Professional Documents
Culture Documents
Ikatan Pada Logam Dan Alloy
Ikatan Pada Logam Dan Alloy
Uploaded by
Kiki LestariCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Ikatan Pada Logam Dan Alloy
Ikatan Pada Logam Dan Alloy
Uploaded by
Kiki LestariCopyright:
Available Formats
Metallic bonding
Metallic bonding is a type of strong chemical bond
that occurs in pure metals and alloys. Metals are giant Did you
kno w …?
three-dimensional structures where layers of positive The me
lt
points o ing and boiling
f metals
metal ions are surrounded by a sea of delocalised to the n a
umber o re related
electron f ou
outer-shell electrons. charge
s. The g ter shell
rea
of the m ter the
the grea et
ter the n al ion,
delocal u
ised ele mber of
the stro ctrons a
nger the nd
bonds.
k now ...?
Did y o u
l l o y s a re used
um a e
Alumini ircraft, becaus
a
to make htweight and
lig
they’re T h e y are also
ong .
very str t o c o rrosion.
t
resistan
Did yo
u know
The c ...?
h
a met emical form
a
for th l is just the ula of
e el sy
lattice ement as m mbol
s do n etallic
fixed o
numb t contain
eg so er of a a
dium toms
is rep –
resen
as Na ted
.
Alloys are mixtures of two or more elements where at least one is a Pure metals only contain one type of metal atom,
metal. Metallic bonds are the strong electrostatic attractions between so the atoms are arranged in layers which can slide
positively charged metal ions and delocalised electrons. over one another. This means they are malleable –
Artwork © Dan Bright/Text by Emma Owens
can be hammered or pressed into shape without
In an alloy, the atoms are different sizes which distorts the layered
breaking or cracking – and ductile, so they can be
structure. This means greater force is needed to make the layers slide
drawn into wires.
over one another, which makes an alloy harder and stronger than the
pure metal.
rsc.li/3P0H9VC
You might also like
- E.M.C. QuestionsDocument38 pagesE.M.C. QuestionsJoshnewfound67% (3)
- Ship Building MaterialsDocument31 pagesShip Building MaterialsgirishNo ratings yet
- Tarsia ColoredDocument4 pagesTarsia ColoredSabeen Ahmed/TCHR/EKNNCNo ratings yet
- MAN Material Measurement: OccurrenceDocument2 pagesMAN Material Measurement: OccurrenceRishi GautamNo ratings yet
- Band Theory, Semiconductors PDFDocument6 pagesBand Theory, Semiconductors PDFpervyguy738No ratings yet
- ThanksDocument4 pagesThanksSandeep Kumar PaloNo ratings yet
- Service FloorDocument1 pageService Floorgulnaz allaudinNo ratings yet
- O Level Nuclear Physics and RadioactivityDocument16 pagesO Level Nuclear Physics and RadioactivityMd Safwat100% (1)
- Progress ChartDocument5 pagesProgress Chartnina andoyNo ratings yet
- Reading Activity Keys 2Document1 pageReading Activity Keys 2Dolores OliveriNo ratings yet
- Product BrochuresDocument12 pagesProduct BrochuresVIHIKA ENGINEERINGNo ratings yet
- Meaning Wheel With Citation 6Document1 pageMeaning Wheel With Citation 6Cilka RamírezNo ratings yet
- Properties of Ionic - Covalent FACTSHEETDocument4 pagesProperties of Ionic - Covalent FACTSHEETh9gfvyjr8gNo ratings yet
