Professional Documents
Culture Documents
Preparation of Toilet Soaps
Preparation of Toilet Soaps
Uploaded by
pradyumansharmaj333Copyright:
Available Formats
You might also like
- Soap Making Guide With Recipes: DIY Homemade Soapmaking Made Easy: DIY Homemade Soapmaking Made EasyFrom EverandSoap Making Guide With Recipes: DIY Homemade Soapmaking Made Easy: DIY Homemade Soapmaking Made EasyRating: 4.5 out of 5 stars4.5/5 (17)
- Practicals InvestagatoryDocument3 pagesPracticals InvestagatorysanyamskismnNo ratings yet
- Preparation of SoapDocument13 pagesPreparation of Soapeyasu milkiasNo ratings yet
- Investigation of Foaming Capacity of Different Washing SoapDocument5 pagesInvestigation of Foaming Capacity of Different Washing SoapAshwin Brahmane40% (15)
- Chapter IV Soap and DetergenDocument6 pagesChapter IV Soap and Detergenpuguh diknaNo ratings yet
- Preaparation of Nail, Polish, Soap, Nail Polish Remover EtcDocument48 pagesPreaparation of Nail, Polish, Soap, Nail Polish Remover EtcShubham Sehgal100% (1)
- Preparation and Properties of A Soap: ObjectiveDocument3 pagesPreparation and Properties of A Soap: ObjectiveBodhi Satwa MishraNo ratings yet
- Soap ReportDocument16 pagesSoap ReportAddison JuttieNo ratings yet
- Sree Narayana Guru Central SchoolDocument14 pagesSree Narayana Guru Central SchoolgreeshmaNo ratings yet
- EXP6 Soap and DetergentheheDocument19 pagesEXP6 Soap and DetergenthehesamengNo ratings yet
- Fatty Material of Different Soap SamplesDocument4 pagesFatty Material of Different Soap Samplesrohini1997No ratings yet
- Chem Proj 1Document10 pagesChem Proj 1Mrinaal RoshanNo ratings yet
- Soap and SaponificationDocument3 pagesSoap and Saponificationemmet11No ratings yet
- Lab 6 (Soaps & Detergents)Document21 pagesLab 6 (Soaps & Detergents)AmeerRashidNo ratings yet
- SoapDocument6 pagesSoapkaram13No ratings yet
- Foaming Capacities of Soaps and DetergentDocument11 pagesFoaming Capacities of Soaps and DetergentShreya GhoshNo ratings yet
- Aim Is To Investigate Foaming Capacity of Different Washing Soap and Effect of Addition of Sodium Carbonate On ThemDocument3 pagesAim Is To Investigate Foaming Capacity of Different Washing Soap and Effect of Addition of Sodium Carbonate On ThemSachin SoniNo ratings yet
- Preparation of SoapDocument16 pagesPreparation of SoapNurr Hada25% (4)
- Investigation of The Foaming Capacity of Different Washing Soaps and The Effect of Addition of Sodium Carbonate On ItDocument10 pagesInvestigation of The Foaming Capacity of Different Washing Soaps and The Effect of Addition of Sodium Carbonate On ItPrasanna kudale100% (1)
- Soap and Detergent ExperimentDocument17 pagesSoap and Detergent Experimentgeek311283% (6)
- Lab 6Document16 pagesLab 6Nurul AinNo ratings yet
- TOPIC 12 Soaps and DetergentsDocument14 pagesTOPIC 12 Soaps and DetergentsKaynine Kiko50% (2)
- Chemistry Investrigatory Project-Analysis On SoapsDocument13 pagesChemistry Investrigatory Project-Analysis On SoapsDharani DharanNo ratings yet
- Chemistry Project: by Shubham Sehgal Class:-Xii Red Roll Number:-10Document48 pagesChemistry Project: by Shubham Sehgal Class:-Xii Red Roll Number:-10Shubham SehgalNo ratings yet
