Professional Documents
Culture Documents
Jurnal Radio 1
Jurnal Radio 1
Uploaded by
Vanessa LeatemiaCopyright:
Available Formats
You might also like
- Chapter 14 Pathology RBC and Bleeding Disorders OutlineDocument19 pagesChapter 14 Pathology RBC and Bleeding Disorders OutlineNatan BabekNo ratings yet
- Respiration in Fish N FrogDocument23 pagesRespiration in Fish N FrogHazirah HussinNo ratings yet
- Fetal Macrocrania: Diagnosis, Delivery and Outcomes: Original ArticleDocument4 pagesFetal Macrocrania: Diagnosis, Delivery and Outcomes: Original ArticleAndreas NatanNo ratings yet
- Det 039Document11 pagesDet 039grimmNo ratings yet
- AmniopatchDocument8 pagesAmniopatchYazmin ZavalaNo ratings yet
- Benign Lesions of PinnaDocument4 pagesBenign Lesions of Pinnamanoj kumarNo ratings yet
- Calcified Cephalohematoma in A Newborn Unusual Presentation Without History of Birth TraumaDocument6 pagesCalcified Cephalohematoma in A Newborn Unusual Presentation Without History of Birth Traumafaizu21No ratings yet
- SWAIMANDocument18 pagesSWAIMANVan John MagallanesNo ratings yet
- Journal AsliDocument4 pagesJournal Aslidesi tethunNo ratings yet
- Alwahab2017 Article OccipitalMeningoencephaloceleC PDFDocument4 pagesAlwahab2017 Article OccipitalMeningoencephaloceleC PDFOvamelia JulioNo ratings yet
- Fetal Ventriculomegaly: Postnatal Management: Special Annual IssueDocument3 pagesFetal Ventriculomegaly: Postnatal Management: Special Annual IssueRendra Artha Ida BagusNo ratings yet
- 0390 6663 48 6 1448Document6 pages0390 6663 48 6 1448فاطمة الزهراء أسامةNo ratings yet
- CC Helps in LocalisationDocument9 pagesCC Helps in LocalisationRavi ChandraNo ratings yet
- Craniopharyngiomas: Clinic Al PearlsDocument18 pagesCraniopharyngiomas: Clinic Al PearlsRanintha SurbaktiNo ratings yet
- Retrospective Examination of Infants With Congenital Neural Tube DefectDocument8 pagesRetrospective Examination of Infants With Congenital Neural Tube Defectözkan ilhanNo ratings yet
- Anticoagulacion Neonatos 2019Document11 pagesAnticoagulacion Neonatos 2019edi21arNo ratings yet
- Embryonal Rhabdomyosarcoma of The Uterine - 2014 - Taiwanese Journal of ObstetrDocument3 pagesEmbryonal Rhabdomyosarcoma of The Uterine - 2014 - Taiwanese Journal of ObstetrSami KahtaniNo ratings yet
- Tumor Teratoma Rabdoide RevisiónDocument16 pagesTumor Teratoma Rabdoide RevisiónPaolo Mendoza LeonNo ratings yet
- Manejo de Las Malformaciones Cavernosas Del Tronco Encefálico Pediátrico - Experiencia de Más de 20 Años en El Hospital de Niños EnfermosDocument7 pagesManejo de Las Malformaciones Cavernosas Del Tronco Encefálico Pediátrico - Experiencia de Más de 20 Años en El Hospital de Niños EnfermosGonzalo Leonidas Rojas DelgadoNo ratings yet
- Placenta AccretaDocument5 pagesPlacenta AccretaF3rcitaNo ratings yet
- Cornell A 2003Document13 pagesCornell A 2003dnazaryNo ratings yet
- Paediatric Neck MassesDocument7 pagesPaediatric Neck MassesGiovanni HenryNo ratings yet
- Kim 2015Document4 pagesKim 2015ruthameliapNo ratings yet
- Testicular Microlithiasis1Document4 pagesTesticular Microlithiasis1Ioannis ValioulisNo ratings yet
- Complex Cesarian SectionDocument11 pagesComplex Cesarian SectionHerald Scholarly Open AccessNo ratings yet
- Seminars in Pediatric Surgery: Immediate Versus Staged Repair of OmphalocelesDocument6 pagesSeminars in Pediatric Surgery: Immediate Versus Staged Repair of OmphalocelesGufi GeorgeNo ratings yet
- Diagnosis and Management of Posterior Plagiocephaly: ObjectiveDocument8 pagesDiagnosis and Management of Posterior Plagiocephaly: ObjectiveSelene MéndezNo ratings yet
- Epidemiology of Brachial Plexus Palsy in NewbornsDocument7 pagesEpidemiology of Brachial Plexus Palsy in NewbornsMardyana MardinNo ratings yet
- Cesarean Scar Ectopic Pregnancies: Etiology, Diagnosis, and ManagementDocument9 pagesCesarean Scar Ectopic Pregnancies: Etiology, Diagnosis, and ManagementDinorah MarcelaNo ratings yet
- Pattern of Encephaloceles A Case SeriesDocument4 pagesPattern of Encephaloceles A Case SeriesSayyid Hakam PerkasaNo ratings yet
- Antenatal Diagnosis ofDocument6 pagesAntenatal Diagnosis ofnskhldNo ratings yet
- Fistula in AnoDocument5 pagesFistula in AnoIoannis ValioulisNo ratings yet
- Diagnosis and Management of Congenital Epulis of New-Born: A Rare Case Report and Literature ReviewDocument3 pagesDiagnosis and Management of Congenital Epulis of New-Born: A Rare Case Report and Literature ReviewIsmail YusufNo ratings yet
- Ghan em 2005Document5 pagesGhan em 2005asfwegereNo ratings yet
- 1 PBDocument7 pages1 PBsahrirNo ratings yet
- Teratoma in ChildrenDocument5 pagesTeratoma in ChildrenRif'aNo ratings yet
- LSM RepairDocument4 pagesLSM RepairDanily Faith VillarNo ratings yet
- Radiology RadiologyDocument6 pagesRadiology RadiologyJonathan ReinaldoNo ratings yet
- Sattar M. Hashim JPMCDocument5 pagesSattar M. Hashim JPMCprastiaNo ratings yet
- Pediatric Regional AnesthesiaDocument6 pagesPediatric Regional Anesthesiatq9prx5s5qNo ratings yet
- PapillomaDocument4 pagesPapillomaNovhy GanggutNo ratings yet
- Implantación Endometrial - GenéticaDocument9 pagesImplantación Endometrial - GenéticaMaríaJoséPinedaNo ratings yet
- Early Postoperative Complications Following Myelomeningocele RepairDocument13 pagesEarly Postoperative Complications Following Myelomeningocele RepairAlemsegedNo ratings yet
- Got Her Strom 2013Document10 pagesGot Her Strom 2013Alexia Nadine PuelNo ratings yet
- 10.1.1.546.6206 BedahDocument7 pages10.1.1.546.6206 Bedahfikri hanifNo ratings yet
- Pos Ok Hova 2012Document1 pagePos Ok Hova 2012Suis MionooNo ratings yet