- (Merge) AmpDocument19 pages(Merge) AmpKathirvel KNo ratings yet
- Unit Review ChargesDocument47 pagesUnit Review Chargeschrisdamato100% (2)
- Charge Comes in Two Types, Positive and Negative Opposite Charges Attract Each Other Identical Charges Repel Each OtherDocument47 pagesCharge Comes in Two Types, Positive and Negative Opposite Charges Attract Each Other Identical Charges Repel Each OtherchrisdamatoNo ratings yet
- Resume 1Document1 pageResume 1jhsizemore5588No ratings yet
- My First in Past E: CeramicDocument4 pagesMy First in Past E: CeramicceramicameoNo ratings yet
- Les Arends Forest Preserve To WN e Be RNDocument1 pageLes Arends Forest Preserve To WN e Be RNapi-26000492No ratings yet
- The Amber Tankard 1-2Document1 pageThe Amber Tankard 1-2Richard PeacockNo ratings yet
- Monitoring ToolsDocument14 pagesMonitoring ToolsStephanie CastillonNo ratings yet
- Ks1 English 2003 Teachers GuideDocument48 pagesKs1 English 2003 Teachers GuideDivya DhayalanNo ratings yet
- How To ReadDocument32 pagesHow To ReadjorgeluiztadeurodriguesfilhoNo ratings yet
- 024 Nov01 TalkDocument29 pages024 Nov01 TalkRusli SiahanNo ratings yet
- KOM PaperDocument1 pageKOM PaperManvith VenugopalNo ratings yet
- Cimeni Mind Map #BrainDocument1 pageCimeni Mind Map #BrainHaahnn CimeniNo ratings yet
- Au Hu 1630639018 Australia Map With Tourist Attractions - Ver - 2Document1 pageAu Hu 1630639018 Australia Map With Tourist Attractions - Ver - 2FLE FranceNo ratings yet
- Acfrogbn1kcg13ocnw2k7bw5lzb Ryfxhqwr2hdrcz6by U5md5fp Zvgf5dztsbanhlcxkg 9i1kij624fmwdyu Epfrg8me7nvlpomuxbzgvs Q0tsubyxj Kwksmg5t5ntritlmiltlbuydgvDocument1 pageAcfrogbn1kcg13ocnw2k7bw5lzb Ryfxhqwr2hdrcz6by U5md5fp Zvgf5dztsbanhlcxkg 9i1kij624fmwdyu Epfrg8me7nvlpomuxbzgvs Q0tsubyxj Kwksmg5t5ntritlmiltlbuydgvMj RarelaNo ratings yet
- Mindmap Kinetic ArchitectureDocument1 pageMindmap Kinetic ArchitectureSiddhant KotakNo ratings yet
- Activities That He Usually Develops:, Ar Ound Ers, His SK in Is Whit e Ebr Own. e Is Thin and His Hair Is BR OwnDocument1 pageActivities That He Usually Develops:, Ar Ound Ers, His SK in Is Whit e Ebr Own. e Is Thin and His Hair Is BR OwnPaola ForeroNo ratings yet
- Site AnalysisDocument1 pageSite AnalysisHappy CornerNo ratings yet
- Lor em Ipsum Dolor Sit Amet, C Onsec - , y Nibh T Ut Laor EetDocument1 pageLor em Ipsum Dolor Sit Amet, C Onsec - , y Nibh T Ut Laor EetTrung Hiếu NguyễnNo ratings yet
- 大众标准PV1303-(英文)耐光照牢度(方法标准)Document10 pages大众标准PV1303-(英文)耐光照牢度(方法标准)zz576614No ratings yet
- FA#1 Abao PDFDocument3 pagesFA#1 Abao PDFRaymond Rey ApuradaNo ratings yet
- 1) Loos Haus: 2) Vi L L A Mul L ErDocument1 page1) Loos Haus: 2) Vi L L A Mul L ErcharuNo ratings yet
- Lesson 1Document18 pagesLesson 1BPISHERENo ratings yet
- Simple Construction PayroleDocument3 pagesSimple Construction PayroleJayson SusaNo ratings yet
- Barcelona Estació de França Tarragona Tortosa / Ulldecona Barcelona Estació de França Salou - Port AventuraDocument2 pagesBarcelona Estació de França Tarragona Tortosa / Ulldecona Barcelona Estació de França Salou - Port AventuraroskienNo ratings yet
