Professional Documents
Culture Documents
GOC 6 Carbocation
GOC 6 Carbocation
Uploaded by
Jain KirtiOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
GOC 6 Carbocation
GOC 6 Carbocation
Uploaded by
Jain KirtiCopyright:
Available Formats
1
Dr. N R PANGULURI (NRP)
+91-9738678240
drnrpanguluri@gmail.com
9. GOC 6 Carbocation Page 1
2
Incomplete octet
Vacant orbital
Free radical
9. GOC 6 Carbocation Page 2
3
(to the system)
(from the system)
9. GOC 6 Carbocation Page 3
4
9. GOC 6 Carbocation Page 4
5
9. GOC 6 Carbocation Page 5
6
only -I
9. GOC 6 Carbocation Page 6
7
Carbocation applications Carbanion applications Carbon freeradical
are used in are used in applications
are used in
Alkenes
Alcohol
Alkylhalide Alkanes
Phenol
Alcohol
Aliphatic acids
Ether
Aromatic acids
carbonyl
9. GOC 6 Carbocation Page 7
8
9. GOC 6 Carbocation Page 8
9
Application of electronic effects
1. Stability of Carbocations :
looking for electrons
2.
neutral
Stabilise Destabilise
-M, -I
+M, HC, +I
EDG always stabilizes carbocation
EWG always destabilizes carbocation
9. GOC 6 Carbocation Page 9
10
3.
strong donating weak donating
strong withdrawing weak withdrawing
4.
Dr. N R PANGULURI (NRP)
+91-9738678240
drnrpanguluri@gmail.com
9. GOC 6 Carbocation Page 10
11
4. Explanation :
Thirsty of water
100 ml, 250 ml, 500 ml
100 rs
10 rs
1 rs
9. GOC 6 Carbocation Page 11
12 (1)
4. Explanation :
9. GOC 6 Carbocation Page 12
12(2) For carbocation
stabilize less
stabilize more Patient
(no effect)
200 km 100 km 50 km
Blood donars
destabilize less destabilizes Patient
no effect more
200 km 100 km 50 km
somebody wants kill and take money
Stability of carbocation can be explained by
1. Resonance or Mesomeric Effect
2. Hyperconjugation & Inductive effect
3. only Inductive Effect
4. HC & M
5. Aromaticity
9. GOC 6 Carbocation Page 13
13
1.
Resonance doesn’t depend on distance
9. GOC 6 Carbocation Page 14
14
2.
9. GOC 6 Carbocation Page 15
15 3. Application of only Inductive effect
I depends on distance
9. GOC 6 Carbocation Page 16
16 3. Application of only Inductive effect
9. GOC 6 Carbocation Page 17
17
moderate
(destabilise less)
9. GOC 6 Carbocation Page 18
18
4.
9. GOC 6 Carbocation Page 19
19
Conclusion :
9. GOC 6 Carbocation Page 20
20
9. GOC 6 Carbocation Page 21
21
5. Stability of carbocation by aromaticity
9. GOC 6 Carbocation Page 22
22 Stability of carbocations in substituted benzenes
1.
3.
2. 4.
