Professional Documents
Culture Documents
Monocyclic Disubstituted
Monocyclic Disubstituted
Uploaded by
Ps SatchithOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Monocyclic Disubstituted
Monocyclic Disubstituted
Uploaded by
Ps SatchithCopyright:
Available Formats
Strain and Stability in Monocyclic Disubstituted Ring Systems
11 November 2023 10:51
Different Types of Strains:
• Eclipsing/Torsional Strain: Torsional strain is the resistance to bond twisting. It occurs when atoms separated by three bonds are
placed in an eclipsed conformation instead of the more stable staggered conformation.
• Bond Angle Strain: It happens when the bond angles in a cycloalkane deviate from the ideal bond angle of 109.5º, causing rise in P.E
• Steric Strain: It happens when atoms are forced to be too close to each other and try to occupy the same space.
NOTE: Transannular strain exists when there is steric repulsion between atoms.
Cyclobutane:
• If cyclobutane were planar, the C-C-C angles would be 90°. However, the planar conformation produces perfect C-H
eclipsing along the C-C bonds, an unfavourable situation. As a result, the molecule puckers, relieving eclipsing but
reducing the C-C-C angles to about 88°. The angle between the two C-C-C planes is 28°. Inversion of the ring to
interconvert the two puckered forms proceeds over a very small barrier (1.45 kcal/mol), and so in many circumstances
one can think of cyclobutane as effectively planar.
• The C-C bond lengths of cyclobutane are 1.55 Å-slightly elongated. The strain energy
is 26.5 kcal/mol, essentially identical to that of cyclopropane. The strain results primarily
from angle bending, but again residual torsional strain (the eclipsing are not completely relieved by puckering) plays a
role.
Cyclopentane:
• Planar cyclopentane would have a C-C-C angle of 108°, very close to the tetrahedral angle. However, as with
cyclobutane, planar cyclopentane would suffer from a large number of C-H eclipsing interactions, and so the planar form
is not a viable conformation. Again, the molecule puckers, even though the puckering compresses the C-C-C bond angles.
Cyclopentane has 6.2 kcal/mol of strain energy, most of it due to torsional (eclipsing) effects, but some due to angle
strain.
• Cyclopentane can distort from the planar form to relieve eclipsing in two different ways. One way is to move just one
carbon out of the plane, producing the envelope conformation, which retains a mirror plane of symmetry.
• Alternatively, cyclopentane can twist about C-C bonds, producing the half-chair form, which has a two-fold rotation axis.
These two forms are very nearly equal in energy, and they interconvert very rapidly (the barrier is < 2 kcal/mol) by a
process termed pseudorotation.
• The rapid interconversion of envelope and half-chair forms makes all hydrogens (and carbons) equivalent, producing a
time-averaged structure that is equivalent to the planar form. Note that the interconversion of envelope and half-chair
forms does not require passing through the fully planar form, which lies - 5 kcal/mol above these structures. Substituents
can cause a cyclopentane to prefer one conformation over the other, or distort this relatively
flexible ring to intermediate conformations.
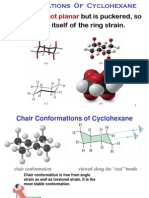
Cyclohexane:
• As in other cycles, the different types of hydrogens (axial and equatorial) interconvert by a conformational process. What
is unique about cyclohexane is that the barrier for this process is - 10.8 kcal/ mol, much higher than for other carbocycles.
Thus, in many circumstances, it is important to distinguish between the axial and equatorial positions.
• The path by which the chair forms interconvert has been extensively studied. After passing through a
half-chair transition state, the molecule enters the twist boat conformer. This conformer is
flexible, like cyclobutane and cyclopentane, and undergoes rapid interconversions among
equivalent forms via a boat transition state, a process known as pseudorotation. The boat form is destabilized by, a long
range steric interaction called the flagpole interaction. Eventually, the system escapes the twist boat regime via another
half-chair transition state to the flipped chair. At no time during the chair flip process are all six carbons in one plane, as
this would be a very highly strained structure.
• When two or more groups are attached to a cyclohexane ring, they can either be cis or trans to each other. The definitions
can be seen from a view of a flat cyclohexane ring where the groups are either on the same side of the ring (cis) or on
opposite sides (trans). This usage is distinct from the axial /equatorial terminology. Hence, cis and
trans groups can occupy various axial and equatorial positions depending upon the substitution pattern. A few examples
are given to the right.