- California Environmental ScorecardDocument7 pagesCalifornia Environmental ScorecardBill Gram-ReeferNo ratings yet
- Barcelona Estació de França Tarragona Tortosa / Ulldecona Barcelona Estació de França Salou - Port AventuraDocument2 pagesBarcelona Estació de França Tarragona Tortosa / Ulldecona Barcelona Estació de França Salou - Port AventurafiestajuguetesNo ratings yet
- Number of Cleavage StepsDocument26 pagesNumber of Cleavage StepsPriyavrat SatheNo ratings yet
- Annual Report 2020 21Document240 pagesAnnual Report 2020 21VyasRmNo ratings yet
- Lightning in A BottleDocument1 pageLightning in A BottleLightning BottleNo ratings yet
- PhenomenaDocument1 pagePhenomenaClariz Angelika EscocioNo ratings yet
- Types of ChipsDocument3 pagesTypes of ChipsRam27092003 GermanNo ratings yet
- Undergraduate PortfolioDocument39 pagesUndergraduate PortfolioChris BordNo ratings yet
- Ryobi 18 Volt Battery Charger ManualDocument24 pagesRyobi 18 Volt Battery Charger ManualBryan JonesNo ratings yet
- Current Trends and Enhancement To The National Geospatial Reference SystemDocument19 pagesCurrent Trends and Enhancement To The National Geospatial Reference SystemNT SpatialNo ratings yet
- 17 - Lubrication: 1 Removing and Installing Parts of Lu Brication SystemDocument9 pages17 - Lubrication: 1 Removing and Installing Parts of Lu Brication SystemFocus MarambaNo ratings yet
- Don't Let The Breezy, Irreverent Style of This Book Fool YouDocument3 pagesDon't Let The Breezy, Irreverent Style of This Book Fool YouArlind LleshiNo ratings yet
- Don't Let The Breezy, Irreverent Style of This Book Fool YouDocument3 pagesDon't Let The Breezy, Irreverent Style of This Book Fool YouArlind LleshiNo ratings yet
- Walmart GRRDocument174 pagesWalmart GRRPatricia DillonNo ratings yet
- SPIE Oral PresentationDocument3 pagesSPIE Oral PresentationvitalakNo ratings yet
- Seba Catalog-2015 PDFDocument44 pagesSeba Catalog-2015 PDFAnonymous 7mJFgWyIqTNo ratings yet
- Academic Year:: Shree Amar Kalyan Secondary School, Maijogmai 1 Nayabazar, IlamDocument2 pagesAcademic Year:: Shree Amar Kalyan Secondary School, Maijogmai 1 Nayabazar, IlamDB BhandariNo ratings yet
- Team 2Document1 pageTeam 2Alejandra Mishell Pacheco CanoNo ratings yet
- Portfolio NFDocument14 pagesPortfolio NFNicholas FitzgeraldNo ratings yet
- Using DDL Statements to Create and Manage Tables: license to use this Student GuideฺDocument40 pagesUsing DDL Statements to Create and Manage Tables: license to use this Student GuideฺSiranjeevi GnanamNo ratings yet
- Meaning Wheel With Citation 6Document1 pageMeaning Wheel With Citation 6Abu HanifahNo ratings yet
- Presentation 2Document31 pagesPresentation 2Tawfiq MahasnehNo ratings yet
- Vol. 2 Komodo DragonsDocument8 pagesVol. 2 Komodo DragonsNugraNo ratings yet
- Lecture 5 Slides and Notes (314 KB)Document30 pagesLecture 5 Slides and Notes (314 KB)Prateek0% (1)
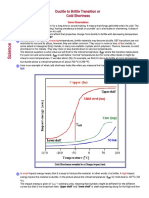
- Ductile To Brittle Transition or Cold ShortnessDocument12 pagesDuctile To Brittle Transition or Cold Shortnessvamsi patnalaNo ratings yet
- The Future of Rock Bolting 1692444089Document4 pagesThe Future of Rock Bolting 1692444089Raul Bracamontes JimenezNo ratings yet