Dr. N R PANGULURI (NRP)
+91-9738678240
drnrpanguluri@gmail.com
9. GOC 6 Carbocation Page 23
23 Stability of substituted benzyl carbocations
M & HC is same at Ortho & Para position (don’t depend on distance )
I is diffeerent at Ortho, Meta & Para positions (depends on distance)
destabilizes less
destabilizes more
9. GOC 6 Carbocation Page 24
24
M & HC is same at Ortho & Para position (don’t depend on distance )
I is different at Ortho, Meta & Para positions (depends on distance)
9. GOC 6 Carbocation Page 25
25
9. GOC 6 Carbocation Page 26
26
destabilizes more
destabilizes less
( ) ( )
9. GOC 6 Carbocation Page 27
27
9. GOC 6 Carbocation Page 28
28
9. GOC 6 Carbocation Page 29
29
weak +I strong +I
9. GOC 6 Carbocation Page 30
30
Remember this chart
Exception :
9. GOC 6 Carbocation Page 31
31
Including Exceptions
9. GOC 6 Carbocation Page 32
32
Aromatic
vacant orbital
Dr. N R PANGULURI (NRP)
+91-9738678240
drnrpanguluri@gmail.com
9. GOC 6 Carbocation Page 33
33
9. GOC 6 Carbocation Page 34
34
9. GOC 6 Carbocation Page 35
35
Dr. N R PANGULURI (NRP)
+91-9738678240
drnrpanguluri@gmail.com
9. GOC 6 Carbocation Page 36
You might also like
- 223A Master Packet 2020F 7-29Document36 pages223A Master Packet 2020F 7-29Ashara DemarestNo ratings yet
- (Hydraulic & Pneumatic) Quiz #4Document3 pages(Hydraulic & Pneumatic) Quiz #4mellon3duwenNo ratings yet
- GOC 9 Acidic Nature 1Document24 pagesGOC 9 Acidic Nature 1Jain KirtiNo ratings yet
- C =Kc C =Kc: B) Ө= 18.6°C, P*=819.7 KpaDocument1 pageC =Kc C =Kc: B) Ө= 18.6°C, P*=819.7 KpaPerak HanNo ratings yet
- Radiopharmaceuticals - Izotop CatalogDocument16 pagesRadiopharmaceuticals - Izotop CatalogAlaa Al masriNo ratings yet
- 2.solder Mask Ink Ksm-6188 ROHSDocument15 pages2.solder Mask Ink Ksm-6188 ROHSmatwan29No ratings yet
- Precision Determination of Precious Metals With ICP-OESDocument25 pagesPrecision Determination of Precious Metals With ICP-OESAbdulrahman JradiNo ratings yet
- Fco 03 2021 Ben - AbiDocument11 pagesFco 03 2021 Ben - Abighoyakartanegara206No ratings yet
- Conformation Analysis of Cyclic Compounds - Class 12-16-11-2023Document22 pagesConformation Analysis of Cyclic Compounds - Class 12-16-11-2023aditya panikkarNo ratings yet
- Modeling Reactions On Uniform SurfacesDocument27 pagesModeling Reactions On Uniform Surfacescebajamundi100% (4)
- 101-9014224-B1-Physical and Thermodynamic Property Rev 00Document72 pages101-9014224-B1-Physical and Thermodynamic Property Rev 00HassanNo ratings yet
- Astm D4692 - 1 (En)Document4 pagesAstm D4692 - 1 (En)Emanuele MastrangeloNo ratings yet
- Ge 4 MMW Practice Set 5, A - FDocument6 pagesGe 4 MMW Practice Set 5, A - FAllysa Galang100% (2)
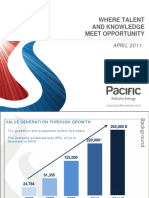
- Where Talent and Knowledge Meet Opportunity: APRIL 2011Document16 pagesWhere Talent and Knowledge Meet Opportunity: APRIL 2011Lin CarlosNo ratings yet
- Department of Defence: ElecteDocument48 pagesDepartment of Defence: ElecteClaudiu RusanNo ratings yet
- Acridones The Fore VisionDocument30 pagesAcridones The Fore VisionKrishnamoorthi BalasubaramanianNo ratings yet
- Sar of BZDDocument8 pagesSar of BZDSomnath MondalNo ratings yet
- (2755205X) Assessed ProblemsDocument12 pages(2755205X) Assessed Problems肖宇翔No ratings yet
- Materials 12 03582 PDFDocument16 pagesMaterials 12 03582 PDFpaulo passeiosNo ratings yet
- Fire Extinguisher Tag Number FinalDocument4 pagesFire Extinguisher Tag Number FinalkambanNo ratings yet