- Chemistry ProjectDocument26 pagesChemistry ProjectMaster PrateekNo ratings yet
- Effect of Sodium Carbonate On Foaming Capacity of A SoapDocument6 pagesEffect of Sodium Carbonate On Foaming Capacity of A SoapAvi ANo ratings yet
- Chemical Engineering Laboratory-1 (CHE F312) Lab Report Engineering Chemistry Lab E-6 SaponificationDocument12 pagesChemical Engineering Laboratory-1 (CHE F312) Lab Report Engineering Chemistry Lab E-6 SaponificationHritik LalNo ratings yet
- SaponificationDocument4 pagesSaponificationtedy yidegNo ratings yet
- The Chemistry of Soaps and DetergentsDocument33 pagesThe Chemistry of Soaps and DetergentsDimas Dwisardi PutraNo ratings yet
- How Soap Cleans: Metal Salt Sodium Carboxylic Acids WaterDocument2 pagesHow Soap Cleans: Metal Salt Sodium Carboxylic Acids WaterV KimNo ratings yet
- Presentation 1Document22 pagesPresentation 1B59 Diganshu JaiswalNo ratings yet
- Soap Formation FlowchartDocument5 pagesSoap Formation FlowchartTarus BrianNo ratings yet
- The Soap Manufacturing ProcessDocument8 pagesThe Soap Manufacturing ProcessSohinee Tinks100% (2)
- Soap PreparationDocument40 pagesSoap PreparationDesiana Anggraeni100% (1)
- Atikah Punya Jugak...Document26 pagesAtikah Punya Jugak...Ika BakarNo ratings yet
- History and Preparation of Soap and DetergentDocument26 pagesHistory and Preparation of Soap and DetergentCafaso Iniyan100% (1)
- Bche Fa19 018Document8 pagesBche Fa19 018Muhammad AliNo ratings yet
- Why Is Foam CreatedDocument5 pagesWhy Is Foam CreatedSumeet JainNo ratings yet
- Chemistry Form 5 Chapter 5 NoteDocument19 pagesChemistry Form 5 Chapter 5 NoteshashababygewlNo ratings yet
- Lab 6 Uitm. Soap Preparation. Comparison Soap and Detergent Properties.Document16 pagesLab 6 Uitm. Soap Preparation. Comparison Soap and Detergent Properties.niniwani59No ratings yet
- Soap and DtergenetsDocument13 pagesSoap and DtergenetsAkanksha PanigrahyNo ratings yet
- Soap PreperationDocument13 pagesSoap PreperationKatrina MillerNo ratings yet
- Soap and Detergents PDFDocument33 pagesSoap and Detergents PDFsab_franc5286100% (5)
- Project SoapsDocument14 pagesProject Soapskeru9718No ratings yet
- 5.1 Analysing Soap and DetergentDocument28 pages5.1 Analysing Soap and DetergentNor HusseinNo ratings yet
- Soaps and DetergentsDocument18 pagesSoaps and DetergentsVince BagguatanNo ratings yet
- Project ChemistryDocument14 pagesProject ChemistryLIYA ASKARNo ratings yet
- Chemistry NotesDocument5 pagesChemistry NotesalbiNo ratings yet
- Chem ProDocument4 pagesChem ProDeepak PatelNo ratings yet
- Experiment 5Document6 pagesExperiment 5DARREN JOHN MUUWILNo ratings yet
- Soaps Project WorkDocument15 pagesSoaps Project WorkSameer SammuNo ratings yet
- Chemistry Project 11-2Document6 pagesChemistry Project 11-2SCHWARTZ. A cseNo ratings yet
- Lab 6 - Soap and DetergentDocument16 pagesLab 6 - Soap and DetergentamiraaikharahNo ratings yet
- Shruthika PtojectDocument15 pagesShruthika PtojectAbhijithNo ratings yet
- Chemistry Project - Foaming Capacity of SoapDocument10 pagesChemistry Project - Foaming Capacity of Soapdivya7charlesNo ratings yet
