Professional Documents
Culture Documents
0 ratings0% found this document useful (0 votes)
10 viewsConcentrationof Ions Concentration of Solution x100: % Ionization
Concentrationof Ions Concentration of Solution x100: % Ionization
Uploaded by
Jose Emanuel AquinoThis document calculates the ionization constant (Ka) and percentage ionization of a weak monoprotic acid that is 3.8% ionized in a 0.2F solution. It is given that the acid is 3.8% ionized in a 0.2F solution. Using this information and the chemical equation for acid dissociation, it calculates the Ka to be 3.002079×10−4. It then calculates that the percentage ionization of this same acid in a 0.01F solution would be 0.3%.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You might also like
- Lab Report Batch Reactor GGDocument25 pagesLab Report Batch Reactor GGFrost Orchid100% (1)
- Sample Problem #1Document2 pagesSample Problem #1Dozdi100% (1)
- 11.1.26 AOAC of Fi Cial Method 974.27 Cad Mium, Chro Mium, Cop Per, Iron, Lead, Mag Ne Sium, Man Ga Nese, Sil Ver, and Zinc in Wa TerDocument2 pages11.1.26 AOAC of Fi Cial Method 974.27 Cad Mium, Chro Mium, Cop Per, Iron, Lead, Mag Ne Sium, Man Ga Nese, Sil Ver, and Zinc in Wa Termttla100% (1)
- Tutorial 1-QuestionsDocument4 pagesTutorial 1-QuestionsSyafiq JaafarNo ratings yet
- 1264 - 1219 - PIC Prak 1 2018Document9 pages1264 - 1219 - PIC Prak 1 2018RafaelNo ratings yet
- KimiaaaaaaDocument11 pagesKimiaaaaaaaimi BatrisyiaNo ratings yet
- Given: F 100 Mol (N-Pentane) 0.60 (N - Heptane) 0.40 101.32 40 60Document6 pagesGiven: F 100 Mol (N-Pentane) 0.60 (N - Heptane) 0.40 101.32 40 60Yasmin KayeNo ratings yet
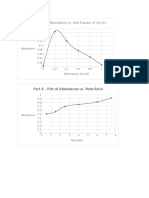
- Part A - Plot of Absorbance vs. Mole Fraction of Iron (II)Document2 pagesPart A - Plot of Absorbance vs. Mole Fraction of Iron (II)Remjohn MagtaasNo ratings yet
- Chem 112 Cu AnalysisDocument2 pagesChem 112 Cu Analysismiami14No ratings yet
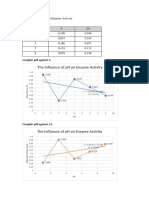
- The Influence of PH On Enzyme Activity: Data in Table FormDocument2 pagesThe Influence of PH On Enzyme Activity: Data in Table FormputriNo ratings yet
- The Influence of PH On Enzyme Activity: Data in Table FormDocument2 pagesThe Influence of PH On Enzyme Activity: Data in Table FormIrvandar NurviandyNo ratings yet
- Concentration vs. AbsorbanceDocument4 pagesConcentration vs. AbsorbanceEllah GutierrezNo ratings yet
- Lab AnalysisDocument4 pagesLab AnalysisErnestasBlaževičNo ratings yet
- Peng Robinson MixturesDocument1 pagePeng Robinson MixturesdckristantoNo ratings yet
- L52 Online Assignment Question 1Document3 pagesL52 Online Assignment Question 1Raj PatelNo ratings yet
- Konservantide (Bensoehappe Ja Sorbiinhappe) Määramine Aura Active Mahlajoogist HPLC MeetodilDocument4 pagesKonservantide (Bensoehappe Ja Sorbiinhappe) Määramine Aura Active Mahlajoogist HPLC MeetodilhedisNo ratings yet
- Practica de Laboratorio 4Document2 pagesPractica de Laboratorio 4Leidy Renteria EstradaNo ratings yet
- Starch Concentration: 1.4 1.6 F (X) 2.7844294722x + 0.0429670884 R 0.9937035548Document4 pagesStarch Concentration: 1.4 1.6 F (X) 2.7844294722x + 0.0429670884 R 0.9937035548Ellah GutierrezNo ratings yet
