Professional Documents
Culture Documents
Elc STD Potentials
Elc STD Potentials
Uploaded by
Archita VCopyright:
Available Formats
You might also like
- Reefer Repair Manual PDFDocument250 pagesReefer Repair Manual PDFjuan.vargas.calle6904No ratings yet
- Kami Export - 2.3.2 Lab - Investigating How Water Affects Earth's Rock (Dry Lab)Document10 pagesKami Export - 2.3.2 Lab - Investigating How Water Affects Earth's Rock (Dry Lab)ISABELLA LOPEZNo ratings yet
- Electrochemical Series PDFDocument10 pagesElectrochemical Series PDFheitorpcents496No ratings yet
- Potenciales Estandar Del ElectrodoDocument3 pagesPotenciales Estandar Del ElectrododavidNo ratings yet
- Standard Electrode and Reduction Potentials at 298 K PrintableDocument3 pagesStandard Electrode and Reduction Potentials at 298 K Printablecarina_yii9690100% (1)
- Standard Reduction PotentialsDocument1 pageStandard Reduction PotentialsCamiloNo ratings yet
- Standard Reduction PotentialsDocument5 pagesStandard Reduction PotentialsnathaloaNo ratings yet
- Kims CopiesDocument17 pagesKims Copieszafarchem_iqbalNo ratings yet
- Chemistry Stage 2 and 3 Data Sheet 2010Document4 pagesChemistry Stage 2 and 3 Data Sheet 2010Edy LiewNo ratings yet
- Tabla de PotencialesDocument6 pagesTabla de PotencialesLuis AntonioNo ratings yet
- DebateDocument3 pagesDebatebbangeles1No ratings yet
- Tabla Potencial Reduccion PDFDocument13 pagesTabla Potencial Reduccion PDFFóxel ArgNo ratings yet
- Standard Calulation For Potentials AcrossDocument2 pagesStandard Calulation For Potentials Acrossmurugan_kribhcoNo ratings yet
- Electrochemical Series - CRC Handbook of Chemistry and PhysicsDocument11 pagesElectrochemical Series - CRC Handbook of Chemistry and Physicsmiguel reynagaNo ratings yet
- Tabla de Potenciales Redox PDFDocument14 pagesTabla de Potenciales Redox PDFAna Altamirano100% (1)
- Standard Reduction PotentialsDocument3 pagesStandard Reduction PotentialsjaverfrivNo ratings yet
- P2 Standard Reduction Potentials by ValueDocument6 pagesP2 Standard Reduction Potentials by ValueASTRID ELIZABET CUEVA GUTIERREZNo ratings yet
- SOA and SRA TableDocument1 pageSOA and SRA TableAhhhhhhhhhhhNo ratings yet
- EMF SeriesDocument5 pagesEMF Seriesmike rosaNo ratings yet
- Wang Battery and EV PDFDocument101 pagesWang Battery and EV PDFMateo DomínguezNo ratings yet
- Appendix EDocument1 pageAppendix EYrhon IbanezNo ratings yet
- Potencial EletroquimicoDocument13 pagesPotencial EletroquimicoMatheus EduardoNo ratings yet
- E ValuesDocument1 pageE ValuesShania LoveresNo ratings yet
- Standard Potentials at 298 K. (A) in Electrochemical Order: Table 6.2Document2 pagesStandard Potentials at 298 K. (A) in Electrochemical Order: Table 6.2Alexander RodriguezNo ratings yet
- UNIT 2 Electrochemistry FinalDocument25 pagesUNIT 2 Electrochemistry FinalPisces SandNo ratings yet
- Chemistry: Written Examination 2Document3 pagesChemistry: Written Examination 2Mohamed MawasNo ratings yet
- Standardreductionpotentials PDFDocument1 pageStandardreductionpotentials PDFBadrus SyamsiNo ratings yet