- 17me3302 - Unit One TheoryDocument70 pages17me3302 - Unit One TheoryZayeem ZehekNo ratings yet
- What Is FractureDocument36 pagesWhat Is FractureMona AwadNo ratings yet
- Understanding The Engineering Polymers. (Annealing of Polymers and Composites), Part 3Document3 pagesUnderstanding The Engineering Polymers. (Annealing of Polymers and Composites), Part 3juan k RestrepoNo ratings yet
- Science DLL Week 4 November 28 29, December 1 2Document9 pagesScience DLL Week 4 November 28 29, December 1 2Ma. Joan Mae MagnoNo ratings yet
- Extended Coverage SprinklerDocument94 pagesExtended Coverage SprinklerbenNo ratings yet
- Failure Analysis For Endineering MaterialsDocument18 pagesFailure Analysis For Endineering MaterialsArielle Joyce de JesusNo ratings yet
- Chapter 4 - MATERIAL TESTINGDocument48 pagesChapter 4 - MATERIAL TESTINGتاج نيسهاNo ratings yet
- Experimental Analysis of Mech Properties of Aluminum AlloysDocument7 pagesExperimental Analysis of Mech Properties of Aluminum AlloysPablo AlcázarNo ratings yet
- Reviewer AjDocument7 pagesReviewer AjMonicDuranNo ratings yet
- General Engineering Knowledge For Marine EngineersDocument272 pagesGeneral Engineering Knowledge For Marine EngineersMANDEEP SINGHNo ratings yet
- Lecture-3-Mechanical Properties and Testing of MaterialsDocument43 pagesLecture-3-Mechanical Properties and Testing of MaterialsTony StarkNo ratings yet
- Best Practice For The Assessment of Defects in Pipelines - CorrosionDocument21 pagesBest Practice For The Assessment of Defects in Pipelines - CorrosionGilletNo ratings yet
- Polypropylene Injection and Extrusion Materials: Standard Classification System and Basis For Specification ForDocument17 pagesPolypropylene Injection and Extrusion Materials: Standard Classification System and Basis For Specification Forist93993No ratings yet
- Li (2023)Document7 pagesLi (2023)Juan JimenezNo ratings yet
- Interpretation and Significance of Reverse Chevron-Shaped Markings On Fracture Surfaces of API X100 Pipeline SteelsDocument10 pagesInterpretation and Significance of Reverse Chevron-Shaped Markings On Fracture Surfaces of API X100 Pipeline SteelsAdil Shahzad QaziNo ratings yet
- GariDocument5 pagesGariBikila MalasaNo ratings yet
- Cryogenics - A Quick GuideDocument7 pagesCryogenics - A Quick GuideAnnamalai Ram JGCNo ratings yet
- METALS PresentationDocument28 pagesMETALS PresentationTheresa TuliaoNo ratings yet
- 9 Test To Check Quality of Bitumen For Use in Road WorkDocument6 pages9 Test To Check Quality of Bitumen For Use in Road WorkEulogio Jamero100% (2)
- ASTM A 197 - A 197M - 00Document4 pagesASTM A 197 - A 197M - 00Er Widodo100% (2)
- Chapter 4 Year 3Document27 pagesChapter 4 Year 3Mohd SoufianNo ratings yet
- Ocbc Ime 2Document40 pagesOcbc Ime 2Hrishabh GroverNo ratings yet
- Section 6 D3 BucketsDocument29 pagesSection 6 D3 Bucketsiman zareieNo ratings yet
- Author's Accepted Manuscript: Materials Science & Engineering ADocument20 pagesAuthor's Accepted Manuscript: Materials Science & Engineering AMeiske HasanNo ratings yet