- Editorial: Mass Screening For Fetal Malformations: The Eurofetus StudyDocument4 pagesEditorial: Mass Screening For Fetal Malformations: The Eurofetus StudyRaluca HabaNo ratings yet
- Editorial: Mass Screening For Fetal Malformations: The Eurofetus StudyDocument4 pagesEditorial: Mass Screening For Fetal Malformations: The Eurofetus StudyRaluca HabaNo ratings yet
- Achondroplasia Natural History Study (CLARITY)Document7 pagesAchondroplasia Natural History Study (CLARITY)jakelinelagoadvNo ratings yet
- Episio PDFDocument4 pagesEpisio PDFEduBarranzuelaNo ratings yet
- Jurnal Reading OtologiDocument19 pagesJurnal Reading OtologiMuhammad gusti haryandiNo ratings yet
- Postnatal Outcomes of Fetuses With Prenatal Diagnosis of - En.esDocument10 pagesPostnatal Outcomes of Fetuses With Prenatal Diagnosis of - En.esgaby3005No ratings yet
- Cross 2023 PediatricepilepsysurgeryDocument11 pagesCross 2023 PediatricepilepsysurgeryGala GonzalezNo ratings yet
- Sijog 47 291-296Document6 pagesSijog 47 291-296IcicNo ratings yet
- Elkhunovich2016 The Utility of Cranial Ultrasound For Detection of IntracranialDocument6 pagesElkhunovich2016 The Utility of Cranial Ultrasound For Detection of IntracranialModou NianeNo ratings yet
- DhirendraDocument3 pagesDhirendraBenny KurniawanNo ratings yet
- Ajns 13 233Document5 pagesAjns 13 233Ade Cahyo IslamiNo ratings yet
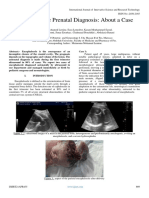
- Encephalocele Prenatal Diagnosis About A CaseDocument3 pagesEncephalocele Prenatal Diagnosis About A CaseInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Sternocleidomastoid Pseudotumor of Infancy A ReporDocument5 pagesSternocleidomastoid Pseudotumor of Infancy A Reporclaudia_isipNo ratings yet
- BCCBenchmarkingDocument48 pagesBCCBenchmarkingوليد خالدNo ratings yet
- Children 09 01560Document9 pagesChildren 09 01560Susana MartinezNo ratings yet
- Stem Cell Biology and Regenerative Medicine in OphthalmologyFrom EverandStem Cell Biology and Regenerative Medicine in OphthalmologyStephen TsangNo ratings yet
- Jurnal THT 1Document7 pagesJurnal THT 1Vanessa LeatemiaNo ratings yet
- Rhs 4Document6 pagesRhs 4Vanessa LeatemiaNo ratings yet
- Rhs 8Document2 pagesRhs 8Vanessa LeatemiaNo ratings yet
- 564-Article Text-1982-2-10-20220426Document7 pages564-Article Text-1982-2-10-20220426Vanessa LeatemiaNo ratings yet
- Cambridge International AS & A Level: Biology 9700/12Document16 pagesCambridge International AS & A Level: Biology 9700/12lllllisaNo ratings yet
- Spontaneous and Re Ex OvulatorsDocument14 pagesSpontaneous and Re Ex OvulatorsFazal Akbar KhaliliNo ratings yet
- Fetal Growth and DevelopmentDocument25 pagesFetal Growth and Developmentniogan anthonyNo ratings yet
- Anatomy of Flowering PlantsDocument16 pagesAnatomy of Flowering PlantsSadik FsNo ratings yet
- Pain MedicineDocument287 pagesPain MedicineSAJID ALINo ratings yet
- 40th DITF Catalogue 2016Document85 pages40th DITF Catalogue 2016Michael HansenNo ratings yet
- Activity Sheet 1 & 2 GenBio1Document12 pagesActivity Sheet 1 & 2 GenBio1Marlou GayaneloNo ratings yet
- Feyer 2020 WvngoshawkDocument13 pagesFeyer 2020 WvngoshawkAgnieszka CzujkowskaNo ratings yet
- Mother and Child Hospital ReportDocument1 pageMother and Child Hospital ReportclinicallabmchNo ratings yet
- Range of Joint Motion Evaluation Chart: 1. Back 2. Lateral (Flexion)Document2 pagesRange of Joint Motion Evaluation Chart: 1. Back 2. Lateral (Flexion)JulesNo ratings yet
- Science 9 Week 1Document18 pagesScience 9 Week 1lenNo ratings yet
- Aad Abdomen WorksheetDocument15 pagesAad Abdomen WorksheetAchNo ratings yet
- CH 10 Somatic and Special Senses Powerpoint 2006Document175 pagesCH 10 Somatic and Special Senses Powerpoint 2006Maikel PakageNo ratings yet
- Physical AssessmentDocument2 pagesPhysical AssessmentEdmar DalmacioNo ratings yet
- Kelainan Morf EritrositDocument12 pagesKelainan Morf EritrositTrio Singgih SaputroNo ratings yet
- The Effect of Trunk Strengthening Exercise Using Oscillation On Trunk Muscle Thickness and BalanceDocument11 pagesThe Effect of Trunk Strengthening Exercise Using Oscillation On Trunk Muscle Thickness and Balancekang soon cheolNo ratings yet
- Week 2 Endocrine Anatomy and Physiology Review & Pituitary DisturbancesDocument27 pagesWeek 2 Endocrine Anatomy and Physiology Review & Pituitary DisturbancesZiqri Dimas SandyNo ratings yet
- Terdapat Hubungan Kadar Estradiol Dengan Ekspresi Kolagen Dan Elastin Pada Vagina Premenopause Dan PascamenopauseDocument9 pagesTerdapat Hubungan Kadar Estradiol Dengan Ekspresi Kolagen Dan Elastin Pada Vagina Premenopause Dan PascamenopausenawangprimailmiafeeNo ratings yet
- Cloning 1 QPDocument11 pagesCloning 1 QPWayne Mc DonaghNo ratings yet
- Quiz 2Document2 pagesQuiz 2Jovie MasongsongNo ratings yet
- TECH 614 Full Spine I (Castellucci)Document10 pagesTECH 614 Full Spine I (Castellucci)Robert StraubNo ratings yet
- Pulmonary Circulation and Acute Lung Injury, The - UnknownDocument616 pagesPulmonary Circulation and Acute Lung Injury, The - UnknownMixalisKaplanisNo ratings yet
- PDF Test Bank For Essentials of Anatomy Physiology 6Th Edition Frederic H Martini Online Ebook Full ChapterDocument68 pagesPDF Test Bank For Essentials of Anatomy Physiology 6Th Edition Frederic H Martini Online Ebook Full Chapterdaniel.duncan540100% (2)
- Test Bank For Advanced Nutrition and Human Metabolism 7th Edition Gropper Smith Carr 1305627857 9781305627857Document22 pagesTest Bank For Advanced Nutrition and Human Metabolism 7th Edition Gropper Smith Carr 1305627857 9781305627857francisNo ratings yet
