Professional Documents
Culture Documents
Article 1685528017
Article 1685528017
Uploaded by
mohdamer161616Copyright:
Available Formats
You might also like
- Narrative Report-Virtual Parent's OrientationnDocument3 pagesNarrative Report-Virtual Parent's OrientationnApril Ramos Dimayuga95% (21)
- 50 Tapping Scripts Gene MonterastelliDocument27 pages50 Tapping Scripts Gene Monterastellilalek00100% (5)
- Rare Case of Bicornuate Uterus With Fetal Death in A Multipara WomanDocument2 pagesRare Case of Bicornuate Uterus With Fetal Death in A Multipara WomanInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Uterine Rupture in Healthy Uterus Complication of Misoprostol (About Two Cases and Review of The Literature)Document3 pagesUterine Rupture in Healthy Uterus Complication of Misoprostol (About Two Cases and Review of The Literature)International Journal of Innovative Science and Research TechnologyNo ratings yet
- Ovarian Pregnancy: Two Case Reports.: Jayeeta Roy, Anindita Sinha BabuDocument5 pagesOvarian Pregnancy: Two Case Reports.: Jayeeta Roy, Anindita Sinha BabuAndriyani MuslimNo ratings yet
- Interstitial Ectopic Pregnancy A Case ReportDocument4 pagesInterstitial Ectopic Pregnancy A Case ReportRina Auliya WahdahNo ratings yet
- Ovarian Ectopic Pregnancy: A Rare Case: Iran J Reprod Med Vol. 12. No. 4. PP: 281-284, April 2014Document4 pagesOvarian Ectopic Pregnancy: A Rare Case: Iran J Reprod Med Vol. 12. No. 4. PP: 281-284, April 2014alif bagusNo ratings yet
- Ruptured Ectopic Pregnancy in The Presence of An Intrauterine DeviceDocument5 pagesRuptured Ectopic Pregnancy in The Presence of An Intrauterine DevicePatrick NunsioNo ratings yet
- Cervical Tear: A Rare Route of Delivery: Case Report: Bhaktii Kohli and Madhu NagpalDocument2 pagesCervical Tear: A Rare Route of Delivery: Case Report: Bhaktii Kohli and Madhu NagpalAisyah MajidNo ratings yet
- 3930 14694 1 PBDocument3 pages3930 14694 1 PBSilvia RNo ratings yet
- Acute Uterine InversionDocument6 pagesAcute Uterine InversionBima GhovaroliyNo ratings yet
- Spontaneous Rupture of An Unscarred GravidDocument4 pagesSpontaneous Rupture of An Unscarred GraviddelaNo ratings yet
- Case Report Ghazanfar Page 130-132Document3 pagesCase Report Ghazanfar Page 130-132sohailurologistNo ratings yet
- Chronic Non-Puerperal Uterine Inversion Mimicking Complete Uterine ProlapsedDocument6 pagesChronic Non-Puerperal Uterine Inversion Mimicking Complete Uterine ProlapsedCentral Asian StudiesNo ratings yet
- Myoma Praevia in A Pregnancy Carried To Term: A Case Report and Updated Review of The LiteratureDocument3 pagesMyoma Praevia in A Pregnancy Carried To Term: A Case Report and Updated Review of The LiteratureInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Artigo Gravidez EctópicaDocument2 pagesArtigo Gravidez EctópicaKevin UchoaNo ratings yet
- Trial of Labor at The Maternity Department of The Sourosanou University Hospital (Chuss) : Clinical Aspects, The Maternal and Perinatal PrognosticsDocument8 pagesTrial of Labor at The Maternity Department of The Sourosanou University Hospital (Chuss) : Clinical Aspects, The Maternal and Perinatal PrognosticsIJAR JOURNALNo ratings yet
- Ectopic Pregnancy: Clinical PracticeDocument9 pagesEctopic Pregnancy: Clinical PracticeNurul WulandariNo ratings yet