• When two substituents are in a cis-1,3 relationship in a cyclohexane, they experience a strong interaction if both are axial.
The energetic consequence of this interaction can be substantial. For the case of the two methyl groups shown, the 1,3-
interaction contributes 3.7 kcal / mol of strain to the diaxial form.
Chemistry Sem 3 Page 1
1,1-Disubstituted Cyclohexanes
• 1,1-dimethylcyclohexane does not have cis or trans isomers, because both methyl groups are on the same carbon. Both
chair conformers have one methyl group in an axial position and one methyl group in an equatorial position giving both
the same relative stability. The steric strain created by the 1,3-diaxial interactions of a methyl group in an axial position
(versus equatorial) is 7.6 kJ/mol, so both conformers will have equal amounts of steric strain. Thus, the equilibrium
between the two conformers does not favor one or the other.
• However, if the two groups are different, as in 1-tert-butyl-1-methylcyclohexane, then the equilibrium favors the
conformer in which the larger group (tert-butyl) is in the more stable equatorial position. The energy cost of having one
tert-butyl group axial (versus equatorial) is approximately 22.8 kJ/mol. The conformer with the tert-butyl group axial is
approximately 15.2 kJ/mol (22.8 kJ/mol - 7.6 kJ/mol) less stable then the conformer with the tert-butyl group equatorial.
This means that 1-tert-butyl-1-methylcyclohexane will spend the majority of its time in the more stable conformation,
with the tert-butyl group in the equatorial position.
Cis and trans stereoisomers of 1,2-dimethylcyclohexane
• In cis-1,2-dimethylcyclohexane, both chair conformations have one methyl group equatorial and one methyl group axial.
As previously discussed, the axial methyl group creates 7.6 kJ/mol of steric strain due to 1,3-diaxial interactions. Overall,
both chair conformations have 11.4 kJ/mol of steric strain and are of equal stability.
• In trans-1,2-dimethylcyclohexane, one chair conformer has both methyl groups axial and the other conformer has both
methyl groups equatorial. The conformer with both methyl groups equatorial has no 1,3-diaxial interactions however
there is till 3.8 kJ/mol of strain created by a gauche interaction. The conformer with both methyl groups axial has four
1,3-Diaxial interactions which creates 2 x 7.6 kJ/mol (15.2 kJ/mol) of steric strain. This conformer is (15.2 kJ/mol -3.8
kJ/mol) 11.4 kJ/mol less stable than the other conformer. The equilibrium will therefore favor the conformer with both
methyl groups in the equatorial position.
Chemistry Sem 3 Page 2
Cis and trans stereoisomers of 1,3-dimethylcyclohexane
• For cis-1,3-dimethylcyclohexane one chair conformation has both methyl groups in axial positions creating 1,3-diaxial
interactions. The other conformer has both methyl groups in equatorial positions thus creating no 1,3-diaxial interaction.
Because the methyl groups are not on adjacent carbons in the cyclohexane rings gauche interactions are not possible.
Even without energy calculations it is simple to determine that the conformer with both methyl groups in the equatorial
position will be the more stable conformer.
• For trans-1,3-dimethylcyclohexane both conformations have one methyl axial and one methyl group equatorial. Each
conformer has one methyl group creating a 1,3-diaxial interaction so both are of equal stability.