- Soap and SaponificationDocument9 pagesSoap and SaponificationGarcia Angela DianneNo ratings yet
- Bar Soap New EditionDocument18 pagesBar Soap New EditionMiracle UzomaNo ratings yet
- Chemistry Investigatory Project 2016-2017: Made By-Rohan Raj ROLL NO-11122 Class-Xi-ADocument5 pagesChemistry Investigatory Project 2016-2017: Made By-Rohan Raj ROLL NO-11122 Class-Xi-AADITYA SAHUNo ratings yet
- Best Tips And Tricks For Soap Making: Time Honored Soap Making TechniquesFrom EverandBest Tips And Tricks For Soap Making: Time Honored Soap Making TechniquesRating: 5 out of 5 stars5/5 (1)
- Grade 9 STE Applied Chem With MELCS TopicsDocument16 pagesGrade 9 STE Applied Chem With MELCS TopicsCaryl Ann C. Sernadilla0% (1)
- Chapter 4-ppt - PDF/ InstrumentationDocument46 pagesChapter 4-ppt - PDF/ Instrumentationregassa rajiNo ratings yet
- CHEM 151 (Quiz 1 (Make-Up) Key)Document3 pagesCHEM 151 (Quiz 1 (Make-Up) Key)Chantel AceveroNo ratings yet
- Shrinkage Values of PolymersDocument4 pagesShrinkage Values of PolymersVijaya SimhaNo ratings yet
- Mass Calculations in Chemical ReactionsDocument61 pagesMass Calculations in Chemical Reactionsrobertbernales2007No ratings yet
- Smooth Endoplasmic ReticulumDocument7 pagesSmooth Endoplasmic Reticulummishiii1006343No ratings yet
- Biosynthesis of PorphyrinsDocument15 pagesBiosynthesis of PorphyrinsShafaqat Ghani Shafaqat GhaniNo ratings yet
- Toxicology Laboratory MIDTERM Autosaved1 MergedDocument49 pagesToxicology Laboratory MIDTERM Autosaved1 MergedrlpmanglicmotNo ratings yet
- Organic Chemistry 2 (CHEM 30) For Bolero Final Exam PDFDocument15 pagesOrganic Chemistry 2 (CHEM 30) For Bolero Final Exam PDFKhangNo ratings yet
- Results and Discussion For CarbohydratesDocument4 pagesResults and Discussion For CarbohydratesDusky25% (4)
- 02 Analytical Profiles of Drug Substances, Vol 02Document571 pages02 Analytical Profiles of Drug Substances, Vol 02nur syaraNo ratings yet
- 1st Week Lec 1-5Document49 pages1st Week Lec 1-5Abhijit NathNo ratings yet
- Chemistry MCQs SSBCrack PDFDocument18 pagesChemistry MCQs SSBCrack PDFutkarsh chaturvediNo ratings yet
- Experiment 8 Chelatometric Analysis of The Complex For Cobalt Using EDTADocument6 pagesExperiment 8 Chelatometric Analysis of The Complex For Cobalt Using EDTAJosef HiltonNo ratings yet
- In Vitro Osteoconductivity of PMMA Y2 - 2022 - Advanced Industrial and EngineeriDocument15 pagesIn Vitro Osteoconductivity of PMMA Y2 - 2022 - Advanced Industrial and EngineeriRauta Robert StefanNo ratings yet
- Many of The Important Properties of Materials Are Due To The Presence of ImperfectionsDocument14 pagesMany of The Important Properties of Materials Are Due To The Presence of ImperfectionsAbdulrahman AlsubieNo ratings yet
- Worksheet Acids Alkalis ks3Document4 pagesWorksheet Acids Alkalis ks3MfanafuthiNo ratings yet
- Calcium-Magnesium by EDTA TitrationDocument5 pagesCalcium-Magnesium by EDTA TitrationnisscriNo ratings yet
- Polymerization: IUPAC DefinitionDocument4 pagesPolymerization: IUPAC DefinitionVeera ManiNo ratings yet