- Case Report: Retained Intrauterine Device (IUD) : Triple Case Report and Review of The LiteratureDocument9 pagesCase Report: Retained Intrauterine Device (IUD) : Triple Case Report and Review of The LiteratureYosie Yulanda PutraNo ratings yet
- Acute Inversion of The Uterus PDFDocument6 pagesAcute Inversion of The Uterus PDFEvelyn TingNo ratings yet
- Diagnosis and Management of Ectopic Pregnancy-A Basic View Through LiteratureDocument2 pagesDiagnosis and Management of Ectopic Pregnancy-A Basic View Through LiteraturefprasasyaaNo ratings yet
- Case Report: Ruptured Ectopic Pregnancy in Caesarean Section Scar: A Case ReportDocument4 pagesCase Report: Ruptured Ectopic Pregnancy in Caesarean Section Scar: A Case ReportwennyNo ratings yet
- Total and Acute Uterine Inversion After Delivery A Case Report PDFDocument4 pagesTotal and Acute Uterine Inversion After Delivery A Case Report PDFatika sgrtNo ratings yet
- CessarDocument4 pagesCessarkknsNo ratings yet
- Cervical Ectopic Pregnancy After in Vitro Fertilization: Case Report Successfully Treated With Cervical Electric AspirationDocument5 pagesCervical Ectopic Pregnancy After in Vitro Fertilization: Case Report Successfully Treated With Cervical Electric AspirationMatias Alarcon ValdesNo ratings yet
- Primary Abdominal Ectopic Pregnancy: A Case ReportDocument4 pagesPrimary Abdominal Ectopic Pregnancy: A Case ReportYared TJNo ratings yet
- Merced 2019Document14 pagesMerced 2019Vanessa CarinoNo ratings yet
- UC Irvine: Clinical Practice and Cases in Emergency MedicineDocument6 pagesUC Irvine: Clinical Practice and Cases in Emergency MedicinePatrick NunsioNo ratings yet
- Ectopic Pregnancy: A ReviewDocument13 pagesEctopic Pregnancy: A ReviewDinorah MarcelaNo ratings yet
- Print 3 FixDocument4 pagesPrint 3 Fixafriskha bulawanNo ratings yet
- Primary Ovarian Abscess in Pregnancy: Case ReportDocument3 pagesPrimary Ovarian Abscess in Pregnancy: Case ReportVinnyRevinaAdrianiNo ratings yet
- Ovarian Torsion in Pregnancy A Case ReportDocument3 pagesOvarian Torsion in Pregnancy A Case ReportCakraEkkyNo ratings yet
- Jurnal Internasional 1Document5 pagesJurnal Internasional 1Ifni AngraeniNo ratings yet
- Complications After Interval Postpartum IntrauterineDocument8 pagesComplications After Interval Postpartum IntrauterinekarlinNo ratings yet
- Insertion and Removal of Intrauterine Devices-AAFPDocument8 pagesInsertion and Removal of Intrauterine Devices-AAFPnouval_iqbalNo ratings yet
- Criog2015 596826Document3 pagesCriog2015 596826charles limbuNo ratings yet
- Spontaneous Heterotopic Pregnancy With Intrauterine Pregnancy Stopped Case Report and Review of LiteratureDocument3 pagesSpontaneous Heterotopic Pregnancy With Intrauterine Pregnancy Stopped Case Report and Review of LiteratureInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Effectiveness of Delayed Absorbable Monofila - 2014 - Taiwanese Journal of ObsteDocument3 pagesEffectiveness of Delayed Absorbable Monofila - 2014 - Taiwanese Journal of ObsteSami KahtaniNo ratings yet
- Bicornuate Uterus With PregnancyDocument2 pagesBicornuate Uterus With Pregnancyain trophisNo ratings yet