- Cileni Cortes Camacho AntologiaDocument62 pagesCileni Cortes Camacho AntologiaCileni CortesNo ratings yet
- Science 5 DLP 1 Human Reproductive SystemDocument12 pagesScience 5 DLP 1 Human Reproductive SystemDyaan TrajicoNo ratings yet
- Host Defense Mechanisms (Non-Specific) : BIO162 Microbiology For Allied Health Page BaluchDocument30 pagesHost Defense Mechanisms (Non-Specific) : BIO162 Microbiology For Allied Health Page Baluchbocah_britpopNo ratings yet
- Gastric Lavage N Gavage Lesson PlanDocument11 pagesGastric Lavage N Gavage Lesson PlanKiran40% (5)
Jurnal Radio 1
Jurnal Radio 1
Uploaded by
Vanessa LeatemiaOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Jurnal Radio 1
Jurnal Radio 1
Uploaded by
Vanessa LeatemiaCopyright:
Available Formats
Open Access Original
Article DOI: 10.7759/cureus.14415
Observational Case Analysis of Neonates With
Large Cephalohematoma
Melih Üçer 1 , Abdullah E. Taçyıldız 2 , Ilhan Aydın 1 , Nesrin Akkoyun Kayran 1 , Semra Işık 1
1. Neurosurgery, University of Health Science Kanuni Sultan Süleyman Research and Training Hospital, Istanbul, TUR
2. Neurosurgery, School of Medicine, Karabuk University, Karabük, TUR
Corresponding author: Melih Üçer, drmucer27@gmail.com
Abstract
Introduction
Cephalohematomas in the newborn period are related to the accumulation of blood between the bone and
periosteum as a result of a series of adverse conditions during labor. The optimal approach to
cephalohematoma cases is still unclear. In this study, we aimed to present the follow-up data of 94
newborns with a cephalohematoma size of >50 mm and a higher risk of ossification.
Methods
This is a single-center, non-randomized, prospective, observational study conducted from May 2014 to May
2019. Records of all newborns with cephalohematoma were reviewed in terms of gender, birth weight,
cephalohematoma region, transverse/vertical diameter of the lesion, delivery method, and rate of
ossification.
Results
The girl-to-boy ratio was 53/41, with a mean gestational age of 38.3±1.4 weeks and a mean birth weight of
3,300±800 grams. The mean transverse/vertical diameter of cephalohematoma was 59±9 mm.
Cephalohematoma was completely resorbed at the first-month control visits in 72 (76.6%) cases, whereas
nine (9.57%) had an ossified cephalohematoma. The ossification was completely or partially resorbed in
these at the end of the one-year follow-up.
Conclusion
Hence, we suggest that an early intervention is not required in the routine treatment of cases with
hematomas with a size of >50 mm in size unless otherwise stipulated with clinical indications.
Categories: Pediatric Surgery, Neurosurgery
Keywords: cephalohematoma, management, ossification
Review began 03/11/2021
Review ended 04/05/2021
Published 04/11/2021
Introduction
© Copyright 2021 Birth traumas include a wide spectrum of incidents that can occur from the initiation and termination of the
Üçer et al. This is an open access article
labor, which might have structural and/or functional effects on the newborn. The result of these traumas
distributed under the terms of the
Creative Commons Attribution License ranges from paralysis to cephalohematoma. Infant cephalohematoma is a subperiosteal blood accumulation
CC-BY 4.0., which permits unrestricted observed in 0.2%-3% of newborns, usually localized over the parietal region, and markedly delimited by
use, distribution, and reproduction in any suture lines [1,2].
medium, provided the original author and
source are credited.
Since the coagulated blood is slowly absorbed, the resorption of cephalohematoma takes several weeks,
while most newborns recover within the first four weeks of the neonatal period. Delayed resorption may
often lead to progressive calcification and ossification [2]. Ossified cephalohematomas are cosmetic
asymmetries and deformities of the skull and mostly heal spontaneously via the gradual absorption of the
ossified tissue by the expanding calvarium, and the physical appearance usually turns to normal before the
age of one year [3].
On rare occasions, surgical intervention is required for the treatment of persistent ossified
cephalohematoma cases in order to prevent a progression to undesirable sequelae during the developmental
duration.
The aim of this prospective study was to evaluate the management outcomes of 94 newborns who were
admitted to the neurosurgery clinic of our hospital due to a cephalohematoma, with a transverse/vertical
diameter of >50 mm, and a higher risk of ossification.
Materials And Methods
This is a single-center, non-randomized, prospective, observational study conducted at the Neurosurgery
How to cite this article
Üçer M, Taçyıldız A E, Aydın I, et al. (April 11, 2021) Observational Case Analysis of Neonates With Large Cephalohematoma. Cureus 13(4):
e14415. DOI 10.7759/cureus.14415
Clinic of Kanuni Sultan Süleyman Research and Training Hospital, Istanbul, Turkey, between May 2014 and
May 2019. The study was conducted in accordance with the Declaration of Helsinki following approval of the
Local Ethical Committee. Informed consent obtained was obtained from the parents or legal guardians of all
patients.
Patients were evaluated in terms of gender, birth weight, delivery method, cephalohematoma region,
transverse/vertical diameter of the hematoma, and rate of ossification.
Infants with an abnormal coagulation profile (suspected or confirmed hemophilia, thrombocytopenia,
disseminated intravascular coagulation) and central nervous system anomalies were excluded from the
study.