- Chapter 4 Oxidation-ReductionDocument68 pagesChapter 4 Oxidation-ReductionPHƯƠNG ĐẶNG YẾNNo ratings yet
- CHEM1 Datasheet May 2020Document4 pagesCHEM1 Datasheet May 2020Miku HatsuneNo ratings yet
- Serie ElectroquímicaDocument10 pagesSerie ElectroquímicaÁngeles LópezNo ratings yet
- Standard Electrode Potentials in Aqueous Solution at 25°C: TablesDocument2 pagesStandard Electrode Potentials in Aqueous Solution at 25°C: TablesLouie G NavaltaNo ratings yet
- Chapter 3 Oxidation ReductionDocument68 pagesChapter 3 Oxidation Reductionlong.vuongbz188No ratings yet
- Standard Redox Potential Table PDFDocument10 pagesStandard Redox Potential Table PDFFercho LotudoNo ratings yet
- UNIT 2 Electrochemistry FinalDocument26 pagesUNIT 2 Electrochemistry FinalA HNo ratings yet
- Standard Reduction Potentials Data Extended PDFDocument2 pagesStandard Reduction Potentials Data Extended PDFAceNo ratings yet
- E° HBCPDocument10 pagesE° HBCPFelipe FariaNo ratings yet
- Chemistry Databook WDocument24 pagesChemistry Databook Wdaemperor216No ratings yet
- Standard Electrode Potential SeriesDocument1 pageStandard Electrode Potential SeriesWONG KEE PING MoeNo ratings yet
- Grade 11 Paper 2 Notes LearnersDocument149 pagesGrade 11 Paper 2 Notes Learnerslethabo.mokoenaNo ratings yet
- Nguyên tố Dạng oxi hoá +ne Dạng khử E, VDocument12 pagesNguyên tố Dạng oxi hoá +ne Dạng khử E, VNhat KhanhNo ratings yet
- A Redox Reactions or Not) ANS Smyu53Document2 pagesA Redox Reactions or Not) ANS Smyu53ams13slaysNo ratings yet
- Exam 4-SolutionsDocument6 pagesExam 4-SolutionsUzo Paul NwabuisiNo ratings yet
- Standard Reduction PotentialDocument7 pagesStandard Reduction Potentialyoyotoonzone1No ratings yet
- A Redox Half Equations) ANS 9284fhDocument1 pageA Redox Half Equations) ANS 9284fhams13slaysNo ratings yet
- 9.12 Electrochemistry Half Reactions IntroDocument5 pages9.12 Electrochemistry Half Reactions IntroPatrick AbidraNo ratings yet
- Electrochemistry Revision-3 OnlineDocument10 pagesElectrochemistry Revision-3 Onlinetumimogotsi14No ratings yet
- High School Science - Redox ReactionsDocument12 pagesHigh School Science - Redox ReactionsPort of Long BeachNo ratings yet
- STD Electrode PontentialsDocument3 pagesSTD Electrode PontentialsVishal PamnaniNo ratings yet
- Sci 10 Data BookletDocument7 pagesSci 10 Data BookletConstanza Vitulli RoqueNo ratings yet
- Tablas Redox 2146Document15 pagesTablas Redox 2146TshikoNo ratings yet
- BalancingpracticeDocument1 pageBalancingpracticehh09anNo ratings yet
- Extract 10 PagesDocument10 pagesExtract 10 PageskuoklukeNo ratings yet
- Standard Electrode Potentials in Aqueous Solution at 25°C: Cathode Cathode Reaction Standard Potential, E° (Volts)Document2 pagesStandard Electrode Potentials in Aqueous Solution at 25°C: Cathode Cathode Reaction Standard Potential, E° (Volts)Mus'abIdriesAbuzetunNo ratings yet
- Standard Reduction Potentials at 25Document1 pageStandard Reduction Potentials at 25Beverly RamosNo ratings yet