SUMMARY
Chemistry Sem 3 Page 3
You might also like
- Isomerism One Shot BouncebackDocument196 pagesIsomerism One Shot BouncebackHarishNo ratings yet
- Barrier To Ring Inversion, Pyramidal Inversion, and 1,3-Diaxial Interaction EditedDocument12 pagesBarrier To Ring Inversion, Pyramidal Inversion, and 1,3-Diaxial Interaction EditedAnuja JoyNo ratings yet
- Cyclohexane Conformational AnalysisDocument17 pagesCyclohexane Conformational AnalysiscclatrumNo ratings yet
- Organic Chemistry - Morrison and BoydDocument390 pagesOrganic Chemistry - Morrison and Boydmadhavdhruv82% (22)
- Octant Rule, Axial Haloketone Rule GoodDocument11 pagesOctant Rule, Axial Haloketone Rule Goodsaheedvk50% (4)
- The Molecules of Life: Physical and Chemical PrinciplesDocument5 pagesThe Molecules of Life: Physical and Chemical PrinciplesMandel MachNo ratings yet
- Cyclohexane Conformational AnalysisDocument1 pageCyclohexane Conformational Analysis휘승No ratings yet
- 3.3 - Conformations of Cyclic Organic Molecules - Chemistry LibreTextsDocument7 pages3.3 - Conformations of Cyclic Organic Molecules - Chemistry LibreTextsFJosue MalaveHNo ratings yet
- Conformational AnalysisDocument10 pagesConformational AnalysisPG Chemistry PG ChemistryNo ratings yet
- Angle Strain Torsional Strain Ring StrainDocument6 pagesAngle Strain Torsional Strain Ring StrainchuasioklengNo ratings yet
- Chapter Four, Cycloalkanes (Part One - Monocyclic Alkane)Document8 pagesChapter Four, Cycloalkanes (Part One - Monocyclic Alkane)Amin JamjahNo ratings yet
- Chapter 4 With Video LinksDocument37 pagesChapter 4 With Video LinksDoom RefugeNo ratings yet
- Chapter 4Document33 pagesChapter 4채종희No ratings yet
- Organic - Class 8Document41 pagesOrganic - Class 8Sajan Singh LUCKYNo ratings yet
- Conformational Analysis For StudentsDocument89 pagesConformational Analysis For Studentsindrapal kumarNo ratings yet
- Conformational IsomersDocument8 pagesConformational Isomersshirodkar_16593No ratings yet
- Organic Lecture 2Document27 pagesOrganic Lecture 2Dhruv RaiNo ratings yet
- Org Lec 2Document27 pagesOrg Lec 2rupayandaripaNo ratings yet
- Cyclo AlkanesDocument38 pagesCyclo Alkanesdinesh111180No ratings yet
- DecalinsDocument25 pagesDecalinstessyNo ratings yet
- Chapter 3: Structure and Stereochemistry of AlkanesDocument42 pagesChapter 3: Structure and Stereochemistry of AlkanesKera Maria AllengerNo ratings yet
- MODULE 5: Conformational Analysis of Alkanes and CyclohexanesDocument8 pagesMODULE 5: Conformational Analysis of Alkanes and CyclohexanesARMAN AKRAM BIN OMAR / UPMNo ratings yet
- Conformation & Conformational IsomersDocument4 pagesConformation & Conformational Isomerspulkit asatiNo ratings yet
- Conformational Analysis of CyclopentaneDocument11 pagesConformational Analysis of CyclopentaneNimra MalikNo ratings yet
- Conformational Analysis:: Chapter - 6 Stereochemistry NotesDocument12 pagesConformational Analysis:: Chapter - 6 Stereochemistry NotesMohit KambojNo ratings yet
- Organic Chemistry: M. R. Naimi-JamalDocument81 pagesOrganic Chemistry: M. R. Naimi-JamalHamidatun NisaNo ratings yet
- GeneralChem LS 25 PDFDocument25 pagesGeneralChem LS 25 PDFSunil NahataNo ratings yet
- Cyclic Alkanes & AlkenesDocument18 pagesCyclic Alkanes & AlkenesbgbscgvNo ratings yet
- Cyclic AlkanesDocument35 pagesCyclic AlkanesKunjal100% (2)
- Conformational Isomerism 2Document8 pagesConformational Isomerism 2ytima uniNo ratings yet