- Cases Journal: Torsion of Ovarian Cyst During Pregnancy: A Case ReportDocument3 pagesCases Journal: Torsion of Ovarian Cyst During Pregnancy: A Case ReportAde Gustina SiahaanNo ratings yet
- DTSCH Arztebl Int-112-0693 PDFDocument13 pagesDTSCH Arztebl Int-112-0693 PDFKhoirunnisaSPazkaNo ratings yet
- Myoma in Necrobiosis and PregnancyDocument2 pagesMyoma in Necrobiosis and PregnancyInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Scar PregnancyDocument11 pagesScar Pregnancyasshagab04No ratings yet
- Case Report Large Uterine Fibroids in Pregnancy With Successful Caesarean MyomectomyDocument4 pagesCase Report Large Uterine Fibroids in Pregnancy With Successful Caesarean MyomectomyChidimma VictoryNo ratings yet
- Case Operation Procedure: Unruptured Ectopic Pregnancy With Risk Factor History of MiscarriageDocument1 pageCase Operation Procedure: Unruptured Ectopic Pregnancy With Risk Factor History of Miscarriageria.janitaNo ratings yet
- Srgype 02 00039 PDFDocument4 pagesSrgype 02 00039 PDFkhusnulNo ratings yet
- Case Report: Ruptured Ectopic Pregnancy in Caesarean Section Scar: A Case ReportDocument3 pagesCase Report: Ruptured Ectopic Pregnancy in Caesarean Section Scar: A Case ReportEga MegawatiNo ratings yet
- Cranney Et Al-2017-Acta Obstetricia Et Gynecologica ScandinavicaDocument11 pagesCranney Et Al-2017-Acta Obstetricia Et Gynecologica ScandinavicaAlejandro Romero LinaresNo ratings yet
- Successful Conservative Treatment of Microinvasive Cervical Cancer During PregnancyDocument3 pagesSuccessful Conservative Treatment of Microinvasive Cervical Cancer During PregnancyEres TriasaNo ratings yet
- Acog Practice Bulletin No 191 Tubal Ectopic.38Document13 pagesAcog Practice Bulletin No 191 Tubal Ectopic.38Uma HamNo ratings yet
- The "Vanishing Twin" Syndrome - A Myth or Clinical Reality in The Obstetric Practice?Document3 pagesThe "Vanishing Twin" Syndrome - A Myth or Clinical Reality in The Obstetric Practice?ariniNo ratings yet
- Toolbox: Ectopic PregnancyDocument4 pagesToolbox: Ectopic PregnancyLuciana LorenzaNo ratings yet
- Atb of Amniotic Fluid SludgeDocument20 pagesAtb of Amniotic Fluid SludgeSantiago RollanNo ratings yet
- Articulo Rotura PrimigestaDocument3 pagesArticulo Rotura PrimigestaYeitzmarNo ratings yet
- Ijmr 136 370Document2 pagesIjmr 136 370Eka MegawatiNo ratings yet
- Safety and Efficacy of Misoprostol in Induction of Labour in Prelabour Rupture of Fetal Membrane in Nigerian Women: A Multicenter StudyDocument6 pagesSafety and Efficacy of Misoprostol in Induction of Labour in Prelabour Rupture of Fetal Membrane in Nigerian Women: A Multicenter StudyAnonymous GssdN5No ratings yet
- Case Report: Puerperal Uterine Inversion Managed by The Uterine Balloon TamponadeDocument4 pagesCase Report: Puerperal Uterine Inversion Managed by The Uterine Balloon TamponadeXubair KiyaniNo ratings yet
- 9.radha Et Al.Document3 pages9.radha Et Al.International Journal of Clinical and Biomedical Research (IJCBR)No ratings yet
- 2016 Article 162 PDFDocument8 pages2016 Article 162 PDFefanNo ratings yet
- Advanced Abdominal Pregnancy: The Challenges of Management: Case ReportDocument3 pagesAdvanced Abdominal Pregnancy: The Challenges of Management: Case ReportaldyNo ratings yet