- SumDocument2 pagesSumShiavm PatelNo ratings yet
- Nathaniel Herod - BalancingpracticeDocument10 pagesNathaniel Herod - BalancingpracticeNathaniel HerodNo ratings yet
- Reduction and Oxidation PotentialDocument1 pageReduction and Oxidation Potentialioan_vNo ratings yet
- Practice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersFrom EverandPractice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersNo ratings yet
- Practice Makes Perfect in Chemistry: Oxidation-ReductionFrom EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionRating: 5 out of 5 stars5/5 (1)
- Volvo Generator Silent V500uc2 IiDocument1 pageVolvo Generator Silent V500uc2 IiGerman E.No ratings yet
- DuraclearDocument2 pagesDuraclearRupesh ParaswarNo ratings yet
- Armacell Thermal Brochure EN 202001 WebDocument8 pagesArmacell Thermal Brochure EN 202001 WebadrianioantomaNo ratings yet
- Influence of Calcined Clay, Limestone, Sulfate and Clinker ProportionsDocument8 pagesInfluence of Calcined Clay, Limestone, Sulfate and Clinker ProportionsPaknubkNo ratings yet
- CorrosionDocument14 pagesCorrosionRAKIB AL MAHDINo ratings yet
- PlasticDocument24 pagesPlasticIlham HabibiNo ratings yet
- A Review Paper On Replacement of Fine AggregateDocument5 pagesA Review Paper On Replacement of Fine AggregateIJRASETPublicationsNo ratings yet
- Helical Auxetic YarnDocument16 pagesHelical Auxetic YarnTehseen MarwatNo ratings yet
- Mom 33Document4 pagesMom 33Aniket DhakeNo ratings yet
- A Coat of Varnish - What Is Duco Paint - How Is It Applied - PDFDocument4 pagesA Coat of Varnish - What Is Duco Paint - How Is It Applied - PDFJohn Rhey Lofranco TagalogNo ratings yet
- Arch29969 - Module 14Document17 pagesArch29969 - Module 14alvychuNo ratings yet
- Manual UtilizareDocument76 pagesManual UtilizarevalerevaliNo ratings yet
- Jet Fuel Colonial Grade 54 - JP54Document1 pageJet Fuel Colonial Grade 54 - JP54BernhardNo ratings yet
- Longitudinal Section of Kitchen Longitudinal Section of Dirty Kitchen Longitudinal Section of T&BDocument1 pageLongitudinal Section of Kitchen Longitudinal Section of Dirty Kitchen Longitudinal Section of T&Bbenj panganibanNo ratings yet
- LBC Fence Main & Addendum Contract Boq & Report ProgressDocument4 pagesLBC Fence Main & Addendum Contract Boq & Report ProgressRoge MingNo ratings yet
- Bauxite Ore To Aluminum MetalDocument12 pagesBauxite Ore To Aluminum MetalmaamounejjehNo ratings yet
- Traistaru Et Al., 2011Document8 pagesTraistaru Et Al., 2011rbonfili4657No ratings yet
- Harga Satuan Material Update: TAHUN 2015: Pt. Mitrabangun AdigrahaDocument41 pagesHarga Satuan Material Update: TAHUN 2015: Pt. Mitrabangun AdigrahaafifNo ratings yet
- 2016 (Book) Regeneration of Spent Catalyst and Impregnation of Catalyst by SDocument188 pages2016 (Book) Regeneration of Spent Catalyst and Impregnation of Catalyst by Scesc321No ratings yet
- Loctite Ea 9394.2 Aero-EnDocument5 pagesLoctite Ea 9394.2 Aero-EnOzgur CimenNo ratings yet