At admission to the neurosurgery outpatient clinic, patients were applied compressive dressing and called
for a check-up at the first month, second month, and first year.
Computed tomography (CT) scan is indicated only when neurological symptoms develop, when there is a
possibility of a collapsed skull fracture, or when a morphologic deformity of the skull is considered. None of
the patients underwent CT scan, except for patients whose cephalohematoma did not regress by the end of
the second postnatal month.
Results
A total of 94 neonates with cephalohematoma were referred to our outpatient clinic, of whom 53 (56.4%)
were girls and 41 (43.6%) were boys, with a mean gestational age of 38.3±1.4 weeks and mean birth weight of
3,300±800 grams. Of the patients, 69 (73.4%) were born via vaginal delivery and 25 (26.6%) were delivered by
cesarean section. The mean transverse/vertical diameter of cephalohematoma measured by ultrasonography
(USG) in the first examination was 59±9 mm. Cephalohematoma was detected at the parietal region in 71
(75.5%) infants, at the occipital region in 12 infants (12.8%), and at the temporoparietal region in 11 infants
(11.7%).
After compressive dressing, cephalohematoma was completely resolved in 72 (76.6%) cases at the first-
month control visits and in 12 (12.7%) cases at the second month of follow-up. Of the remaining 10
newborns who were followed up with intermittent controls, ossification formation in cephalohematomas
was observed in nine (9.57%) patients. At the end of the one-year follow-up, the ossification was completely
or partially resorbed in all nine patients (Figures 1, 2). None of the patients had additional clinical findings
or adverse events related to the condition, and surgery was not required for any patients.
FIGURE 1: (A) An ossified cephalohematoma was seen in the left
parietal region in a two-month-old baby. (B) Ossification was completely
resorbed in the control CT taken at the ninth month.
2021 Üçer et al. Cureus 13(4): e14415. DOI 10.7759/cureus.14415 2 of 7
FIGURE 2: (A) An ossified cephalohematoma was seen in the right
parietal region in a two-month-old baby. (B) At the 12th month, the
ossified hematoma is seen to be resorbed in the control CT scan, but
cranium thickening is observed in the area where the cephalohematoma
is present.
Discussion
The exact mechanism of development of cephalohematoma during labor is unknown; however, it is thought
to be an injury caused by forceful and repeated compression of the skull by the pelvic bones alongside
contractions during labor. This shear action leads to bleeding of the diploic vessels to the subperiosteal layer
of the skull [4] (Figure 3). Common risk factors are prolonged labor, macrosomia, weak and ineffective
uterine contractions, male gender, abnormal presentation and position of the fetus, forceps- or vacuum-
assisted delivery, polycyesia, and cephalopelvic disproportion [5]. It has been reported that the risk of
cephalohematoma development increases by up to 10.8% with assisted vaginal delivery [6].
Cephalohematomas are mostly located on the parietal bone and on the right side, but they are bilateral in
10% of the cases [7].
2021 Üçer et al. Cureus 13(4): e14415. DOI 10.7759/cureus.14415 3 of 7
FIGURE 3: Cephalohematoma mechanism.
Printed with permission from Dr. Gokhan CANAZ©, Department of Neurosurgery, Arnavutkoy State Hospital.
Despite the fact that for unknown reasons the incidence of cephalohematoma is reported to be twice as
higher in males than females in the literature, in our series the majority of the cases were female [8]. The
overall incidence of cephalohematoma was approximately 1.25% per year for our center, which is one of the
largest hospitals in a European metropole and specializes in women’s and children’s health with a birth
incidence of more than 5,000 cases per year [9].
Although the tissue formed when cephalohematoma is not resorbed was previously considered to be
calcification, studies have shown that the phenomenon is ossification [10]. It is essential to be thoroughly
familiar with the embryology of the scalp in order to better understand the ossification process of
cephalohematoma. During the developmental process, the scalp begins to appear as a vascular plexus on the
49th day of gestation. A few weeks after the first hair begins to appear on the eyebrows at the 16th week,
they begin to cover the scalp, and the fetal hair, which is longer than lanugo, begins to grow at the seventh
2021 Üçer et al. Cureus 13(4): e14415. DOI 10.7759/cureus.14415 4 of 7
month of the intrauterine period. The structure of the scalp is different from the skin structures of other
body parts owing to the inductive interaction between the two different germ layers, ectoderm and
mesoderm [11]. On the other hand, the periosteum, which is very effective in the growth, repair, and blood
supply of the bone, is not yet fully understood and has been a subject of still existing controversy and debate
[12].
The periosteum histologically consists of outer and inner layers. The superficial part of the outer "fibrous
layer” contains a higher level of vascularization with a rich neural network and significantly contributes to
the blood flow of the bone and adjacent muscle tissue. The profound section of the outer layer has less
vascularization and is collagenous with a small number of cells. On the other hand, the inner "cambium
layer" is rich in cells, consisting of mesenchymal progenitor cells, differentiated osteogenic progenitors,
osteoblasts, and fibroblasts. Osteoblasts are in contact with the bone layer, and pericytes with high
osteoblastic potential are also found in the region due to rich vascular network [12]. The cambium layer is
thickest in fetal life and gets thinner with age [13]. The calvarial periosteum, which is the area of interest in
our study, has less osteogenic potential compared to the other forms of periosteum [14,15].
A reciprocal interplay between osteogenic progenitor cells of the periosteum, cytokines, and growth factors
in the hematoma along with birth trauma have been found to play a role in the formation of
cephalohematoma ossification [16]. Cephalohematoma progresses to ossification in approximately 3%-5%
of cases [17,18]. Although there is a controversy between the initial size of cephalohematoma and its
progression to ossification in the literature, data from case reports suggest that massive cephalohematomas
at presentation have a higher potential for progression to ossifying cephalohematomas [19].
In our study, the rate of ossification was 9.57%, and we believe that the relatively higher ratio in our study
might be due to a selection bias since we included only the cases with a transverse/vertical diameter of >50
mm. No ossification was observed in any newborns with a cephalohematoma transverse/vertical diameter
below 50 mm, which we did not include in the study (data not shown).
The optimum treatment of choice for cephalohematoma is still controversial due to its potential for a benign
clinical course. Hence, the common approach is usually observational and conservative as the
cephalohematoma is gradually absorbed as the skull develops; however, this process usually takes three to
six months [20] (Figure 4).
FIGURE 4: The ossified tissue by the expanding calvarium, and the
physical appearance usually turns to normal before the age of one year.