- Maintenance Works Done in O&M Division, Dindi For The Month of September-2019Document4 pagesMaintenance Works Done in O&M Division, Dindi For The Month of September-2019sudhakarNo ratings yet
- MS2019 - Progress Report For LCT and Cleaner Flotation Test ResultsDocument9 pagesMS2019 - Progress Report For LCT and Cleaner Flotation Test ResultsMartin.c.figueroaNo ratings yet
- Specs 10-12 QUEEN DNG71Document5 pagesSpecs 10-12 QUEEN DNG71ABCO SA de CVNo ratings yet
- Superabsorbent PolymerDocument21 pagesSuperabsorbent PolymerIsha MeshramNo ratings yet
- The Procter & Gamble Co.: Issue Date: See TS FormDocument7 pagesThe Procter & Gamble Co.: Issue Date: See TS FormIssam LahlouNo ratings yet
- Inorganic Reactions and Methods, The Formation of Bonds to Transition and Inner-Transition MetalsFrom EverandInorganic Reactions and Methods, The Formation of Bonds to Transition and Inner-Transition MetalsA. P. HagenNo ratings yet
- Mass Balance Data For Mass Balance M.W G/gmolDocument39 pagesMass Balance Data For Mass Balance M.W G/gmolHarshil TejaniNo ratings yet
- Production, Import/Export, Use, and DisposalDocument5 pagesProduction, Import/Export, Use, and DisposalValeria BarbaranNo ratings yet
- FCO 14a 2021 BEN - KDI - Id.enDocument2 pagesFCO 14a 2021 BEN - KDI - Id.enghoyakartanegara206No ratings yet
- (Download PDF) Organic Chemistry 1st Edition Klein Solutions Manual Full ChapterDocument55 pages(Download PDF) Organic Chemistry 1st Edition Klein Solutions Manual Full Chapterkrebermariya5100% (7)
- GeoSL 2Document3 pagesGeoSL 2jaimesreinoNo ratings yet
- Backwash Characteristics of Granular Activated Carbons (GAC) From AsiaDocument18 pagesBackwash Characteristics of Granular Activated Carbons (GAC) From AsiaMiguel EspañolNo ratings yet
- Aipg 2007 PDFDocument26 pagesAipg 2007 PDFteju patneediNo ratings yet
- SGS Test Report For SNCC Nylon Ink PDFDocument11 pagesSGS Test Report For SNCC Nylon Ink PDFKamruzzaman Sheikh100% (1)
- Emulsões Aula 3 Caracterização de EmulsõesDocument29 pagesEmulsões Aula 3 Caracterização de EmulsõesIsabela CarvalhoNo ratings yet
- MgO in CementDocument10 pagesMgO in Cementchandra shekharNo ratings yet
- 5990 6943enDocument8 pages5990 6943enHoanghanh LequangNo ratings yet
- Total NH NH NH NH 53306.524011 Q (KW) - 145.23864592945: Energy Balance For ConverterDocument3 pagesTotal NH NH NH NH 53306.524011 Q (KW) - 145.23864592945: Energy Balance For ConverterAhmed Qutb AkmalNo ratings yet
- Poly (N.vinylcarbazole) A Selective Review of Its Polymerization, Structure, Properties, and Electrical CharacteristicsDocument98 pagesPoly (N.vinylcarbazole) A Selective Review of Its Polymerization, Structure, Properties, and Electrical CharacteristicsSofía Basurto CerecedaNo ratings yet
- Descriptives: Post Hoc TestsDocument1 pageDescriptives: Post Hoc TestsAhmad FadhilahNo ratings yet
- Smartsil 450 ResultsDocument1 pageSmartsil 450 ResultsAnkita Baban GavadeNo ratings yet
- 1978, SETO - CoarctatinDocument3 pages1978, SETO - CoarctatinMayra FonsecaNo ratings yet
- 6109257Document72 pages6109257varadjoshi41No ratings yet
- Bio Report On TransportDocument9 pagesBio Report On TransportRene Louis SingsonNo ratings yet
- Widatra Otsuka Santoria Widatra Otsuka Santoria Widatra: Nego Tahap IiDocument7 pagesWidatra Otsuka Santoria Widatra Otsuka Santoria Widatra: Nego Tahap Iikholisoh linawatiNo ratings yet