- 3.9 - Conformations of Disubstituted CyclohexanesDocument5 pages3.9 - Conformations of Disubstituted CyclohexanesHajra NaeemNo ratings yet
- Conformational Analysis: Carey & Sundberg: Part A Chapter 3Document56 pagesConformational Analysis: Carey & Sundberg: Part A Chapter 3swastikNo ratings yet
- Conformaciones Diferentes Configuraciones DiferentesDocument45 pagesConformaciones Diferentes Configuraciones DiferentesJuan CarlosNo ratings yet
- Org Chem Text - Chapter 1 - Sec1-14 - 1-14Document3 pagesOrg Chem Text - Chapter 1 - Sec1-14 - 1-14TET2005No ratings yet
- Cycloalkane - Bayer Strain Theory and Limitation of Bayer Strain TheoryDocument5 pagesCycloalkane - Bayer Strain Theory and Limitation of Bayer Strain TheorySayed AltamashNo ratings yet
- UntitledDocument46 pagesUntitled양우경No ratings yet
- StereochemistryDocument94 pagesStereochemistryNelvianaNo ratings yet
- 3 SKO3023 Student Note - STEREOCHEMISTRYDocument94 pages3 SKO3023 Student Note - STEREOCHEMISTRYKHISHALINNI A/P M.MEGANATHANNo ratings yet
- BSES Topic 06 Cycloalkanes and Aromatic CompoundsDocument42 pagesBSES Topic 06 Cycloalkanes and Aromatic CompoundsJhunessa Mae JalagatNo ratings yet
- Hydroarbons - NotesDocument3 pagesHydroarbons - NotesxxxclanexeNo ratings yet
- U UNIT-2: Rmational Is Omerism Is Mational Iso Omers or Co Onformers A MersDocument6 pagesU UNIT-2: Rmational Is Omerism Is Mational Iso Omers or Co Onformers A MersImran AhmadNo ratings yet
- Strain 02Document7 pagesStrain 02Sherlock Wesley ConanNo ratings yet
- 1515564149CHE P1 M16 EtextDocument22 pages1515564149CHE P1 M16 EtextElangovan NatarajanNo ratings yet
- 2m Engl Isomerism 2021 For StudDocument96 pages2m Engl Isomerism 2021 For StudGhost ShooterNo ratings yet
- Lecture 31 PDFDocument17 pagesLecture 31 PDFRachit ShahNo ratings yet
- Conformations IIDocument16 pagesConformations IIsndNo ratings yet
- Organic Compounds:: Cycloalkanes and Their StereochemistryDocument34 pagesOrganic Compounds:: Cycloalkanes and Their StereochemistryrilaNo ratings yet
- Lesson StructureDocument19 pagesLesson Structurer karthickNo ratings yet
- Stereochemistry of Alkanes and Cycloalkanes: Based On Mcmurry'S Organic Chemistry, 6 Edition, Chapter 4 ©2003Document29 pagesStereochemistry of Alkanes and Cycloalkanes: Based On Mcmurry'S Organic Chemistry, 6 Edition, Chapter 4 ©2003hei chuNo ratings yet
- Conformational Isomerism by MuneebDocument11 pagesConformational Isomerism by MuneebMuneeb Ur RehmanNo ratings yet
- Types of Isomerisms Chain IsomerismDocument5 pagesTypes of Isomerisms Chain IsomerismRahul DubeyNo ratings yet
- LecturerNotes BPharmIIISem PCISyllabus UNIT V CycloalkanesStrainTheoryDocument8 pagesLecturerNotes BPharmIIISem PCISyllabus UNIT V CycloalkanesStrainTheoryKevalNo ratings yet
- Geometric IsomerismDocument68 pagesGeometric IsomerismRx Nadeem ChhipaNo ratings yet
- Chap 4Document29 pagesChap 4hy2023056744No ratings yet
- Conformations IIIDocument21 pagesConformations IIIsndNo ratings yet
- Week 3.2 Slide DitaDocument28 pagesWeek 3.2 Slide DitaAnnisah MardiyyahNo ratings yet
- Chair & BoatDocument9 pagesChair & BoatpardeepbthNo ratings yet
- Stereochemistry of Alkanes and CycloalkanesDocument29 pagesStereochemistry of Alkanes and Cycloalkanessuci wulansariNo ratings yet
- Loudon5ech07sec04 Cyclohexane ConformationsDocument8 pagesLoudon5ech07sec04 Cyclohexane ConformationsF1234TNo ratings yet
- CONFORMATIONSDocument3 pagesCONFORMATIONSElaaf AnzarNo ratings yet
- Chm102a Oc-L3-SdDocument34 pagesChm102a Oc-L3-SdDanish VasdevNo ratings yet