Printed with permission from Dr. Gokhan CANAZ©, Department of Neurosurgery, Arnavutkoy State Hospital.
In some reports, aspiration is recommended for nonabsorbable cephalohematoma after one month, whereas
some do not advise aspiration due to the increased risk of infection [21-23].
Firlik and Adelson suggested puncture for all cephalohematoma cases after one month of observation in
order to avoid scalp deformity and delayed open surgery [24]. Another study advocated aspiration under local
anesthesia earlier than one month to avoid difficult evacuation as a result of premature ossification [25]. The
procedure was performed using a 20G spinal needle, without a drain insertion, and a routine coverage with
sterile surgical dressing for 24 hours. Eseonu et al. reported an early surgical evacuation of a large
cephalohematoma (>70 mm) by small scalp incision under general anesthesia in a neonate at two weeks of
age, suggesting that a complicated surgery might be avoided by early intervention [26].
Despite their promising data with the surgical aspiration of both large and unossified cephalohematoma
cases, both methods have some potential restraints. Firstly, although local anesthetic drugs of the amide
group are usually considered safe, they may cause adverse events and toxic effects in infants even in case of
an administration of the minimal dose. The reports have suggested toxic activity and methemoglobinemia in
2021 Üçer et al. Cureus 13(4): e14415. DOI 10.7759/cureus.14415 5 of 7
various surgical practices of the infant with these agents [27]. It should be noted that systemic interference
might be possible with the absorption of the anesthetic agent when applied on the areas with higher
absorption rate, and minimal portions of local anesthetic agent might also penetrate through the ruptured
small blood vessels in the cephalohematoma cases into the systemic circulation. Also, the possible causes of
impaired clearance and G6PD deficiency should be of concern while local anesthetics are given to neonates,
and the procedures might be performed in operating room conditions instead of an outpatient setting in
order to provide access to emergency intervention condition in case of toxicity.
On the other hand, surgery of infants possesses its own specific drawbacks, including the requirement of
fasting, insertion of a drain for larger hematoma cases, and limited access of the mother and caregiving
medical team to the newborn to nurse and calm down the newborn during the postoperative follow-up
period. In addition, needle aspiration is related to an increased incidence of infection mainly with strains of
Escherichia coli and Staphylococcus aureus; however, cephalohematoma itself is a risk factor for spontaneous
infection with these agents [28]. Hence, close observation of the infant is essential following an invasive
procedure, in the presence of a large hematoma, or during any incidents of irritability, fever, or lethargy in
parallel to laboratory examination with markers of local or systemic infection. An USG-guidance and use of
an echogenic needle are recommended for the needle aspiration process to rule of puncture-related
complications.
Despite the diversity and lack of definite consensus on treatment opinions, the most widely accepted criteria
for surgery includes cases with cosmetic deformity, confirmed cases of restricted brain growth, and cases
with associated craniosynostosis [2,29]. Surgery might be challenging since complicated cases with
ossification undergo surgical reshaping of the skull via trimming the deformed bony prominences, and
experience is needed to reconstruct the depressed skull portions in order to correct the cosmetic deformity
and restore the anatomical form [30].
Although the ossification rate was 9.57% in our series, we did not apply any kind of surgical intervention
and observed that all ossifications of the developing cases are resorbed within one year; thus, we
recommend a close observation with concomitant follow-up visits until the age of one year prior to an
ultimate surgical decision.
Conclusions
Cephalohematoma is usually a lesion that disappears spontaneously within the first few months of
postnatal life without any cosmetic deformities. Ossifying cephalohematoma is a rare clinical condition.
Since any intervention is generally not required in the routine treatment of cases with hematomas > 50 mm
in size, unless there is a clinical indication, surgery should be performed only for cosmetic purposes after the
age of one year.
Additional Information
Disclosures
Human subjects: Consent was obtained or waived by all participants in this study. University of Health
Sciences Clinical Audit Team issued approval 2014/112. Animal subjects: All authors have confirmed that
this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE
uniform disclosure form, all authors declare the following: Payment/services info: All authors have
declared that no financial support was received from any organization for the submitted work. Financial
relationships: All authors have declared that they have no financial relationships at present or within the
previous three years with any organizations that might have an interest in the submitted work. Other
relationships: All authors have declared that there are no other relationships or activities that could appear
to have influenced the submitted work.
References
1. Tan KL: Cephalhaematoma. Aust NZ J Obstet Gynaecol. 1970, 10:101-6. 10.1111/j.1479-828x.1970.tb00410.x
2. Yoon SD, Cho BM, Oh SM, Park SH: Spontaneous resorption of calcified cephalhematoma in a 9-month-old
child: case report. Childs Nerv Syst. 2013, 29:517-9. 10.1007/s00381-012-2008-1
3. Singh I, Rohilla S, Siddiqui SA, Kumar P: Growing skull fractures: guidelines for early diagnosis and surgical
management. Childs Nerv Syst. 2016, 32:1117-22. 10.1007/s00381-016-3061-y
4. Nicholson L: Caput succedaneum and cephalohematoma: the cs that leave bumps on the head . Neonatal
Netw. 2007, 26:277-81. 10.1891/0730-0832.26.5.277
5. Petrikovsky BM, Schneider E, Smith-Levitin M: Cephalhematoma and caput succedaneum: do they always
occur in labor?. Am J Obstet Gynecol. 1998, 179:906-8. 10.1016/s0002-9378(98)70187-5
6. Kendall N, Woloshin H: Cephalhematoma associated with fracture of the skull . J Pediatr. 1952, 41:125-32.
10.1016/s0022-3476(52)80047-2
7. Fan HC, Hua YM, Juan CJ, Fang YM, Cheng SN, Wang CC: Infected cephalohematoma associated with sepsis
and scalp cellulitis: a case report. J Microbiol Immunol Infect. 2002, 35:125-8.
8. Wong CH, Foo CL, Seow WT: Calcified cephalohematoma: classification, indications for surgery and
techniques. J Craniofac Surg. 2006, 17:970-9. 10.1097/01.scs.0000229552.82081.de
9. Gynecology and Obstetrics Commission Report . (2017). Accessed: January 15, 2021:
http://www.istanbulsaglik.gov.tr/w/anasayfalinkler/belge/ekutuphane/kadin_hastaliklari_ve_dogum_bransi_komisyon_calis...
