yet
- Tablet FormulationDocument34 pagesTablet FormulationWicharn KetjindaNo ratings yet
- C-8 TabletsDocument3 pagesC-8 TabletsAli Uy100% (2)
- Tablets: By: Katryn PunsalangDocument69 pagesTablets: By: Katryn PunsalangKatryn PunsalangNo ratings yet
- Bab IDocument18 pagesBab IasmaraniNo ratings yet
- Preps 1 5Document19 pagesPreps 1 5Angela ArcamoNo ratings yet
- A Review On Importance of SuperdisintegrDocument5 pagesA Review On Importance of Superdisintegr72 - Wasim Akhtar AnsariNo ratings yet
- Assignment 4 and 5Document7 pagesAssignment 4 and 5Trisha ArgaoNo ratings yet
- NEW Tablet Manufacturing Process PDFDocument32 pagesNEW Tablet Manufacturing Process PDFShmmon Ahmad0% (2)
- Industrial Training at Benham Pharmaceutical LTDDocument37 pagesIndustrial Training at Benham Pharmaceutical LTDRabbiiKhanNo ratings yet
- D TabletsDocument19 pagesD TabletsMadhav MadyNo ratings yet
- Tablet SIBDocument45 pagesTablet SIBMd Sayeed100% (1)
- Compressed Tablets: Biopharmaceutics and PharmacokineticsDocument13 pagesCompressed Tablets: Biopharmaceutics and Pharmacokineticskhara teanoNo ratings yet
- Quality Control of Tablets Lecture 2Document24 pagesQuality Control of Tablets Lecture 2sakrishnagoud BingiNo ratings yet
- TS TabletDocument44 pagesTS TabletAkrimul Khalqi100% (1)
- Metode Pembuatan Tablet PDFDocument53 pagesMetode Pembuatan Tablet PDFezaNo ratings yet
- Physics of Tablet With Compaction and Compression Process For Novel Drug Dosage FormDocument7 pagesPhysics of Tablet With Compaction and Compression Process For Novel Drug Dosage FormSangram KendreNo ratings yet
- Pharmaceutical Manufacturing Lecture Tablets & Tablet CoatingDocument14 pagesPharmaceutical Manufacturing Lecture Tablets & Tablet CoatingDaena TimtimanNo ratings yet
- CapsulesDocument5 pagesCapsulesL1485 WeareoneNo ratings yet
- TABLETSDocument1 pageTABLETSCHARLOTTE MAY SAPIERANo ratings yet
- TabletDocument80 pagesTabletParizal Very100% (4)
- Technology of Making Tablets: Murat KizaibekDocument79 pagesTechnology of Making Tablets: Murat KizaibekSangram KendreNo ratings yet
- Tablets: Prepared By: Roshni Mehta PHD Research Scholar Guided By: Dr. Yamini Shah Associate ProfessorDocument169 pagesTablets: Prepared By: Roshni Mehta PHD Research Scholar Guided By: Dr. Yamini Shah Associate ProfessorMithu SarkerNo ratings yet
- Quality Control of TabletsDocument33 pagesQuality Control of TabletsSohail SheikhNo ratings yet
- Tablet Technology StepsDocument9 pagesTablet Technology StepsVikas JhawatNo ratings yet
- Flow charts of pharmaceutical quality control tests for different dosage formsFrom EverandFlow charts of pharmaceutical quality control tests for different dosage formsNo ratings yet
- Science 9 Second Quarter ExamDocument4 pagesScience 9 Second Quarter ExamAllan Roloma100% (1)
- Decoding - MSDS PPT 3Document17 pagesDecoding - MSDS PPT 3VidyaNo ratings yet
- Foramtive Assessment - I Subject: Intergrated Science Duration: 2 Hrs Class: Viii Maximum Marks: 20 Marks Name of The CandidateDocument10 pagesForamtive Assessment - I Subject: Intergrated Science Duration: 2 Hrs Class: Viii Maximum Marks: 20 Marks Name of The CandidateelizabethNo ratings yet
- HILICDocument11 pagesHILICMaria TănaseNo ratings yet