- Hydrothermal Processing of Materials: Past, Present and FutureDocument21 pagesHydrothermal Processing of Materials: Past, Present and FutureSiti AmirahNo ratings yet
- Common Ions Anions and Cations PDFDocument2 pagesCommon Ions Anions and Cations PDFJM GNo ratings yet
- PISR1-En-US SiC Shell & Tube Heat Exchanger - SR SeriesDocument2 pagesPISR1-En-US SiC Shell & Tube Heat Exchanger - SR SeriesViajante_santosNo ratings yet
- ElectrolysisDocument24 pagesElectrolysisSMELLY CATNo ratings yet
- Composition of Carbonated Drinks: Chemistry ProjectDocument16 pagesComposition of Carbonated Drinks: Chemistry ProjectNisith K DasNo ratings yet
- Natsci1 Reference2 Lesson19 PDFDocument8 pagesNatsci1 Reference2 Lesson19 PDFPercen7No ratings yet
- Chemical BondingDocument8 pagesChemical BondingAhmed AbdelghanyNo ratings yet
- Soal ElektrolisisDocument4 pagesSoal ElektrolisisWahyu UswaNo ratings yet
- Elimination Reactions Mechanism Lecture NotesDocument17 pagesElimination Reactions Mechanism Lecture NotesveluselvamaniNo ratings yet
- HL Bonding Questions 2Document4 pagesHL Bonding Questions 2ehodariNo ratings yet
- Corrosion Presentation 1Document27 pagesCorrosion Presentation 1Ishu AttriNo ratings yet
Preparation of Toilet Soaps
Preparation of Toilet Soaps
Uploaded by
pradyumansharmaj333Original Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Preparation of Toilet Soaps
Preparation of Toilet Soaps
Uploaded by
pradyumansharmaj333Copyright:
Available Formats
www.thechemistryguru.
com 1
Preparation of Toilet Soaps
Abstract
Preparation of Toilet Soaps.
Theory
Soaps are water soluble sodium or potassium salt of higher fatty acids.
Soaps are made from fats, arid oils, their acide by treating them
chemically with a strong alkali. The fats and oils used in soap, asking
come from animal or plant sources. Each fat or oil is made up of a
distinctive mixture of several different triglyceride molecule, three fatty
acid molecules are attached to one molecule of glycerin.
There are many types of triglycerides, each type consists of its own
particular combination of fatty acids. Fatty acids are the components of
fats and oils that are used in making soap .they are weak acids and are
composed of two parts. A carboxylic acid group consisting of one
hydrogen (H) atom, two oxygen atoms and one carbon atom, plus a
hydrocarbon chain attached to the carboxylic acid group. The alkali
used in soap making was obtained from ashes of plants, but they are
now made commercially.
Today the term alkali describes a substance that chemically is a base
and that reacts with and neutralizes an acid. The common alkalis used
in soap making are sodium hydroxide, also called caustic soda and
www.thechemistryguru.com 2
potassium hydroxide, also called caustic potash. When soap is shaken,
it produces foam which is responsible for removal of dirt. A soap which
produces more foam is more effective in cleaning. So a best soap among
a group of much soap can be identified by comparing their foaming
capacities. More the time taken for the disappearance of foam more is
its foaming capacity and more efficient it is, in its cleansing action.