2021 Üçer et al. Cureus 13(4): e14415. DOI 10.7759/cureus.14415 6 of 7
10. Liu L, Dong C, Chen L: Surgical treatment of ossified cephalhematoma: a case report and review of the
literature. World Neurosurg. 2016, 96:614-7. 10.1016/j.wneu.2016.08.024
11. Carlson BM: Human Embryology and Developmental Biology. 6th Edition. Elsevier Health Sciences, New
York, NY; 2018.
12. Dwek JR: The periosteum: what is it, where is it, and what mimics it in its absence? . Skeletal Radiol. 2010,
39:319-23. 10.1007/s00256-009-0849-9
13. Allen MR, Hock JM, Burr DB: Periosteum: biology, regulation, and response to osteoporosis therapies . Bone.
2004, 35:1003-12. 10.1016/j.bone.2004.07.014
14. Uddströmer L: The osteogenic capacity of tubular and membranous bone periosteum. A qualitative and
quantitative experimental study in growing rabbits. Scand J Plast Reconstr Surg. 1978, 12:195-205.
10.3109/02844317809012995
15. Bilkay U, Tokat C, Helvaci E, Ozek C, Zekioglu O, Onat T, Songur E: Osteogenic capacities of tibial and
cranial periosteum: a biochemical and histologic study. J Craniofac Surg. 2008, 19:453-8.
10.1097/SCS.0b013e318052fe3d
16. Rosenberg AE: Bone, joints and soft tissue tumors . Robbins & Cotran Pathologic Basis of Disease. 7th
Edition. Kumar V, Abbas AK, Fausto N (ed): Elsevier Saunders, Philadelphia, PA; 2005. 1288.
17. Nguyen DC, Patel KB, Woo AS, Kane AA, Smyth MD: Endoscopic-assisted treatment of sagittal
craniosynostosis and calcified cephalohematoma. J Craniofac Surg. 2014, 25:2127-9.
10.1097/SCS.0000000000001092
18. Daglioglu E, Okay O, Hatipoglu HG, Dalgic A, Ergungor F: Spontaneous resolution of calcified
cephalhematomas of infancy: report of two cases. Turk Neurosurg. 2010, 20:96-9.
19. Kaiser GL, Oesch V: Sagittal craniosynostosis combined with ossified cephalhematoma - a tricky and
demanding puzzle. Childs Nerv Syst. 2009, 25:103-10. 10.1007/s00381-008-0726-1
20. Guclu B, Yalcinkaya U, Kazanci B, Adilay U, Ekici MA: Diagnosis and treatment of ossified cephalhematoma .
J Craniofac Surg. 2012, 23:505-7. 10.1097/SCS.0b013e318266893c
21. Gupta PK, Mathew GS, Malik AK, Al Derazi T: Ossified cephalhematoma. Pediatr Neurosurg. 2007, 43:492-7.
10.1159/000108793
22. Blom NA, Vreede WB: Infected cephalhematomas associated with osteomyelitis, sepsis and meningitis .
Pediatr Infect Dis J. 1993, 12:1015-7. 10.1097/00006454-199312000-00011
23. Wong CS, Cheah FC: Cephalhematoma infected by Escherichia coli presenting as an extensive scalp abscess .
J Pediatr Surg. 2012, 47:2336-40. 10.1016/j.jpedsurg.2012.09.029
24. Firlik KS, Adelson PD: Large chronic cephalohematoma without calcification. Pediatr Neurosurg. 1999,
30:39-42. 10.1159/000028759
25. Blanc F, Bigorre M, Lamouroux A, Captier G: Early needle aspiration of large infant cephalohematoma: a
safe procedure to avoid esthetic complications. Eur J Pediatr. 2020, 179:265-9. 10.1007/s00431-019-03487-5
26. Eseonu CI, Sacino AN, Ahn ES: Early surgical intervention for a large newborn cephalohematoma . Pediatr
Neurosurg. 2016, 51:210-3. 10.1159/000444195
27. Kuiper-Prins E, Kerkhof GF, Reijnen CG, van Dijken PJ: A 12-day-old boy with methemoglobinemia after
circumcision with local anesthesia (lidocaine/prilocaine). Drug Saf Case Rep. 2016, 3:12. 10.1007/s40800-
016-0033-9
28. Chung HY, Chung JY, Lee DG, Yang JD, Baik BS, Hwang SG, Cho BC: Surgical treatment of ossified
cephalhematoma. J Craniofac Surg. 2004, 15:774-9. 10.1097/00001665-200409000-00015
29. Kandemirli SG, Cingoz M, Bilgin C, Olmaz B: Temporal evolution of imaging Findings in ossified
cephalohematoma. J Craniofac Surg. 2020, 31:375-8. 10.1097/SCS.0000000000006319
30. Liu L, Dong C, Chen L: Surgical treatment of ossified cephalhematoma: a case report and review of the
literature. World Neurosurg. 2016, 96:614-7. 10.1016/j.wneu.2016.08.024
2021 Üçer et al. Cureus 13(4): e14415. DOI 10.7759/cureus.14415 7 of 7
You might also like
- Chapter 14 Pathology RBC and Bleeding Disorders OutlineDocument19 pagesChapter 14 Pathology RBC and Bleeding Disorders OutlineNatan BabekNo ratings yet
- Respiration in Fish N FrogDocument23 pagesRespiration in Fish N FrogHazirah HussinNo ratings yet
- Fetal Macrocrania: Diagnosis, Delivery and Outcomes: Original ArticleDocument4 pagesFetal Macrocrania: Diagnosis, Delivery and Outcomes: Original ArticleAndreas NatanNo ratings yet
- Det 039Document11 pagesDet 039grimmNo ratings yet
- AmniopatchDocument8 pagesAmniopatchYazmin ZavalaNo ratings yet
- Benign Lesions of PinnaDocument4 pagesBenign Lesions of Pinnamanoj kumarNo ratings yet
- Calcified Cephalohematoma in A Newborn Unusual Presentation Without History of Birth TraumaDocument6 pagesCalcified Cephalohematoma in A Newborn Unusual Presentation Without History of Birth Traumafaizu21No ratings yet
- SWAIMANDocument18 pagesSWAIMANVan John MagallanesNo ratings yet
- Journal AsliDocument4 pagesJournal Aslidesi tethunNo ratings yet
- Alwahab2017 Article OccipitalMeningoencephaloceleC PDFDocument4 pagesAlwahab2017 Article OccipitalMeningoencephaloceleC PDFOvamelia JulioNo ratings yet