- Starch Concentration: 1.4 1.6 F (X) 2.7844294722x + 0.0429670884 R 0.9937035548Document4 pagesStarch Concentration: 1.4 1.6 F (X) 2.7844294722x + 0.0429670884 R 0.9937035548Ellah GutierrezNo ratings yet
- Of 0.1M Na S O Solution (ML) of Distilled Water (ML) Concent Ration of Na S O (M) of 0.1M HCL Solution (ML) Time, T (S) 1/T (S)Document4 pagesOf 0.1M Na S O Solution (ML) of Distilled Water (ML) Concent Ration of Na S O (M) of 0.1M HCL Solution (ML) Time, T (S) 1/T (S)aimi BatrisyiaNo ratings yet
- Hasil Analisa Air FormasiDocument27 pagesHasil Analisa Air FormasiRizki FadliNo ratings yet
- Assignment 5 Ionization (LEC)Document8 pagesAssignment 5 Ionization (LEC)Poison PinkNo ratings yet
- Spreadsheet 5Document3 pagesSpreadsheet 5DanKimberleyNo ratings yet
- Curva de Calibración para 432: A MtraDocument6 pagesCurva de Calibración para 432: A MtraMaira Alejandra Cubillos TorresNo ratings yet
- Exepermint 1Document4 pagesExepermint 1Jhone SaaimonNo ratings yet
- Exp1 Phychem 2 Lab 2 1Document14 pagesExp1 Phychem 2 Lab 2 1Nicole ValmonteNo ratings yet
- Acid-Base Group WorkDocument2 pagesAcid-Base Group WorkTebarek SitotawNo ratings yet
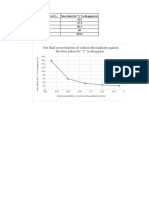
- The Final Concentration of Sodium Thiosulphate Against The Time Taken For "X" To DisappearDocument3 pagesThe Final Concentration of Sodium Thiosulphate Against The Time Taken For "X" To DisappearLohNo ratings yet
- Template Lengkap KunDocument13 pagesTemplate Lengkap Kun시우민SeohyunNo ratings yet
- Absorbance at λmax 260nm for PBS: Calibration curvesDocument3 pagesAbsorbance at λmax 260nm for PBS: Calibration curvesNaveedNo ratings yet
- Abaco de MoodyDocument3 pagesAbaco de MoodyjhordanNo ratings yet
- Absorbance Vs Concentration: Experiment 2Document2 pagesAbsorbance Vs Concentration: Experiment 2Amy MusaNo ratings yet
- PBL NewDocument3 pagesPBL NewALAMEL MANZGHAI A/P GANESONNo ratings yet
- Tugas 1 - Eka Kurniati Kombong Datu - 05201026Document9 pagesTugas 1 - Eka Kurniati Kombong Datu - 05201026시우민SeohyunNo ratings yet
- KurvaDocument2 pagesKurvaRizal LaksanaNo ratings yet
- Problem 2.79 PDFDocument1 pageProblem 2.79 PDFKauê BrittoNo ratings yet
- ZN (PPM) ZN (PPM)Document2 pagesZN (PPM) ZN (PPM)Putri Pah Kumala DewiNo ratings yet
- KurvaDocument2 pagesKurvaRizal LaksanaNo ratings yet
- MT Example LeachingDocument19 pagesMT Example LeachingAshish RajakNo ratings yet
- Results and Data CMT 463 Exp 3Document7 pagesResults and Data CMT 463 Exp 3IzzyanIsaNo ratings yet
- IntroductionDocument5 pagesIntroductionberjalankehadapanNo ratings yet
- Chem Lab 7Document4 pagesChem Lab 7Norayr GulumainNo ratings yet
- TMP 82Document2 pagesTMP 82ZubairNo ratings yet
- Curva Estandar MineralesDocument2 pagesCurva Estandar MineralesMichel Jer AbzNo ratings yet
- Chart Title: 0.09 0.1 F (X) 0.0017563907x + 0.0041799266 R 0.9937284888 Column G Linear (Column G)Document2 pagesChart Title: 0.09 0.1 F (X) 0.0017563907x + 0.0041799266 R 0.9937284888 Column G Linear (Column G)Edwin SaputraNo ratings yet