Cleansing Action of Soap
Soap is sodium or potassium salt of higher fatty acid and may be
represented as RCOO¯Na+.when dissolved in water, it dissociates into
as RCOO¯ and Na+ ions. The RCOO¯ ions however consist of two parts
a long hydrocarbon chain R (also called non polar tail)which is
hydrophobic (water loving).The RCOO¯ ions are therefore, present on
the surface with their COO¯ group in water and the hydrocarbon chains
are staying away from it and remains at the surface. But at critical
micelle concentration, the anions are pulled into the bulk of the solution
and aggregate to form a spherical shape with their hydrocarbon chains
pointing towards the center of the spheres with COO¯ part remaining
outward on the surphase of the sphere. An aggregate thus formed is
known as ‘ionic micelle’
Soap is an excellent cleanser because of its ability to act as emulsifying
agent. An emulsifier is capable of dispersing one liquid into another
immiscible liquid. This means that while oil does not naturally mix with
water, soap can suspend oil/dirt in such a way that it can be removed.
www.thechemistryguru.com 3
The cleansing action of soap is due to the fact that soap molecules form
micelle around the oil droplet in such a way that hydrophobic parts of
RCOO¯ ions is in the oil droplet and hydrophilic part projects out of
grease droplet like the bristles. Since the polar groups can interact with
water. The oil droplet surrounded by RCOO¯ ions is now pulled in
water and removed from the dirty surface. Thus soaps helps in
emulsification and washing away of oils and fats. The negatively
charged sheath around the globules prevent them from coming together
and forming aggregates
Requirements
COCONUT OIL – 1200mL
STONE POWDER -200g
CAUSTIC SODA -100g
PERFUME -10mL
Water -600Ml
COLOUR -200g
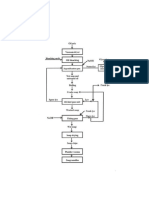
Procedure
1. First of all, NaOH is added to 600mL of water and kept aside for 5
hours.
2. To another container containing 200 mL oil, colour is added and
stireed well.
www.thechemistryguru.com 4
3. To this oil- colour mixture, then added soap stone powder and
stirred well till no precipitate or solid component exists.
4. Then add 1L of oil and mixed well.
5. Now add the dilute NaOH solution prepared at the initial step to
the oil-colour mixture
6. Then the solution is stirred in the clockwise direction for 35-45
minutes in a medium speed.
7. To this perfume is added and stirred well for 1 minute.
8. It is then transferred into the mould.
9. Next day, the mould is removed and the soap is covered with
wrapper.
Result
o Toilet soaps of 30 numbers oval in shape 150g each is prepared
o By preparing the soaps, it is possible to find the contents of it
The soap thus obtained will contain less impurities as it is made
under our presence
o In short, this project is the humble contribution in the quest for
knowledge exploration
You might also like
- Soap Making Guide With Recipes: DIY Homemade Soapmaking Made Easy: DIY Homemade Soapmaking Made EasyFrom EverandSoap Making Guide With Recipes: DIY Homemade Soapmaking Made Easy: DIY Homemade Soapmaking Made EasyRating: 4.5 out of 5 stars4.5/5 (17)
- Practicals InvestagatoryDocument3 pagesPracticals InvestagatorysanyamskismnNo ratings yet
- Preparation of SoapDocument13 pagesPreparation of Soapeyasu milkiasNo ratings yet