- Static and Cyclic Properties of Clay Subgrade Stabilised With Rice Husk Ash and Portland Slag CementDocument12 pagesStatic and Cyclic Properties of Clay Subgrade Stabilised With Rice Husk Ash and Portland Slag CementAndrea RinconNo ratings yet
- Copper and Copper Alloys - R A WilkinsDocument376 pagesCopper and Copper Alloys - R A WilkinsFabio Fonceca de SousaNo ratings yet
- Adhesive and Coating ScienceDocument24 pagesAdhesive and Coating ScienceHarsh ModiNo ratings yet
- Alligation Sheet 2Document4 pagesAlligation Sheet 2Ranjan RajNo ratings yet
- Terrell 1981Document55 pagesTerrell 1981oreamigNo ratings yet
- 3350503 (2)Document2 pages3350503 (2)Neerav Indrajit GadhviNo ratings yet
- Science RevisionDocument17 pagesScience RevisionHend HamedNo ratings yet
- IS 2185 Part1 2005Document1 pageIS 2185 Part1 2005stoneghoo2618No ratings yet
Elc STD Potentials
Elc STD Potentials
Uploaded by
Archita VOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Elc STD Potentials
Elc STD Potentials
Uploaded by
Archita VCopyright:
Available Formats
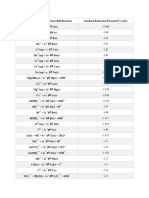
Electrochemistry
Lorenz Gubler
Standard Electrode Potentials – Data Page
Values relative to the standard hydrogen electrode (SHE). (Source: Wikipedia)
T0 = 298.15 K (25.00°C)
p0 = 1.01325 bar = 1 atm (most literature data are at 1 atm, despite the current standard of 1 bar)
activity a = 1 or pure solid, pure liquid, or for water
for ionic species dissolved in water, a = 1 = ∙ c / c0, where c0 = 1 mol/L
E
Half-cell reaction
(V vs. SHE)
F2 + 2 e– 2 F– +2.87
Ce4+ + e– Ce3+ +1.61
MnO4– +
+ 8 H + 5 e Mn + 4 H2O – 2+
+1.51
Cl2 + 2e– 2 Cl– +1.36
2– + – 3+
Cr2O7 + 14 H + 6 e 2 Cr + 7 H2O +1.33
O2 + 4 H+ + 4 e– 2 H2O +1.23
+ – 2+
MnO2 + 4 H + 2 e Mn + 2 H2O +1.23
Br2 + 2e– 2 Br– +1.06
NO3– +
+ 4 H + 3 e NO + H2O –
+0.96
Ag+ + e– Ag +0.80
3+ – 2+
Fe + e Fe +0.77
Q + 2 H+ + 2 e– H2Q (*) +0.699
+ –
O2 + 2 H + 2 e H2O2 +0.68
MnO4– + 2 H2O + 3 e– MnO2 + 4 OH– +0.59
– –
I2 + 2 e 2 I +0.54
O2 + 2 H2O + 4e– 4 OH– +0.40
3– – 4–
[Fe(CN)6] + e [Fe(CN)6] +0.370
Cu2+ + 2 e– Cu +0.34
– –
AgCl(s) + e Ag + Cl +0.222
2 H+ + 2 e– H2 0.00
2+ –
Ni + 2 e Ni –0.28
Cd2+ + 2 e– Cd –0.40
2+ –
Fe + 2 e Fe –0.44
Zn2+ + 2 e– Zn –0.76
– –
2 H2O + 2 e H2 + 2 OH –0.83
Al3+ + 3 e– Al –1.66
+ –
Na + e Na –2.71
Li+ + e– → Li –3.05
* Q = quinone, H2Q = hydroquinone
http://www.psi.ch/ene/elc 1
You might also like
- Reefer Repair Manual PDFDocument250 pagesReefer Repair Manual PDFjuan.vargas.calle6904No ratings yet
- Kami Export - 2.3.2 Lab - Investigating How Water Affects Earth's Rock (Dry Lab)Document10 pagesKami Export - 2.3.2 Lab - Investigating How Water Affects Earth's Rock (Dry Lab)ISABELLA LOPEZNo ratings yet
- Electrochemical Series PDFDocument10 pagesElectrochemical Series PDFheitorpcents496No ratings yet
- Potenciales Estandar Del ElectrodoDocument3 pagesPotenciales Estandar Del ElectrododavidNo ratings yet
- Standard Electrode and Reduction Potentials at 298 K PrintableDocument3 pagesStandard Electrode and Reduction Potentials at 298 K Printablecarina_yii9690100% (1)