- Minerals Unit Formulae - ExerciseDocument5 pagesMinerals Unit Formulae - ExerciseTapiwa MandazaNo ratings yet
- TMP 2 1Document2 pagesTMP 2 1mj03127477706No ratings yet
- Quantitative Application PotentiometricDocument4 pagesQuantitative Application PotentiometricSuci Fitria I.No ratings yet
- Abaco de MoodyDocument3 pagesAbaco de Moodydamaris murgaNo ratings yet
- Lab 4Document3 pagesLab 4Olivia PowerNo ratings yet
- ElkimmmDocument6 pagesElkimmmElemental12No ratings yet
- Table S1 Analytical Figures of Merit of ICP-OES Determination of The Mineral Composition of Baby Food SamplesDocument6 pagesTable S1 Analytical Figures of Merit of ICP-OES Determination of The Mineral Composition of Baby Food SamplesAna RamírezNo ratings yet
- Curva CalibracionDocument2 pagesCurva CalibracionSofiaNo ratings yet
- TMP 83Document2 pagesTMP 83ZubairNo ratings yet
- Determination of Fe in Water by Absorption SpectrophotometryDocument2 pagesDetermination of Fe in Water by Absorption SpectrophotometryLUTHFIA PRATIWINo ratings yet
- LABO3Document9 pagesLABO3Kevin GarciaNo ratings yet
- Julia Silviani: 0.025 0.03 F (X) 0.028x - 0.0006666667 R 0.9811680572 Column D Linear (Column D)Document3 pagesJulia Silviani: 0.025 0.03 F (X) 0.028x - 0.0006666667 R 0.9811680572 Column D Linear (Column D)efli pratama dinaNo ratings yet
- Kurva Larutan Standar CaDocument2 pagesKurva Larutan Standar CaEstu RahajengNo ratings yet
- Task 2Document1 pageTask 2Jose Emanuel AquinoNo ratings yet
- M3 Performance TaskDocument3 pagesM3 Performance TaskJose Emanuel AquinoNo ratings yet
- The Philippines Is A Country With Rich and Different CulturesDocument5 pagesThe Philippines Is A Country With Rich and Different CulturesJose Emanuel AquinoNo ratings yet
- Safety PrecautionsDocument4 pagesSafety PrecautionsJose Emanuel AquinoNo ratings yet
- The Philippines Is A Country With Rich and Different CulturesDocument5 pagesThe Philippines Is A Country With Rich and Different CulturesJose Emanuel AquinoNo ratings yet
- Models in Understanding The SelfDocument7 pagesModels in Understanding The SelfJose Emanuel AquinoNo ratings yet
- Models in Understanding The SelfDocument3 pagesModels in Understanding The SelfJose Emanuel AquinoNo ratings yet
- Taking of The VesselDocument7 pagesTaking of The VesselJose Emanuel AquinoNo ratings yet
- Aspect of The Self PhilosophicalDocument1 pageAspect of The Self PhilosophicalJose Emanuel AquinoNo ratings yet
Concentrationof Ions Concentration of Solution x100: % Ionization
Concentrationof Ions Concentration of Solution x100: % Ionization
Uploaded by
Jose Emanuel Aquino0 ratings0% found this document useful (0 votes)
10 views1 pageThis document calculates the ionization constant (Ka) and percentage ionization of a weak monoprotic acid that is 3.8% ionized in a 0.2F solution. It is given that the acid is 3.8% ionized in a 0.2F solution. Using this information and the chemical equation for acid dissociation, it calculates the Ka to be 3.002079×10−4. It then calculates that the percentage ionization of this same acid in a 0.01F solution would be 0.3%.