- Investigation of Foaming Capacity of Different Washing SoapDocument5 pagesInvestigation of Foaming Capacity of Different Washing SoapAshwin Brahmane40% (15)
- Chapter IV Soap and DetergenDocument6 pagesChapter IV Soap and Detergenpuguh diknaNo ratings yet
- Preaparation of Nail, Polish, Soap, Nail Polish Remover EtcDocument48 pagesPreaparation of Nail, Polish, Soap, Nail Polish Remover EtcShubham Sehgal100% (1)
- Preparation and Properties of A Soap: ObjectiveDocument3 pagesPreparation and Properties of A Soap: ObjectiveBodhi Satwa MishraNo ratings yet
- Soap ReportDocument16 pagesSoap ReportAddison JuttieNo ratings yet
- Sree Narayana Guru Central SchoolDocument14 pagesSree Narayana Guru Central SchoolgreeshmaNo ratings yet
- EXP6 Soap and DetergentheheDocument19 pagesEXP6 Soap and DetergenthehesamengNo ratings yet
- Fatty Material of Different Soap SamplesDocument4 pagesFatty Material of Different Soap Samplesrohini1997No ratings yet
- Chem Proj 1Document10 pagesChem Proj 1Mrinaal RoshanNo ratings yet
- Soap and SaponificationDocument3 pagesSoap and Saponificationemmet11No ratings yet
- Lab 6 (Soaps & Detergents)Document21 pagesLab 6 (Soaps & Detergents)AmeerRashidNo ratings yet
- SoapDocument6 pagesSoapkaram13No ratings yet
- Foaming Capacities of Soaps and DetergentDocument11 pagesFoaming Capacities of Soaps and DetergentShreya GhoshNo ratings yet
- Aim Is To Investigate Foaming Capacity of Different Washing Soap and Effect of Addition of Sodium Carbonate On ThemDocument3 pagesAim Is To Investigate Foaming Capacity of Different Washing Soap and Effect of Addition of Sodium Carbonate On ThemSachin SoniNo ratings yet
- Preparation of SoapDocument16 pagesPreparation of SoapNurr Hada25% (4)
- Investigation of The Foaming Capacity of Different Washing Soaps and The Effect of Addition of Sodium Carbonate On ItDocument10 pagesInvestigation of The Foaming Capacity of Different Washing Soaps and The Effect of Addition of Sodium Carbonate On ItPrasanna kudale100% (1)
- Soap and Detergent ExperimentDocument17 pagesSoap and Detergent Experimentgeek311283% (6)
- Lab 6Document16 pagesLab 6Nurul AinNo ratings yet
- TOPIC 12 Soaps and DetergentsDocument14 pagesTOPIC 12 Soaps and DetergentsKaynine Kiko50% (2)
- Chemistry Investrigatory Project-Analysis On SoapsDocument13 pagesChemistry Investrigatory Project-Analysis On SoapsDharani DharanNo ratings yet
- Chemistry Project: by Shubham Sehgal Class:-Xii Red Roll Number:-10Document48 pagesChemistry Project: by Shubham Sehgal Class:-Xii Red Roll Number:-10Shubham SehgalNo ratings yet
- Chemistry ProjectDocument26 pagesChemistry ProjectMaster PrateekNo ratings yet
- Effect of Sodium Carbonate On Foaming Capacity of A SoapDocument6 pagesEffect of Sodium Carbonate On Foaming Capacity of A SoapAvi ANo ratings yet
- Chemical Engineering Laboratory-1 (CHE F312) Lab Report Engineering Chemistry Lab E-6 SaponificationDocument12 pagesChemical Engineering Laboratory-1 (CHE F312) Lab Report Engineering Chemistry Lab E-6 SaponificationHritik LalNo ratings yet
- SaponificationDocument4 pagesSaponificationtedy yidegNo ratings yet
- The Chemistry of Soaps and DetergentsDocument33 pagesThe Chemistry of Soaps and DetergentsDimas Dwisardi PutraNo ratings yet