- Tutorial07b OrganicCycloalkanesDocument24 pagesTutorial07b OrganicCycloalkanesHana NisrinaNo ratings yet
- A-Level Chemistry Revision: Cheeky Revision ShortcutsFrom EverandA-Level Chemistry Revision: Cheeky Revision ShortcutsRating: 4 out of 5 stars4/5 (5)
- Molecular Modeling of The Enantioselectivity in Lipase-Catalyzed Transesterification ReactionsDocument12 pagesMolecular Modeling of The Enantioselectivity in Lipase-Catalyzed Transesterification ReactionsDavid AlvarezNo ratings yet
- MCAT Organic ChemistryDocument7 pagesMCAT Organic ChemistryjoNo ratings yet
- Stereoisomers Part 1Document14 pagesStereoisomers Part 1Mabelle DucusinNo ratings yet
- Question Bank 3051Document3 pagesQuestion Bank 3051Ashish AmbekarNo ratings yet
- Stereochemistry of Organic CompoundsDocument31 pagesStereochemistry of Organic CompoundsSrinivasulu KonetiNo ratings yet
- Acs Accounts 7b00638Document11 pagesAcs Accounts 7b00638Giggly HadidNo ratings yet
- Wileys Solomons Fryhle Snyder Organic Chemistry For Jee Main Advanced 3Rd Edition M S Chouhan All ChapterDocument67 pagesWileys Solomons Fryhle Snyder Organic Chemistry For Jee Main Advanced 3Rd Edition M S Chouhan All Chapteralfred.hale973100% (21)
- Potential Energy SurfaceDocument21 pagesPotential Energy SurfaceSIDDHARTH MARATHANo ratings yet
- Cap 2 - Chain Conformations in PolymersDocument20 pagesCap 2 - Chain Conformations in Polymersbrian delgado de lucioNo ratings yet
- Chem Workshop - Question Paper Compilation (Class Xi)Document212 pagesChem Workshop - Question Paper Compilation (Class Xi)aarzoopatel08100% (3)
- Isomerism and Stereochemistry: Answers To Worked ExamplesDocument15 pagesIsomerism and Stereochemistry: Answers To Worked ExamplesDana CapbunNo ratings yet
- Grade 11 Chemistry Notes Vol II (2022-2023)Document52 pagesGrade 11 Chemistry Notes Vol II (2022-2023)Timothy SaxenaNo ratings yet
- Coformational Isomerism PDFDocument13 pagesCoformational Isomerism PDFJeetNo ratings yet
- Part Test-6: Allindiatest SeriesDocument16 pagesPart Test-6: Allindiatest SeriesafasdfasdNo ratings yet
- Confirmational AnalysisDocument15 pagesConfirmational AnalysisNitya BhartiNo ratings yet
- Chapter 05 Wade 8thDocument66 pagesChapter 05 Wade 8thanupamgupta112No ratings yet
- Handout 1 - IsomerismDocument14 pagesHandout 1 - Isomerismboriwat thaengthonglang100% (1)
- Stereochemistrychem 2Document91 pagesStereochemistrychem 2Mary Ann DimacaliNo ratings yet
- UNIT 13 Hydrocarbons For More Visit HTTP://WWW - Ncert.nic - In/textbooks/testing/index - HTMDocument33 pagesUNIT 13 Hydrocarbons For More Visit HTTP://WWW - Ncert.nic - In/textbooks/testing/index - HTMArun Kumar100% (1)
- Houk Hydroboration TetDocument18 pagesHouk Hydroboration TetSuavo Tekka MukherjeeNo ratings yet
- Slides Ch34 Diastereoselectivity (Felkin-Ahn)Document15 pagesSlides Ch34 Diastereoselectivity (Felkin-Ahn)Rahn NaNo ratings yet
- Loudon, Organic Chemistry Study Guide ErrataDocument10 pagesLoudon, Organic Chemistry Study Guide Errataig12345No ratings yet
- Vinod Final Polyplex-1Document103 pagesVinod Final Polyplex-1Fan of carry minatiNo ratings yet
- Organic Chemistry 7th Edition Carey Test BankDocument15 pagesOrganic Chemistry 7th Edition Carey Test Bankmanuelhuynhv2zNo ratings yet
- CH 4Document8 pagesCH 4PolikNo ratings yet
- Representations of 3 Dimensional Structures: CY101: Engineering ChemistryDocument9 pagesRepresentations of 3 Dimensional Structures: CY101: Engineering Chemistryc.No ratings yet