Original Description:
Original Title
4
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document calculates the ionization constant (Ka) and percentage ionization of a weak monoprotic acid that is 3.8% ionized in a 0.2F solution. It is given that the acid is 3.8% ionized in a 0.2F solution. Using this information and the chemical equation for acid dissociation, it calculates the Ka to be 3.002079×10−4. It then calculates that the percentage ionization of this same acid in a 0.01F solution would be 0.3%.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
0 ratings0% found this document useful (0 votes)
10 views1 pageConcentrationof Ions Concentration of Solution x100: % Ionization
Concentrationof Ions Concentration of Solution x100: % Ionization
Uploaded by
Jose Emanuel AquinoThis document calculates the ionization constant (Ka) and percentage ionization of a weak monoprotic acid that is 3.8% ionized in a 0.2F solution. It is given that the acid is 3.8% ionized in a 0.2F solution. Using this information and the chemical equation for acid dissociation, it calculates the Ka to be 3.002079×10−4. It then calculates that the percentage ionization of this same acid in a 0.01F solution would be 0.3%.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
You are on page 1of 1
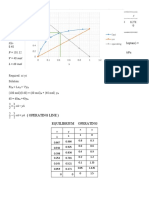
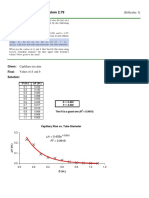
4. If a weak monoprotic acid is 3.8 percent ionized in a 0.
2F solution, calculate its
ionization constant. What is the percentage ionization of this acid in a 0.01F
solution?
Given: % ionization = 3.8% [HA] = 0.2 F
Reqd: Ka
Soln:
HA + H20 = H30+ + OH-
I 0.2 F 0 0
C -0.2(0.038) +0.2(0.038) +0.2(0.038)
E 0.1924 0.0076 0.0076
Ka=¿ ¿
[ 0.0076 ] [0.0076]
Ka = [0.1924]
Ka= 3.002079×10−4
What is the percentage ionization of this acid in a 0.01F
Concentration of ions
% ionization = x100
concentration of solution
3.002079 x 10−4
% ionization= x100
0.1
% ionization= 0.3 %
You might also like
- Lab Report Batch Reactor GGDocument25 pagesLab Report Batch Reactor GGFrost Orchid100% (1)
- Sample Problem #1Document2 pagesSample Problem #1Dozdi100% (1)
- 11.1.26 AOAC of Fi Cial Method 974.27 Cad Mium, Chro Mium, Cop Per, Iron, Lead, Mag Ne Sium, Man Ga Nese, Sil Ver, and Zinc in Wa TerDocument2 pages11.1.26 AOAC of Fi Cial Method 974.27 Cad Mium, Chro Mium, Cop Per, Iron, Lead, Mag Ne Sium, Man Ga Nese, Sil Ver, and Zinc in Wa Termttla100% (1)
- Tutorial 1-QuestionsDocument4 pagesTutorial 1-QuestionsSyafiq JaafarNo ratings yet
- 1264 - 1219 - PIC Prak 1 2018Document9 pages1264 - 1219 - PIC Prak 1 2018RafaelNo ratings yet
- KimiaaaaaaDocument11 pagesKimiaaaaaaaimi BatrisyiaNo ratings yet
- Given: F 100 Mol (N-Pentane) 0.60 (N - Heptane) 0.40 101.32 40 60Document6 pagesGiven: F 100 Mol (N-Pentane) 0.60 (N - Heptane) 0.40 101.32 40 60Yasmin KayeNo ratings yet
- Part A - Plot of Absorbance vs. Mole Fraction of Iron (II)Document2 pagesPart A - Plot of Absorbance vs. Mole Fraction of Iron (II)Remjohn MagtaasNo ratings yet
- Chem 112 Cu AnalysisDocument2 pagesChem 112 Cu Analysismiami14No ratings yet
- The Influence of PH On Enzyme Activity: Data in Table FormDocument2 pagesThe Influence of PH On Enzyme Activity: Data in Table FormputriNo ratings yet