- How Soap Cleans: Metal Salt Sodium Carboxylic Acids WaterDocument2 pagesHow Soap Cleans: Metal Salt Sodium Carboxylic Acids WaterV KimNo ratings yet
- Presentation 1Document22 pagesPresentation 1B59 Diganshu JaiswalNo ratings yet
- Soap Formation FlowchartDocument5 pagesSoap Formation FlowchartTarus BrianNo ratings yet
- The Soap Manufacturing ProcessDocument8 pagesThe Soap Manufacturing ProcessSohinee Tinks100% (2)
- Soap PreparationDocument40 pagesSoap PreparationDesiana Anggraeni100% (1)
- Atikah Punya Jugak...Document26 pagesAtikah Punya Jugak...Ika BakarNo ratings yet
- History and Preparation of Soap and DetergentDocument26 pagesHistory and Preparation of Soap and DetergentCafaso Iniyan100% (1)
- Bche Fa19 018Document8 pagesBche Fa19 018Muhammad AliNo ratings yet
- Why Is Foam CreatedDocument5 pagesWhy Is Foam CreatedSumeet JainNo ratings yet
- Chemistry Form 5 Chapter 5 NoteDocument19 pagesChemistry Form 5 Chapter 5 NoteshashababygewlNo ratings yet
- Lab 6 Uitm. Soap Preparation. Comparison Soap and Detergent Properties.Document16 pagesLab 6 Uitm. Soap Preparation. Comparison Soap and Detergent Properties.niniwani59No ratings yet
- Soap and DtergenetsDocument13 pagesSoap and DtergenetsAkanksha PanigrahyNo ratings yet
- Soap PreperationDocument13 pagesSoap PreperationKatrina MillerNo ratings yet
- Soap and Detergents PDFDocument33 pagesSoap and Detergents PDFsab_franc5286100% (5)
- Project SoapsDocument14 pagesProject Soapskeru9718No ratings yet
- 5.1 Analysing Soap and DetergentDocument28 pages5.1 Analysing Soap and DetergentNor HusseinNo ratings yet
- Soaps and DetergentsDocument18 pagesSoaps and DetergentsVince BagguatanNo ratings yet
- Project ChemistryDocument14 pagesProject ChemistryLIYA ASKARNo ratings yet
- Chemistry NotesDocument5 pagesChemistry NotesalbiNo ratings yet
- Chem ProDocument4 pagesChem ProDeepak PatelNo ratings yet
- Experiment 5Document6 pagesExperiment 5DARREN JOHN MUUWILNo ratings yet
- Soaps Project WorkDocument15 pagesSoaps Project WorkSameer SammuNo ratings yet
- Chemistry Project 11-2Document6 pagesChemistry Project 11-2SCHWARTZ. A cseNo ratings yet
- Lab 6 - Soap and DetergentDocument16 pagesLab 6 - Soap and DetergentamiraaikharahNo ratings yet
- Shruthika PtojectDocument15 pagesShruthika PtojectAbhijithNo ratings yet
- Chemistry Project - Foaming Capacity of SoapDocument10 pagesChemistry Project - Foaming Capacity of Soapdivya7charlesNo ratings yet
- Soap and SaponificationDocument9 pagesSoap and SaponificationGarcia Angela DianneNo ratings yet
- Bar Soap New EditionDocument18 pagesBar Soap New EditionMiracle UzomaNo ratings yet
- Chemistry Investigatory Project 2016-2017: Made By-Rohan Raj ROLL NO-11122 Class-Xi-ADocument5 pagesChemistry Investigatory Project 2016-2017: Made By-Rohan Raj ROLL NO-11122 Class-Xi-AADITYA SAHUNo ratings yet
- Best Tips And Tricks For Soap Making: Time Honored Soap Making TechniquesFrom EverandBest Tips And Tricks For Soap Making: Time Honored Soap Making TechniquesRating: 5 out of 5 stars5/5 (1)
- Grade 9 STE Applied Chem With MELCS TopicsDocument16 pagesGrade 9 STE Applied Chem With MELCS TopicsCaryl Ann C. Sernadilla0% (1)
- Chapter 4-ppt - PDF/ InstrumentationDocument46 pagesChapter 4-ppt - PDF/ Instrumentationregassa rajiNo ratings yet
- CHEM 151 (Quiz 1 (Make-Up) Key)Document3 pagesCHEM 151 (Quiz 1 (Make-Up) Key)Chantel AceveroNo ratings yet
