Professional Documents
Culture Documents
Chemistry Note The Law of Pression K27
Chemistry Note The Law of Pression K27
Uploaded by
Animation Toysuprise0 ratings0% found this document useful (0 votes)
3 views2 pagesThe law of partial pressures states that in a gas mixture, the total pressure is equal to the sum of the individual pressures of each gas if it occupied the container alone. John Dalton observed that each gas in a mixture acts independently and exerts the same pressure as if it was the only gas present. The pressure exerted by each individual gas in a mixture is called its partial pressure, and the total pressure is equal to the sum of the partial pressures according to Dalton's law of partial pressures.
Original Description:
Original Title
Chemistry note the law of pression K27
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe law of partial pressures states that in a gas mixture, the total pressure is equal to the sum of the individual pressures of each gas if it occupied the container alone. John Dalton observed that each gas in a mixture acts independently and exerts the same pressure as if it was the only gas present. The pressure exerted by each individual gas in a mixture is called its partial pressure, and the total pressure is equal to the sum of the partial pressures according to Dalton's law of partial pressures.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
3 views2 pagesChemistry Note The Law of Pression K27
Chemistry Note The Law of Pression K27
Uploaded by
Animation ToysupriseThe law of partial pressures states that in a gas mixture, the total pressure is equal to the sum of the individual pressures of each gas if it occupied the container alone. John Dalton observed that each gas in a mixture acts independently and exerts the same pressure as if it was the only gas present. The pressure exerted by each individual gas in a mixture is called its partial pressure, and the total pressure is equal to the sum of the partial pressures according to Dalton's law of partial pressures.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 2
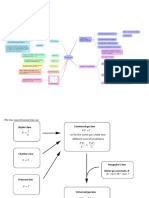
The law of partial pressure
The law of partial pressures states that, at a given temperature,
the total pressure of a gas mixture is equal to the sum of the
partial pressures exerted by each of the gases composing the
mixture.
The English scientist John Dalton (1766-1844) observed that, in
a mixture consisting of several gases, the sum of each of the
pressures exerted by the different gases corresponds to the total
pressure of the mixture. Thus, each gas acts in a mixture as if it
were the only one to occupy all the space available in the
container. Each gas therefore exerts an identical pressure as if it
were alone, its behavior not being influenced by the presence of
other gases.
Graphical representation of the law of partial pressures: On the
left, we find the pressures exerted by gases A and B if they were
alone in the container. On the right, we find the pressure exerted
by the mixture of gases A and B in the same container. It can be
noted that the pressure of the mixture is equal to the sum of the
pressures exerted by each gas individually.
The individual pressure exerted by each gas in a container is called
partial pressure.
This pressure is only part of the total pressure exerted by all the
gases contained in the container. Mathematically, we can express
the law of partial pressures, also called Dalton's law, as follows:
This law applies to any mixture of gases, regardless of the number
of gases that make up the mixture. It also applies when a gas is
collected by displacement of water. In this technique, a certain
amount of water vapour is mixed with the resulting gas. To
calculate the actual pressure exerted by the gas, it is therefore
necessary to subtract the pressure of the water vapour from the
total pressure of the mixture collected.
The partial pressure exerted by a gas can also be determined by
multiplying the total pressure of the mixture by the molar
proportion that the gas represents in the mixture.
You might also like
- The Properties of GasesDocument26 pagesThe Properties of GasesHitesh Swami100% (1)
- GASES SendingDocument2 pagesGASES Sendingyoow.youthNo ratings yet
- Henry's Law Is One of The Gas Laws, Formulated by William Henry in 1803. ItDocument7 pagesHenry's Law Is One of The Gas Laws, Formulated by William Henry in 1803. ItysuntherenNo ratings yet
- General Chemistry 1 (Grade 11-STEM) : Gas LawDocument5 pagesGeneral Chemistry 1 (Grade 11-STEM) : Gas Lawlui yangyangNo ratings yet
- Week 7: Dalton'S Law of Partial PressuresDocument16 pagesWeek 7: Dalton'S Law of Partial PressuresLeonilo Olanda JrNo ratings yet
- Chem Assignment 2 (E)Document13 pagesChem Assignment 2 (E)misganamarcos10No ratings yet
- Dalton's Law (Law of Partial Pressures)Document1 pageDalton's Law (Law of Partial Pressures)structuredes.1No ratings yet
- Chapter 1 .Properties of Gases - Lecture 2.Document23 pagesChapter 1 .Properties of Gases - Lecture 2.Mahmoud MahmoudNo ratings yet
- Chapter3 IdealgaslawDocument45 pagesChapter3 Idealgaslaw翁绍棠No ratings yet