- The Influence of PH On Enzyme Activity: Data in Table FormDocument2 pagesThe Influence of PH On Enzyme Activity: Data in Table FormIrvandar NurviandyNo ratings yet
- Concentration vs. AbsorbanceDocument4 pagesConcentration vs. AbsorbanceEllah GutierrezNo ratings yet
- Lab AnalysisDocument4 pagesLab AnalysisErnestasBlaževičNo ratings yet
- Peng Robinson MixturesDocument1 pagePeng Robinson MixturesdckristantoNo ratings yet
- L52 Online Assignment Question 1Document3 pagesL52 Online Assignment Question 1Raj PatelNo ratings yet
- Konservantide (Bensoehappe Ja Sorbiinhappe) Määramine Aura Active Mahlajoogist HPLC MeetodilDocument4 pagesKonservantide (Bensoehappe Ja Sorbiinhappe) Määramine Aura Active Mahlajoogist HPLC MeetodilhedisNo ratings yet
- Practica de Laboratorio 4Document2 pagesPractica de Laboratorio 4Leidy Renteria EstradaNo ratings yet
- Starch Concentration: 1.4 1.6 F (X) 2.7844294722x + 0.0429670884 R 0.9937035548Document4 pagesStarch Concentration: 1.4 1.6 F (X) 2.7844294722x + 0.0429670884 R 0.9937035548Ellah GutierrezNo ratings yet
- Starch Concentration: 1.4 1.6 F (X) 2.7844294722x + 0.0429670884 R 0.9937035548Document4 pagesStarch Concentration: 1.4 1.6 F (X) 2.7844294722x + 0.0429670884 R 0.9937035548Ellah GutierrezNo ratings yet
- Of 0.1M Na S O Solution (ML) of Distilled Water (ML) Concent Ration of Na S O (M) of 0.1M HCL Solution (ML) Time, T (S) 1/T (S)Document4 pagesOf 0.1M Na S O Solution (ML) of Distilled Water (ML) Concent Ration of Na S O (M) of 0.1M HCL Solution (ML) Time, T (S) 1/T (S)aimi BatrisyiaNo ratings yet
- Hasil Analisa Air FormasiDocument27 pagesHasil Analisa Air FormasiRizki FadliNo ratings yet
- Assignment 5 Ionization (LEC)Document8 pagesAssignment 5 Ionization (LEC)Poison PinkNo ratings yet
- Spreadsheet 5Document3 pagesSpreadsheet 5DanKimberleyNo ratings yet
- Curva de Calibración para 432: A MtraDocument6 pagesCurva de Calibración para 432: A MtraMaira Alejandra Cubillos TorresNo ratings yet
- Exepermint 1Document4 pagesExepermint 1Jhone SaaimonNo ratings yet
- Exp1 Phychem 2 Lab 2 1Document14 pagesExp1 Phychem 2 Lab 2 1Nicole ValmonteNo ratings yet
- Acid-Base Group WorkDocument2 pagesAcid-Base Group WorkTebarek SitotawNo ratings yet
- The Final Concentration of Sodium Thiosulphate Against The Time Taken For "X" To DisappearDocument3 pagesThe Final Concentration of Sodium Thiosulphate Against The Time Taken For "X" To DisappearLohNo ratings yet
- Template Lengkap KunDocument13 pagesTemplate Lengkap Kun시우민SeohyunNo ratings yet
- Absorbance at λmax 260nm for PBS: Calibration curvesDocument3 pagesAbsorbance at λmax 260nm for PBS: Calibration curvesNaveedNo ratings yet
- Abaco de MoodyDocument3 pagesAbaco de MoodyjhordanNo ratings yet
- Absorbance Vs Concentration: Experiment 2Document2 pagesAbsorbance Vs Concentration: Experiment 2Amy MusaNo ratings yet
- PBL NewDocument3 pagesPBL NewALAMEL MANZGHAI A/P GANESONNo ratings yet
- Tugas 1 - Eka Kurniati Kombong Datu - 05201026Document9 pagesTugas 1 - Eka Kurniati Kombong Datu - 05201026시우민SeohyunNo ratings yet
- KurvaDocument2 pagesKurvaRizal LaksanaNo ratings yet
- Problem 2.79 PDFDocument1 pageProblem 2.79 PDFKauê BrittoNo ratings yet
- ZN (PPM) ZN (PPM)Document2 pagesZN (PPM) ZN (PPM)Putri Pah Kumala DewiNo ratings yet