- Fetal Ventriculomegaly: Postnatal Management: Special Annual IssueDocument3 pagesFetal Ventriculomegaly: Postnatal Management: Special Annual IssueRendra Artha Ida BagusNo ratings yet
- 0390 6663 48 6 1448Document6 pages0390 6663 48 6 1448فاطمة الزهراء أسامةNo ratings yet
- CC Helps in LocalisationDocument9 pagesCC Helps in LocalisationRavi ChandraNo ratings yet
- Craniopharyngiomas: Clinic Al PearlsDocument18 pagesCraniopharyngiomas: Clinic Al PearlsRanintha SurbaktiNo ratings yet
- Retrospective Examination of Infants With Congenital Neural Tube DefectDocument8 pagesRetrospective Examination of Infants With Congenital Neural Tube Defectözkan ilhanNo ratings yet
- Anticoagulacion Neonatos 2019Document11 pagesAnticoagulacion Neonatos 2019edi21arNo ratings yet
- Embryonal Rhabdomyosarcoma of The Uterine - 2014 - Taiwanese Journal of ObstetrDocument3 pagesEmbryonal Rhabdomyosarcoma of The Uterine - 2014 - Taiwanese Journal of ObstetrSami KahtaniNo ratings yet
- Tumor Teratoma Rabdoide RevisiónDocument16 pagesTumor Teratoma Rabdoide RevisiónPaolo Mendoza LeonNo ratings yet
- Manejo de Las Malformaciones Cavernosas Del Tronco Encefálico Pediátrico - Experiencia de Más de 20 Años en El Hospital de Niños EnfermosDocument7 pagesManejo de Las Malformaciones Cavernosas Del Tronco Encefálico Pediátrico - Experiencia de Más de 20 Años en El Hospital de Niños EnfermosGonzalo Leonidas Rojas DelgadoNo ratings yet
- Placenta AccretaDocument5 pagesPlacenta AccretaF3rcitaNo ratings yet
- Cornell A 2003Document13 pagesCornell A 2003dnazaryNo ratings yet
- Paediatric Neck MassesDocument7 pagesPaediatric Neck MassesGiovanni HenryNo ratings yet
- Kim 2015Document4 pagesKim 2015ruthameliapNo ratings yet
- Testicular Microlithiasis1Document4 pagesTesticular Microlithiasis1Ioannis ValioulisNo ratings yet
- Complex Cesarian SectionDocument11 pagesComplex Cesarian SectionHerald Scholarly Open AccessNo ratings yet
- Seminars in Pediatric Surgery: Immediate Versus Staged Repair of OmphalocelesDocument6 pagesSeminars in Pediatric Surgery: Immediate Versus Staged Repair of OmphalocelesGufi GeorgeNo ratings yet
- Diagnosis and Management of Posterior Plagiocephaly: ObjectiveDocument8 pagesDiagnosis and Management of Posterior Plagiocephaly: ObjectiveSelene MéndezNo ratings yet
- Epidemiology of Brachial Plexus Palsy in NewbornsDocument7 pagesEpidemiology of Brachial Plexus Palsy in NewbornsMardyana MardinNo ratings yet
- Cesarean Scar Ectopic Pregnancies: Etiology, Diagnosis, and ManagementDocument9 pagesCesarean Scar Ectopic Pregnancies: Etiology, Diagnosis, and ManagementDinorah MarcelaNo ratings yet
- Pattern of Encephaloceles A Case SeriesDocument4 pagesPattern of Encephaloceles A Case SeriesSayyid Hakam PerkasaNo ratings yet
- Antenatal Diagnosis ofDocument6 pagesAntenatal Diagnosis ofnskhldNo ratings yet
- Fistula in AnoDocument5 pagesFistula in AnoIoannis ValioulisNo ratings yet
- Diagnosis and Management of Congenital Epulis of New-Born: A Rare Case Report and Literature ReviewDocument3 pagesDiagnosis and Management of Congenital Epulis of New-Born: A Rare Case Report and Literature ReviewIsmail YusufNo ratings yet
- Ghan em 2005Document5 pagesGhan em 2005asfwegereNo ratings yet
- 1 PBDocument7 pages1 PBsahrirNo ratings yet
- Teratoma in ChildrenDocument5 pagesTeratoma in ChildrenRif'aNo ratings yet
- LSM RepairDocument4 pagesLSM RepairDanily Faith VillarNo ratings yet
- Radiology RadiologyDocument6 pagesRadiology RadiologyJonathan ReinaldoNo ratings yet
- Sattar M. Hashim JPMCDocument5 pagesSattar M. Hashim JPMCprastiaNo ratings yet
- Pediatric Regional AnesthesiaDocument6 pagesPediatric Regional Anesthesiatq9prx5s5qNo ratings yet
- PapillomaDocument4 pagesPapillomaNovhy GanggutNo ratings yet
- Implantación Endometrial - GenéticaDocument9 pagesImplantación Endometrial - GenéticaMaríaJoséPinedaNo ratings yet
- Early Postoperative Complications Following Myelomeningocele RepairDocument13 pagesEarly Postoperative Complications Following Myelomeningocele RepairAlemsegedNo ratings yet
- Got Her Strom 2013Document10 pagesGot Her Strom 2013Alexia Nadine PuelNo ratings yet
- 10.1.1.546.6206 BedahDocument7 pages10.1.1.546.6206 Bedahfikri hanifNo ratings yet
- Pos Ok Hova 2012Document1 pagePos Ok Hova 2012Suis MionooNo ratings yet
- Editorial: Mass Screening For Fetal Malformations: The Eurofetus StudyDocument4 pagesEditorial: Mass Screening For Fetal Malformations: The Eurofetus StudyRaluca HabaNo ratings yet
- Editorial: Mass Screening For Fetal Malformations: The Eurofetus StudyDocument4 pagesEditorial: Mass Screening For Fetal Malformations: The Eurofetus StudyRaluca HabaNo ratings yet
- Achondroplasia Natural History Study (CLARITY)Document7 pagesAchondroplasia Natural History Study (CLARITY)jakelinelagoadvNo ratings yet
- Episio PDFDocument4 pagesEpisio PDFEduBarranzuelaNo ratings yet
- Jurnal Reading OtologiDocument19 pagesJurnal Reading OtologiMuhammad gusti haryandiNo ratings yet
- Postnatal Outcomes of Fetuses With Prenatal Diagnosis of - En.esDocument10 pagesPostnatal Outcomes of Fetuses With Prenatal Diagnosis of - En.esgaby3005No ratings yet
- Cross 2023 PediatricepilepsysurgeryDocument11 pagesCross 2023 PediatricepilepsysurgeryGala GonzalezNo ratings yet