- Test Report: Signed For and On Behalf of SGS-CSTC Standards Technical Services (Shanghai) Co., LTDDocument12 pagesTest Report: Signed For and On Behalf of SGS-CSTC Standards Technical Services (Shanghai) Co., LTDigoratmelNo ratings yet
- Unlocking The Potential of Ferrous Sulfate: C.E.R.E.S. Corporation's Innovative SolutionsDocument269 pagesUnlocking The Potential of Ferrous Sulfate: C.E.R.E.S. Corporation's Innovative SolutionsC.E.R.E.S. CorporationNo ratings yet
- Chapter 5. Potential For Human Exposure: Figure 5-1. Number of NPL Sites With Ethylene Oxide ContaminationDocument27 pagesChapter 5. Potential For Human Exposure: Figure 5-1. Number of NPL Sites With Ethylene Oxide Contaminationhassan.extndNo ratings yet
- Smithsonian LABprimertableDocument1 pageSmithsonian LABprimertablelacosNo ratings yet
- 2022 Ke Kelekema Elimination Round Questions 1 PDFDocument5 pages2022 Ke Kelekema Elimination Round Questions 1 PDFXave BajetNo ratings yet
- FINAL Taganito HPAL Nickel CorporationDocument30 pagesFINAL Taganito HPAL Nickel CorporationFabiano, Jr. BarcenalNo ratings yet
- Cesium Carbonate S 2004 834787Document2 pagesCesium Carbonate S 2004 834787transitory.reificationNo ratings yet
- 4928 4972 4980 4983 Rohs 20230920Document7 pages4928 4972 4980 4983 Rohs 20230920Nerissa CastilloNo ratings yet
- Materials: Quinacridone-Diketopyrrolopyrrole-Based Polymers For Organic Field-Effect TransistorsDocument11 pagesMaterials: Quinacridone-Diketopyrrolopyrrole-Based Polymers For Organic Field-Effect TransistorsData KiswaraNo ratings yet
- CC5 Resin SGSDocument9 pagesCC5 Resin SGSRAJ ANKITNo ratings yet
- Flow Correlation Selection PDFDocument14 pagesFlow Correlation Selection PDFNigel RamkhalawanNo ratings yet
- CHAINGUARD-DB For Filler Dispersion (General)Document29 pagesCHAINGUARD-DB For Filler Dispersion (General)muhammad haziqNo ratings yet
- Coa Aspartame Exp 2023Document1 pageCoa Aspartame Exp 2023libertiNo ratings yet
- CR Talim Bevbour 30092020Document2 pagesCR Talim Bevbour 30092020Sofiane KhelfaouiNo ratings yet
- Cappadocia 2017Document30 pagesCappadocia 2017Rigol GradnjaNo ratings yet
- RF Module ROHSDocument9 pagesRF Module ROHStbws666No ratings yet
- GOC 9 Acidic Nature 1Document24 pagesGOC 9 Acidic Nature 1Jain KirtiNo ratings yet
- Adobe Scan 23 Mar 2023Document4 pagesAdobe Scan 23 Mar 2023Jain KirtiNo ratings yet
- GOC 7 Carbanion and FreeradicalDocument30 pagesGOC 7 Carbanion and FreeradicalJain KirtiNo ratings yet
- GOC 12 TautomerismDocument14 pagesGOC 12 TautomerismJain KirtiNo ratings yet
- 1 GocDocument9 pages1 GocJain KirtiNo ratings yet
- Benzene NotesDocument30 pagesBenzene NotesJain KirtiNo ratings yet
- Topic 5 - Energetics: Average Bond Enthalpy: Born-Haber Cycle Electron Affinity EnthalpyDocument4 pagesTopic 5 - Energetics: Average Bond Enthalpy: Born-Haber Cycle Electron Affinity EnthalpyoscarbecNo ratings yet
- Anthracite PDFDocument2 pagesAnthracite PDFmramos4191No ratings yet
- Wandamassey Resume FinalDocument2 pagesWandamassey Resume Finalapi-385522539No ratings yet
- Introduction To Titrimetric AnalysisDocument50 pagesIntroduction To Titrimetric AnalysisMartha Phasha100% (1)
- Book & Media Reviews: Chemistry Updated by Girolami and Rauchfuss (2), TanakaDocument2 pagesBook & Media Reviews: Chemistry Updated by Girolami and Rauchfuss (2), TanakaMosisa DugasaNo ratings yet
- 03 Modular Skid SystemsDocument16 pages03 Modular Skid Systemsjosethompson100% (2)