- Shrinkage Values of PolymersDocument4 pagesShrinkage Values of PolymersVijaya SimhaNo ratings yet
- Mass Calculations in Chemical ReactionsDocument61 pagesMass Calculations in Chemical Reactionsrobertbernales2007No ratings yet
- Smooth Endoplasmic ReticulumDocument7 pagesSmooth Endoplasmic Reticulummishiii1006343No ratings yet
- Biosynthesis of PorphyrinsDocument15 pagesBiosynthesis of PorphyrinsShafaqat Ghani Shafaqat GhaniNo ratings yet
- Toxicology Laboratory MIDTERM Autosaved1 MergedDocument49 pagesToxicology Laboratory MIDTERM Autosaved1 MergedrlpmanglicmotNo ratings yet
- Organic Chemistry 2 (CHEM 30) For Bolero Final Exam PDFDocument15 pagesOrganic Chemistry 2 (CHEM 30) For Bolero Final Exam PDFKhangNo ratings yet
- Results and Discussion For CarbohydratesDocument4 pagesResults and Discussion For CarbohydratesDusky25% (4)
- 02 Analytical Profiles of Drug Substances, Vol 02Document571 pages02 Analytical Profiles of Drug Substances, Vol 02nur syaraNo ratings yet
- 1st Week Lec 1-5Document49 pages1st Week Lec 1-5Abhijit NathNo ratings yet
- Chemistry MCQs SSBCrack PDFDocument18 pagesChemistry MCQs SSBCrack PDFutkarsh chaturvediNo ratings yet
- Experiment 8 Chelatometric Analysis of The Complex For Cobalt Using EDTADocument6 pagesExperiment 8 Chelatometric Analysis of The Complex For Cobalt Using EDTAJosef HiltonNo ratings yet
- In Vitro Osteoconductivity of PMMA Y2 - 2022 - Advanced Industrial and EngineeriDocument15 pagesIn Vitro Osteoconductivity of PMMA Y2 - 2022 - Advanced Industrial and EngineeriRauta Robert StefanNo ratings yet
- Many of The Important Properties of Materials Are Due To The Presence of ImperfectionsDocument14 pagesMany of The Important Properties of Materials Are Due To The Presence of ImperfectionsAbdulrahman AlsubieNo ratings yet
- Worksheet Acids Alkalis ks3Document4 pagesWorksheet Acids Alkalis ks3MfanafuthiNo ratings yet
- Calcium-Magnesium by EDTA TitrationDocument5 pagesCalcium-Magnesium by EDTA TitrationnisscriNo ratings yet
- Polymerization: IUPAC DefinitionDocument4 pagesPolymerization: IUPAC DefinitionVeera ManiNo ratings yet
- Hydrothermal Processing of Materials: Past, Present and FutureDocument21 pagesHydrothermal Processing of Materials: Past, Present and FutureSiti AmirahNo ratings yet
- Common Ions Anions and Cations PDFDocument2 pagesCommon Ions Anions and Cations PDFJM GNo ratings yet
- PISR1-En-US SiC Shell & Tube Heat Exchanger - SR SeriesDocument2 pagesPISR1-En-US SiC Shell & Tube Heat Exchanger - SR SeriesViajante_santosNo ratings yet
- ElectrolysisDocument24 pagesElectrolysisSMELLY CATNo ratings yet
- Composition of Carbonated Drinks: Chemistry ProjectDocument16 pagesComposition of Carbonated Drinks: Chemistry ProjectNisith K DasNo ratings yet
- Natsci1 Reference2 Lesson19 PDFDocument8 pagesNatsci1 Reference2 Lesson19 PDFPercen7No ratings yet
- Chemical BondingDocument8 pagesChemical BondingAhmed AbdelghanyNo ratings yet
- Soal ElektrolisisDocument4 pagesSoal ElektrolisisWahyu UswaNo ratings yet
- Elimination Reactions Mechanism Lecture NotesDocument17 pagesElimination Reactions Mechanism Lecture NotesveluselvamaniNo ratings yet
- HL Bonding Questions 2Document4 pagesHL Bonding Questions 2ehodariNo ratings yet
- Corrosion Presentation 1Document27 pagesCorrosion Presentation 1Ishu AttriNo ratings yet