- Standard Reduction PotentialsDocument1 pageStandard Reduction PotentialsCamiloNo ratings yet
- Standard Reduction PotentialsDocument5 pagesStandard Reduction PotentialsnathaloaNo ratings yet
- Kims CopiesDocument17 pagesKims Copieszafarchem_iqbalNo ratings yet
- Chemistry Stage 2 and 3 Data Sheet 2010Document4 pagesChemistry Stage 2 and 3 Data Sheet 2010Edy LiewNo ratings yet
- Tabla de PotencialesDocument6 pagesTabla de PotencialesLuis AntonioNo ratings yet
- DebateDocument3 pagesDebatebbangeles1No ratings yet
- Tabla Potencial Reduccion PDFDocument13 pagesTabla Potencial Reduccion PDFFóxel ArgNo ratings yet
- Standard Calulation For Potentials AcrossDocument2 pagesStandard Calulation For Potentials Acrossmurugan_kribhcoNo ratings yet
- Electrochemical Series - CRC Handbook of Chemistry and PhysicsDocument11 pagesElectrochemical Series - CRC Handbook of Chemistry and Physicsmiguel reynagaNo ratings yet
- Tabla de Potenciales Redox PDFDocument14 pagesTabla de Potenciales Redox PDFAna Altamirano100% (1)
- Standard Reduction PotentialsDocument3 pagesStandard Reduction PotentialsjaverfrivNo ratings yet
- P2 Standard Reduction Potentials by ValueDocument6 pagesP2 Standard Reduction Potentials by ValueASTRID ELIZABET CUEVA GUTIERREZNo ratings yet
- SOA and SRA TableDocument1 pageSOA and SRA TableAhhhhhhhhhhhNo ratings yet
- EMF SeriesDocument5 pagesEMF Seriesmike rosaNo ratings yet
- Wang Battery and EV PDFDocument101 pagesWang Battery and EV PDFMateo DomínguezNo ratings yet
- Appendix EDocument1 pageAppendix EYrhon IbanezNo ratings yet
- Potencial EletroquimicoDocument13 pagesPotencial EletroquimicoMatheus EduardoNo ratings yet
- E ValuesDocument1 pageE ValuesShania LoveresNo ratings yet
- Standard Potentials at 298 K. (A) in Electrochemical Order: Table 6.2Document2 pagesStandard Potentials at 298 K. (A) in Electrochemical Order: Table 6.2Alexander RodriguezNo ratings yet
- UNIT 2 Electrochemistry FinalDocument25 pagesUNIT 2 Electrochemistry FinalPisces SandNo ratings yet
- Chemistry: Written Examination 2Document3 pagesChemistry: Written Examination 2Mohamed MawasNo ratings yet
- Standardreductionpotentials PDFDocument1 pageStandardreductionpotentials PDFBadrus SyamsiNo ratings yet
- Chapter 4 Oxidation-ReductionDocument68 pagesChapter 4 Oxidation-ReductionPHƯƠNG ĐẶNG YẾNNo ratings yet
- CHEM1 Datasheet May 2020Document4 pagesCHEM1 Datasheet May 2020Miku HatsuneNo ratings yet
- Serie ElectroquímicaDocument10 pagesSerie ElectroquímicaÁngeles LópezNo ratings yet
- Standard Electrode Potentials in Aqueous Solution at 25°C: TablesDocument2 pagesStandard Electrode Potentials in Aqueous Solution at 25°C: TablesLouie G NavaltaNo ratings yet
- Chapter 3 Oxidation ReductionDocument68 pagesChapter 3 Oxidation Reductionlong.vuongbz188No ratings yet
- Standard Redox Potential Table PDFDocument10 pagesStandard Redox Potential Table PDFFercho LotudoNo ratings yet
- UNIT 2 Electrochemistry FinalDocument26 pagesUNIT 2 Electrochemistry FinalA HNo ratings yet