- Dalton's Law: Chemistry Physics Pressure Partial Pressures Empirical John Dalton Ideal Gas LawsDocument3 pagesDalton's Law: Chemistry Physics Pressure Partial Pressures Empirical John Dalton Ideal Gas LawsJillian BrownNo ratings yet
- DALTON'SDocument3 pagesDALTON'Sjowelantonio20No ratings yet
- GasesDocument30 pagesGasesWHAT'S SUPNo ratings yet
- Practical ApplicationsDocument3 pagesPractical ApplicationsELvin Jay CasiñoNo ratings yet
- Gas Laws - Wikipedia PDFDocument17 pagesGas Laws - Wikipedia PDFEmegu MosesNo ratings yet
- Gas LawDocument3 pagesGas LawJohn Reynard PacsonNo ratings yet
- Source 1: Daghay Char Dalton's Law (Law of Partial Pressures)Document24 pagesSource 1: Daghay Char Dalton's Law (Law of Partial Pressures)marielNo ratings yet
- Dalton's Law of Partial PressureDocument11 pagesDalton's Law of Partial PressureJohn Eric TajorNo ratings yet
- Week 3 PPT AD CHEMDocument8 pagesWeek 3 PPT AD CHEMSophia Ysabelle EstradaNo ratings yet
- Derivation of Dalton's Law of Partial PressureDocument9 pagesDerivation of Dalton's Law of Partial PressureRichard TumbagaNo ratings yet
- Chapter 5 KimiaDocument3 pagesChapter 5 KimiaelmishaenandaeNo ratings yet
- Che 1001 Cha. 4 GasesDocument108 pagesChe 1001 Cha. 4 Gasessamsung.kadir56No ratings yet
- 651add4e06b372214ad7e2af 93246937559Document4 pages651add4e06b372214ad7e2af 93246937559mya thet htar sweNo ratings yet
- Alkali: Latin PH Standard State Acetic Acid Vinegar Sulfuric Acid Car Batteries Redox Oxidation StateDocument4 pagesAlkali: Latin PH Standard State Acetic Acid Vinegar Sulfuric Acid Car Batteries Redox Oxidation StateFatima Erika Ayessa IngkohNo ratings yet
- Vpy I KP Paudel Laws Related To Solubility of Gas, Physical Principles of Gas Exchange and Exchange of Gases in Lungs and TissuesDocument38 pagesVpy I KP Paudel Laws Related To Solubility of Gas, Physical Principles of Gas Exchange and Exchange of Gases in Lungs and TissuesKNo ratings yet
- Gas Laws in RespirationDocument3 pagesGas Laws in Respirationroberto543No ratings yet
- Vapor Pressure PDFDocument8 pagesVapor Pressure PDFChri ShaNo ratings yet
- Gas Mixtures: Dalton's LawDocument6 pagesGas Mixtures: Dalton's LawPratyay RayNo ratings yet
- 4th QTR - Module - Week 3Document3 pages4th QTR - Module - Week 3Avirel Reynante PodadorNo ratings yet
- Amagat's LawDocument2 pagesAmagat's LawEn CsakNo ratings yet
- Henry's LawDocument6 pagesHenry's LawHg RosmeriNo ratings yet
- Gas Laws: Solids LiquidsDocument2 pagesGas Laws: Solids LiquidsRaymond CabanillaNo ratings yet
- Equation of State PDFDocument2 pagesEquation of State PDFEdwardNo ratings yet
- PVT ExperimentDocument23 pagesPVT ExperimentAbdullah FarhanNo ratings yet
- GasesDocument55 pagesGasesMMacalua, FranzylNo ratings yet
- Defence Engineering College: Applied Thermodynamics MV2012Document18 pagesDefence Engineering College: Applied Thermodynamics MV2012Getachew TikueNo ratings yet
- Ideal Gas: General Chemistry 1Document9 pagesIdeal Gas: General Chemistry 1Daniel Corcino100% (1)
- Name: Krisha Shane Magsaysay Section: STEM 301Document2 pagesName: Krisha Shane Magsaysay Section: STEM 301KrishaNo ratings yet
- Module 3 Gas LawsDocument12 pagesModule 3 Gas Lawsmarcarvielloydperla3No ratings yet
- What Is Pressure?: The Ideal Gas LawDocument1 pageWhat Is Pressure?: The Ideal Gas LawAbdulNo ratings yet
- Dalton's Law (Law of Partial Pressures)Document3 pagesDalton's Law (Law of Partial Pressures)Luyando LikubangwaNo ratings yet
- Gas LawsDocument2 pagesGas LawsJJAMPPONG PS100% (1)
- Study of Gas LawDocument15 pagesStudy of Gas LawKushagra jaiswalNo ratings yet
- Assignment: Science IV: 1.) Boyle's LawDocument6 pagesAssignment: Science IV: 1.) Boyle's LawIrish WahidNo ratings yet
- Gas LawsDocument2 pagesGas LawsPrathapan KPNo ratings yet
- Pressure: Force Area Perpendicular Gauge PressureDocument6 pagesPressure: Force Area Perpendicular Gauge PressureSyed Rameez MohiuddinNo ratings yet
- April 12 Catch Up Reading MaterialseeeDocument3 pagesApril 12 Catch Up Reading MaterialseeeClarkskieNo ratings yet
- Gas Mixture Lec 2Document18 pagesGas Mixture Lec 2Muhammad Ilyas Qaiser KhanNo ratings yet
- Raoult vs. HenryDocument2 pagesRaoult vs. Henryseraj ibramemNo ratings yet
- Chapter 2 PDF MeteoDocument24 pagesChapter 2 PDF MeteosecretzNo ratings yet
- Lec 3Document14 pagesLec 3Not EmeraruduNo ratings yet
- The Molar Volume of A GasDocument12 pagesThe Molar Volume of A GasabeerNo ratings yet
- Chemistry Gas Laws AssignmentDocument6 pagesChemistry Gas Laws AssignmentHans Webster LabordoNo ratings yet
- Ch. 12Document38 pagesCh. 12Sophia StewartNo ratings yet
- Lecture 4 Gas Laws and RelationsDocument28 pagesLecture 4 Gas Laws and RelationsArsal SohrabNo ratings yet
- Gas LawsDocument3 pagesGas Lawsphebbz_phunky24No ratings yet
- NB: The 'Handful' Story Is My Own Simplified Way of Understanding This. It's Not Really A ThingDocument2 pagesNB: The 'Handful' Story Is My Own Simplified Way of Understanding This. It's Not Really A ThingMohammed Zaakir AllyNo ratings yet
- Respiratory System: A Tutorial Study GuideFrom EverandRespiratory System: A Tutorial Study GuideRating: 5 out of 5 stars5/5 (1)