- Sijog 47 291-296Document6 pagesSijog 47 291-296IcicNo ratings yet
- Elkhunovich2016 The Utility of Cranial Ultrasound For Detection of IntracranialDocument6 pagesElkhunovich2016 The Utility of Cranial Ultrasound For Detection of IntracranialModou NianeNo ratings yet
- DhirendraDocument3 pagesDhirendraBenny KurniawanNo ratings yet
- Ajns 13 233Document5 pagesAjns 13 233Ade Cahyo IslamiNo ratings yet
- Encephalocele Prenatal Diagnosis About A CaseDocument3 pagesEncephalocele Prenatal Diagnosis About A CaseInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Sternocleidomastoid Pseudotumor of Infancy A ReporDocument5 pagesSternocleidomastoid Pseudotumor of Infancy A Reporclaudia_isipNo ratings yet
- BCCBenchmarkingDocument48 pagesBCCBenchmarkingوليد خالدNo ratings yet
- Children 09 01560Document9 pagesChildren 09 01560Susana MartinezNo ratings yet
- Stem Cell Biology and Regenerative Medicine in OphthalmologyFrom EverandStem Cell Biology and Regenerative Medicine in OphthalmologyStephen TsangNo ratings yet
- Jurnal THT 1Document7 pagesJurnal THT 1Vanessa LeatemiaNo ratings yet
- Rhs 4Document6 pagesRhs 4Vanessa LeatemiaNo ratings yet
- Rhs 8Document2 pagesRhs 8Vanessa LeatemiaNo ratings yet
- 564-Article Text-1982-2-10-20220426Document7 pages564-Article Text-1982-2-10-20220426Vanessa LeatemiaNo ratings yet
- Cambridge International AS & A Level: Biology 9700/12Document16 pagesCambridge International AS & A Level: Biology 9700/12lllllisaNo ratings yet
- Spontaneous and Re Ex OvulatorsDocument14 pagesSpontaneous and Re Ex OvulatorsFazal Akbar KhaliliNo ratings yet
- Fetal Growth and DevelopmentDocument25 pagesFetal Growth and Developmentniogan anthonyNo ratings yet
- Anatomy of Flowering PlantsDocument16 pagesAnatomy of Flowering PlantsSadik FsNo ratings yet
- Pain MedicineDocument287 pagesPain MedicineSAJID ALINo ratings yet
- 40th DITF Catalogue 2016Document85 pages40th DITF Catalogue 2016Michael HansenNo ratings yet
- Activity Sheet 1 & 2 GenBio1Document12 pagesActivity Sheet 1 & 2 GenBio1Marlou GayaneloNo ratings yet
- Feyer 2020 WvngoshawkDocument13 pagesFeyer 2020 WvngoshawkAgnieszka CzujkowskaNo ratings yet
- Mother and Child Hospital ReportDocument1 pageMother and Child Hospital ReportclinicallabmchNo ratings yet
- Range of Joint Motion Evaluation Chart: 1. Back 2. Lateral (Flexion)Document2 pagesRange of Joint Motion Evaluation Chart: 1. Back 2. Lateral (Flexion)JulesNo ratings yet
- Science 9 Week 1Document18 pagesScience 9 Week 1lenNo ratings yet
- Aad Abdomen WorksheetDocument15 pagesAad Abdomen WorksheetAchNo ratings yet
- CH 10 Somatic and Special Senses Powerpoint 2006Document175 pagesCH 10 Somatic and Special Senses Powerpoint 2006Maikel PakageNo ratings yet
- Physical AssessmentDocument2 pagesPhysical AssessmentEdmar DalmacioNo ratings yet
- Kelainan Morf EritrositDocument12 pagesKelainan Morf EritrositTrio Singgih SaputroNo ratings yet
- The Effect of Trunk Strengthening Exercise Using Oscillation On Trunk Muscle Thickness and BalanceDocument11 pagesThe Effect of Trunk Strengthening Exercise Using Oscillation On Trunk Muscle Thickness and Balancekang soon cheolNo ratings yet
- Week 2 Endocrine Anatomy and Physiology Review & Pituitary DisturbancesDocument27 pagesWeek 2 Endocrine Anatomy and Physiology Review & Pituitary DisturbancesZiqri Dimas SandyNo ratings yet
- Terdapat Hubungan Kadar Estradiol Dengan Ekspresi Kolagen Dan Elastin Pada Vagina Premenopause Dan PascamenopauseDocument9 pagesTerdapat Hubungan Kadar Estradiol Dengan Ekspresi Kolagen Dan Elastin Pada Vagina Premenopause Dan PascamenopausenawangprimailmiafeeNo ratings yet
- Cloning 1 QPDocument11 pagesCloning 1 QPWayne Mc DonaghNo ratings yet
- Quiz 2Document2 pagesQuiz 2Jovie MasongsongNo ratings yet
- TECH 614 Full Spine I (Castellucci)Document10 pagesTECH 614 Full Spine I (Castellucci)Robert StraubNo ratings yet
- Pulmonary Circulation and Acute Lung Injury, The - UnknownDocument616 pagesPulmonary Circulation and Acute Lung Injury, The - UnknownMixalisKaplanisNo ratings yet
- PDF Test Bank For Essentials of Anatomy Physiology 6Th Edition Frederic H Martini Online Ebook Full ChapterDocument68 pagesPDF Test Bank For Essentials of Anatomy Physiology 6Th Edition Frederic H Martini Online Ebook Full Chapterdaniel.duncan540100% (2)
- Test Bank For Advanced Nutrition and Human Metabolism 7th Edition Gropper Smith Carr 1305627857 9781305627857Document22 pagesTest Bank For Advanced Nutrition and Human Metabolism 7th Edition Gropper Smith Carr 1305627857 9781305627857francisNo ratings yet
- Cileni Cortes Camacho AntologiaDocument62 pagesCileni Cortes Camacho AntologiaCileni CortesNo ratings yet
- Science 5 DLP 1 Human Reproductive SystemDocument12 pagesScience 5 DLP 1 Human Reproductive SystemDyaan TrajicoNo ratings yet
- Host Defense Mechanisms (Non-Specific) : BIO162 Microbiology For Allied Health Page BaluchDocument30 pagesHost Defense Mechanisms (Non-Specific) : BIO162 Microbiology For Allied Health Page Baluchbocah_britpopNo ratings yet
- Gastric Lavage N Gavage Lesson PlanDocument11 pagesGastric Lavage N Gavage Lesson PlanKiran40% (5)