- Towards Micromachine Intelligence Potential of PolymersDocument16 pagesTowards Micromachine Intelligence Potential of PolymersFurkan KolayNo ratings yet
- Diafragma Neumatica-Av-04960Document8 pagesDiafragma Neumatica-Av-04960Karen VásconezNo ratings yet
- Aerogel Insulation Mat Super Light For Industrial ApplicationsDocument7 pagesAerogel Insulation Mat Super Light For Industrial ApplicationsCam EllNo ratings yet
- 5baee5df 4235 4aee 930d 13671813b398 Investment Workshop 3 Oilfield ChemicalsDocument40 pages5baee5df 4235 4aee 930d 13671813b398 Investment Workshop 3 Oilfield ChemicalsamitNo ratings yet
- Corrosion of Metals (Aluminium) in Hydrocarbons (Kerosene)Document22 pagesCorrosion of Metals (Aluminium) in Hydrocarbons (Kerosene)Ajibola AjiboyeNo ratings yet
- 11protein Purification and AnalysisDocument26 pages11protein Purification and AnalysisFidiya Septi Kusma WardaniNo ratings yet
- Microwave-Mediated Synthesis of Lophine: W Developing A Mechanism To Explain A ProductDocument3 pagesMicrowave-Mediated Synthesis of Lophine: W Developing A Mechanism To Explain A ProductDannyNo ratings yet
- Tegomer P 121Document2 pagesTegomer P 121javiera1983No ratings yet
- FAQ's - NDT - MFL Magnetic Flux Leakage Storage Tank Inspection PDFDocument2 pagesFAQ's - NDT - MFL Magnetic Flux Leakage Storage Tank Inspection PDFJai PatelNo ratings yet
- Chapter 3 Mass Relationships in Chemical ReactionsDocument96 pagesChapter 3 Mass Relationships in Chemical ReactionsDoom Refuge100% (2)
- NephronMap Copy2Document1 pageNephronMap Copy2Anonymous KxwaMDhANo ratings yet
- Chemical Reactions and Quantities: Types of Reactions Oxidation-Reduction ReactionsDocument21 pagesChemical Reactions and Quantities: Types of Reactions Oxidation-Reduction ReactionsHakakNo ratings yet
- Safety Data Sheet: Product Name: Mobil Dte Oil Heavy MediumDocument12 pagesSafety Data Sheet: Product Name: Mobil Dte Oil Heavy MediumOm Prakash RajNo ratings yet
- Explicit Lesson-Plan SalardaDocument6 pagesExplicit Lesson-Plan SalardaJhon Ivy Jhon IvyNo ratings yet
- EP0257845A2Document56 pagesEP0257845A2Jen RealNo ratings yet
- EpoxyDocument27 pagesEpoxyBui Xuan Loc100% (2)
- CHM Unit 1 & 2 Mcq'sDocument15 pagesCHM Unit 1 & 2 Mcq'sC - 01 ÃshwinNo ratings yet
- R Up Tura ReviewDocument61 pagesR Up Tura ReviewCecilia MoryNo ratings yet
- Organic Compounds: Are They Useful?: Science 9 Week 4 WorksheetsDocument4 pagesOrganic Compounds: Are They Useful?: Science 9 Week 4 WorksheetsGEROME REY LINONo ratings yet
- 2 Preparation of Chemical ReagentsDocument9 pages2 Preparation of Chemical ReagentsMagnus JordanNo ratings yet
- CMQ 2003 42 3 365Document13 pagesCMQ 2003 42 3 365RB CreationNo ratings yet
- Chem of BreathalyserDocument11 pagesChem of Breathalysersroy191006No ratings yet
- Gold Standard MCAT General Chemistry Review: StoichiometryDocument12 pagesGold Standard MCAT General Chemistry Review: StoichiometryMaxine Taeyeon100% (2)
- University of Gondar: College of Natural and Computational ScienceDocument8 pagesUniversity of Gondar: College of Natural and Computational ScienceAkalu AmereNo ratings yet
- White Rust On Galvanized and Galvanized Pre-Painted SteelDocument8 pagesWhite Rust On Galvanized and Galvanized Pre-Painted SteelNabendu BhaumikNo ratings yet