- Standard Reduction Potentials Data Extended PDFDocument2 pagesStandard Reduction Potentials Data Extended PDFAceNo ratings yet
- E° HBCPDocument10 pagesE° HBCPFelipe FariaNo ratings yet
- Chemistry Databook WDocument24 pagesChemistry Databook Wdaemperor216No ratings yet
- Standard Electrode Potential SeriesDocument1 pageStandard Electrode Potential SeriesWONG KEE PING MoeNo ratings yet
- Grade 11 Paper 2 Notes LearnersDocument149 pagesGrade 11 Paper 2 Notes Learnerslethabo.mokoenaNo ratings yet
- Nguyên tố Dạng oxi hoá +ne Dạng khử E, VDocument12 pagesNguyên tố Dạng oxi hoá +ne Dạng khử E, VNhat KhanhNo ratings yet
- A Redox Reactions or Not) ANS Smyu53Document2 pagesA Redox Reactions or Not) ANS Smyu53ams13slaysNo ratings yet
- Exam 4-SolutionsDocument6 pagesExam 4-SolutionsUzo Paul NwabuisiNo ratings yet
- Standard Reduction PotentialDocument7 pagesStandard Reduction Potentialyoyotoonzone1No ratings yet
- A Redox Half Equations) ANS 9284fhDocument1 pageA Redox Half Equations) ANS 9284fhams13slaysNo ratings yet
- 9.12 Electrochemistry Half Reactions IntroDocument5 pages9.12 Electrochemistry Half Reactions IntroPatrick AbidraNo ratings yet
- Electrochemistry Revision-3 OnlineDocument10 pagesElectrochemistry Revision-3 Onlinetumimogotsi14No ratings yet
- High School Science - Redox ReactionsDocument12 pagesHigh School Science - Redox ReactionsPort of Long BeachNo ratings yet
- STD Electrode PontentialsDocument3 pagesSTD Electrode PontentialsVishal PamnaniNo ratings yet
- Sci 10 Data BookletDocument7 pagesSci 10 Data BookletConstanza Vitulli RoqueNo ratings yet
- Tablas Redox 2146Document15 pagesTablas Redox 2146TshikoNo ratings yet
- BalancingpracticeDocument1 pageBalancingpracticehh09anNo ratings yet
- Extract 10 PagesDocument10 pagesExtract 10 PageskuoklukeNo ratings yet
- Standard Electrode Potentials in Aqueous Solution at 25°C: Cathode Cathode Reaction Standard Potential, E° (Volts)Document2 pagesStandard Electrode Potentials in Aqueous Solution at 25°C: Cathode Cathode Reaction Standard Potential, E° (Volts)Mus'abIdriesAbuzetunNo ratings yet
- Standard Reduction Potentials at 25Document1 pageStandard Reduction Potentials at 25Beverly RamosNo ratings yet
- SumDocument2 pagesSumShiavm PatelNo ratings yet
- Nathaniel Herod - BalancingpracticeDocument10 pagesNathaniel Herod - BalancingpracticeNathaniel HerodNo ratings yet
- Reduction and Oxidation PotentialDocument1 pageReduction and Oxidation Potentialioan_vNo ratings yet
- Practice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersFrom EverandPractice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersNo ratings yet
- Practice Makes Perfect in Chemistry: Oxidation-ReductionFrom EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionRating: 5 out of 5 stars5/5 (1)
- Volvo Generator Silent V500uc2 IiDocument1 pageVolvo Generator Silent V500uc2 IiGerman E.No ratings yet
- DuraclearDocument2 pagesDuraclearRupesh ParaswarNo ratings yet