- Type of MicroscopesDocument2 pagesType of MicroscopesNina SuzetteNo ratings yet
- Hydranautics - Nitto Membrane Applications, Case Studies, Lessons LearntDocument37 pagesHydranautics - Nitto Membrane Applications, Case Studies, Lessons Learntkalyan patilNo ratings yet
- Modeling and Simulation of Ammonia Synthesis Reactor: Petroleum and Coal January 2006Document10 pagesModeling and Simulation of Ammonia Synthesis Reactor: Petroleum and Coal January 2006Sarang GohNo ratings yet
- DSEAR Risk Assessment FormDocument4 pagesDSEAR Risk Assessment FormAndrei VNo ratings yet
- Differential CentrifugationDocument2 pagesDifferential CentrifugationdevbaljinderNo ratings yet
- Eksmal-K: Special Admixture For The Preparation of Cementitious Mixtures For GroutsDocument1 pageEksmal-K: Special Admixture For The Preparation of Cementitious Mixtures For Grouts1aleksandar_t5349No ratings yet
- Current Situation of Aqueous Arsenic Contamination in Pakistan Focusedon Sindh and Punjab Province Pakistan A Review 2375 4397 1000207Document8 pagesCurrent Situation of Aqueous Arsenic Contamination in Pakistan Focusedon Sindh and Punjab Province Pakistan A Review 2375 4397 1000207aroosa basharatNo ratings yet
- Annealed or Cold-Worked Austenitic Stainless Steel Sheet, Strip, Plate, and Flat BarDocument8 pagesAnnealed or Cold-Worked Austenitic Stainless Steel Sheet, Strip, Plate, and Flat Baralucard375No ratings yet
- Photoinitiated Cross-Linking of A Thiol Methacrylate SystemDocument10 pagesPhotoinitiated Cross-Linking of A Thiol Methacrylate SystemElena SirbuNo ratings yet
- Asme Sec Viii D1 C PT UhtDocument14 pagesAsme Sec Viii D1 C PT Uhtkcp1986No ratings yet
- Transforming Lives. Transforming Materials.: Advanced Co-Rotating Twin Screw ExtruderDocument16 pagesTransforming Lives. Transforming Materials.: Advanced Co-Rotating Twin Screw ExtruderReha YelkenNo ratings yet
- Atmospheric Crude TowerDocument17 pagesAtmospheric Crude Towerhussein ayoubNo ratings yet
- Example Problems - 2017 - Hydraulic Fracturing in Unconventional ReservoirsDocument8 pagesExample Problems - 2017 - Hydraulic Fracturing in Unconventional ReservoirsAndré BrasilNo ratings yet
- Sedimentation and Elutration PDFDocument14 pagesSedimentation and Elutration PDFTapas Das50% (4)
- G-Protein Coupled Receptors and Their Signaling MechanismDocument21 pagesG-Protein Coupled Receptors and Their Signaling Mechanismaroob9mahamoodaurinNo ratings yet
- Answers & Solutions: For For For For For JEE (MAIN) - 2021 (Online) Phase-4Document25 pagesAnswers & Solutions: For For For For For JEE (MAIN) - 2021 (Online) Phase-4Insta GramNo ratings yet
- 1 s2.0 S0360319921005115 MainDocument14 pages1 s2.0 S0360319921005115 MainPatrice PariNo ratings yet
- C21 APR 2024 Regular TimetableDocument44 pagesC21 APR 2024 Regular Timetabledarkstore024No ratings yet
- CVE - 131 - 1 - Lecture 2Document15 pagesCVE - 131 - 1 - Lecture 2ZxeroNo ratings yet
- Sika Solutions For Concrete BridgesDocument17 pagesSika Solutions For Concrete BridgescaapromoNo ratings yet
- L23-30H GenSet TierIIDocument325 pagesL23-30H GenSet TierIIyogi sandiNo ratings yet
- Earth-Science-exogenic ProcessesDocument33 pagesEarth-Science-exogenic ProcessesKc MandingNo ratings yet
- Analysis of Some Cod-Desalting Process VariablesDocument6 pagesAnalysis of Some Cod-Desalting Process VariablesMarion MarchettiNo ratings yet