- KurvaDocument2 pagesKurvaRizal LaksanaNo ratings yet
- MT Example LeachingDocument19 pagesMT Example LeachingAshish RajakNo ratings yet
- Results and Data CMT 463 Exp 3Document7 pagesResults and Data CMT 463 Exp 3IzzyanIsaNo ratings yet
- IntroductionDocument5 pagesIntroductionberjalankehadapanNo ratings yet
- Chem Lab 7Document4 pagesChem Lab 7Norayr GulumainNo ratings yet
- TMP 82Document2 pagesTMP 82ZubairNo ratings yet
- Curva Estandar MineralesDocument2 pagesCurva Estandar MineralesMichel Jer AbzNo ratings yet
- Chart Title: 0.09 0.1 F (X) 0.0017563907x + 0.0041799266 R 0.9937284888 Column G Linear (Column G)Document2 pagesChart Title: 0.09 0.1 F (X) 0.0017563907x + 0.0041799266 R 0.9937284888 Column G Linear (Column G)Edwin SaputraNo ratings yet
- Minerals Unit Formulae - ExerciseDocument5 pagesMinerals Unit Formulae - ExerciseTapiwa MandazaNo ratings yet
- TMP 2 1Document2 pagesTMP 2 1mj03127477706No ratings yet
- Quantitative Application PotentiometricDocument4 pagesQuantitative Application PotentiometricSuci Fitria I.No ratings yet
- Abaco de MoodyDocument3 pagesAbaco de Moodydamaris murgaNo ratings yet
- Lab 4Document3 pagesLab 4Olivia PowerNo ratings yet
- ElkimmmDocument6 pagesElkimmmElemental12No ratings yet
- Table S1 Analytical Figures of Merit of ICP-OES Determination of The Mineral Composition of Baby Food SamplesDocument6 pagesTable S1 Analytical Figures of Merit of ICP-OES Determination of The Mineral Composition of Baby Food SamplesAna RamírezNo ratings yet
- Curva CalibracionDocument2 pagesCurva CalibracionSofiaNo ratings yet
- TMP 83Document2 pagesTMP 83ZubairNo ratings yet
- Determination of Fe in Water by Absorption SpectrophotometryDocument2 pagesDetermination of Fe in Water by Absorption SpectrophotometryLUTHFIA PRATIWINo ratings yet
- LABO3Document9 pagesLABO3Kevin GarciaNo ratings yet
- Julia Silviani: 0.025 0.03 F (X) 0.028x - 0.0006666667 R 0.9811680572 Column D Linear (Column D)Document3 pagesJulia Silviani: 0.025 0.03 F (X) 0.028x - 0.0006666667 R 0.9811680572 Column D Linear (Column D)efli pratama dinaNo ratings yet
- Kurva Larutan Standar CaDocument2 pagesKurva Larutan Standar CaEstu RahajengNo ratings yet
- Task 2Document1 pageTask 2Jose Emanuel AquinoNo ratings yet
- M3 Performance TaskDocument3 pagesM3 Performance TaskJose Emanuel AquinoNo ratings yet
- The Philippines Is A Country With Rich and Different CulturesDocument5 pagesThe Philippines Is A Country With Rich and Different CulturesJose Emanuel AquinoNo ratings yet
- Safety PrecautionsDocument4 pagesSafety PrecautionsJose Emanuel AquinoNo ratings yet
- The Philippines Is A Country With Rich and Different CulturesDocument5 pagesThe Philippines Is A Country With Rich and Different CulturesJose Emanuel AquinoNo ratings yet
- Models in Understanding The SelfDocument7 pagesModels in Understanding The SelfJose Emanuel AquinoNo ratings yet
- Models in Understanding The SelfDocument3 pagesModels in Understanding The SelfJose Emanuel AquinoNo ratings yet
- Taking of The VesselDocument7 pagesTaking of The VesselJose Emanuel AquinoNo ratings yet
- Aspect of The Self PhilosophicalDocument1 pageAspect of The Self PhilosophicalJose Emanuel AquinoNo ratings yet