- Armacell Thermal Brochure EN 202001 WebDocument8 pagesArmacell Thermal Brochure EN 202001 WebadrianioantomaNo ratings yet
- Influence of Calcined Clay, Limestone, Sulfate and Clinker ProportionsDocument8 pagesInfluence of Calcined Clay, Limestone, Sulfate and Clinker ProportionsPaknubkNo ratings yet
- CorrosionDocument14 pagesCorrosionRAKIB AL MAHDINo ratings yet
- PlasticDocument24 pagesPlasticIlham HabibiNo ratings yet
- A Review Paper On Replacement of Fine AggregateDocument5 pagesA Review Paper On Replacement of Fine AggregateIJRASETPublicationsNo ratings yet
- Helical Auxetic YarnDocument16 pagesHelical Auxetic YarnTehseen MarwatNo ratings yet
- Mom 33Document4 pagesMom 33Aniket DhakeNo ratings yet
- A Coat of Varnish - What Is Duco Paint - How Is It Applied - PDFDocument4 pagesA Coat of Varnish - What Is Duco Paint - How Is It Applied - PDFJohn Rhey Lofranco TagalogNo ratings yet
- Arch29969 - Module 14Document17 pagesArch29969 - Module 14alvychuNo ratings yet
- Manual UtilizareDocument76 pagesManual UtilizarevalerevaliNo ratings yet
- Jet Fuel Colonial Grade 54 - JP54Document1 pageJet Fuel Colonial Grade 54 - JP54BernhardNo ratings yet
- Longitudinal Section of Kitchen Longitudinal Section of Dirty Kitchen Longitudinal Section of T&BDocument1 pageLongitudinal Section of Kitchen Longitudinal Section of Dirty Kitchen Longitudinal Section of T&Bbenj panganibanNo ratings yet
- LBC Fence Main & Addendum Contract Boq & Report ProgressDocument4 pagesLBC Fence Main & Addendum Contract Boq & Report ProgressRoge MingNo ratings yet
- Bauxite Ore To Aluminum MetalDocument12 pagesBauxite Ore To Aluminum MetalmaamounejjehNo ratings yet
- Traistaru Et Al., 2011Document8 pagesTraistaru Et Al., 2011rbonfili4657No ratings yet
- Harga Satuan Material Update: TAHUN 2015: Pt. Mitrabangun AdigrahaDocument41 pagesHarga Satuan Material Update: TAHUN 2015: Pt. Mitrabangun AdigrahaafifNo ratings yet
- 2016 (Book) Regeneration of Spent Catalyst and Impregnation of Catalyst by SDocument188 pages2016 (Book) Regeneration of Spent Catalyst and Impregnation of Catalyst by Scesc321No ratings yet
- Loctite Ea 9394.2 Aero-EnDocument5 pagesLoctite Ea 9394.2 Aero-EnOzgur CimenNo ratings yet
- Static and Cyclic Properties of Clay Subgrade Stabilised With Rice Husk Ash and Portland Slag CementDocument12 pagesStatic and Cyclic Properties of Clay Subgrade Stabilised With Rice Husk Ash and Portland Slag CementAndrea RinconNo ratings yet
- Copper and Copper Alloys - R A WilkinsDocument376 pagesCopper and Copper Alloys - R A WilkinsFabio Fonceca de SousaNo ratings yet
- Adhesive and Coating ScienceDocument24 pagesAdhesive and Coating ScienceHarsh ModiNo ratings yet
- Alligation Sheet 2Document4 pagesAlligation Sheet 2Ranjan RajNo ratings yet
- Terrell 1981Document55 pagesTerrell 1981oreamigNo ratings yet
- 3350503 (2)Document2 pages3350503 (2)Neerav Indrajit GadhviNo ratings yet
- Science RevisionDocument17 pagesScience RevisionHend HamedNo ratings yet
- IS 2185 Part1 2005Document1 pageIS 2185 Part1 2005stoneghoo2618No ratings yet