- A Randomized Trial of Misoprostol Compared With Manual Vacuum Aspiration For Incomplete AbortionDocument8 pagesA Randomized Trial of Misoprostol Compared With Manual Vacuum Aspiration For Incomplete AbortionElena ZepedaNo ratings yet
- Treatment Strategy for Unexplained Infertility and Recurrent MiscarriageFrom EverandTreatment Strategy for Unexplained Infertility and Recurrent MiscarriageKeiji KurodaNo ratings yet
- v3.2 Data Risk Analytics User Guide 3-21-2023Document236 pagesv3.2 Data Risk Analytics User Guide 3-21-2023singhpriyank.psNo ratings yet
- Soul Winning TractDocument2 pagesSoul Winning TractPaul Moiloa50% (2)
- Canadian Business English Canadian 7th Edition Guffey Test Bank 1Document20 pagesCanadian Business English Canadian 7th Edition Guffey Test Bank 1tedNo ratings yet
- Service Operation Management: Session 8Document59 pagesService Operation Management: Session 8mukul3087_305865623No ratings yet
- INCREASE CARDIAC OUTPUT Related To Altered Heart Rate RythmDocument3 pagesINCREASE CARDIAC OUTPUT Related To Altered Heart Rate RythmSenyorita KHayeNo ratings yet
- Curriculum Vitae: Dawn M. McbrideDocument15 pagesCurriculum Vitae: Dawn M. McbrideAndrea NemenzoNo ratings yet
- Matabuena vs. CervantesDocument1 pageMatabuena vs. CervantesAndrew GallardoNo ratings yet
- If You Liked Selling The 740 Series You'll Love Selling The New 753/754Document8 pagesIf You Liked Selling The 740 Series You'll Love Selling The New 753/754HINANo ratings yet
- Discovery Products (BD) Ltd-ConsDocument1 pageDiscovery Products (BD) Ltd-ConsUnimart DhanmondiNo ratings yet
- A Management Information SystemDocument15 pagesA Management Information SystemFRAkfurdNo ratings yet
- Chapter 8 Bonding Powerpoint AP ChemDocument68 pagesChapter 8 Bonding Powerpoint AP ChemAbdul jan sultaniNo ratings yet
- The Physical Life of Woman:Advice To The Maiden, Wife and Mother by Napheys, George H. (George Henry), 1842-1876Document192 pagesThe Physical Life of Woman:Advice To The Maiden, Wife and Mother by Napheys, George H. (George Henry), 1842-1876Gutenberg.orgNo ratings yet
- Pile CapDocument44 pagesPile Capbhavik modiNo ratings yet
- Compounding Self AssessmentDocument15 pagesCompounding Self AssessmentLara LaiNo ratings yet
- Identification and Prioritisation of Risk Factors in R&D Projects Based On An R&D Process ModelDocument18 pagesIdentification and Prioritisation of Risk Factors in R&D Projects Based On An R&D Process ModelSravani JonnadulaNo ratings yet
- The Moderation Effect of Age On Adopting E-Payment TechnologyDocument8 pagesThe Moderation Effect of Age On Adopting E-Payment TechnologypirehNo ratings yet
- Purser - Review of Bellanger's OrientiusDocument35 pagesPurser - Review of Bellanger's OrientiusRaphael SoaresNo ratings yet
- At 01 - Introduction To Assurance and Related Services (Incl. Intro To Audit) - QuizzerDocument6 pagesAt 01 - Introduction To Assurance and Related Services (Incl. Intro To Audit) - QuizzerRei-Anne Rea100% (1)
- Week 9 - STS The Good LifeDocument4 pagesWeek 9 - STS The Good LifeGilda Genive Ariola100% (4)
- BattleNight - Red HalidomsDocument47 pagesBattleNight - Red HalidomsWinner ElbertNo ratings yet
- Potassium FormateDocument4 pagesPotassium FormateHeris SitompulNo ratings yet
- Skip Morris Trout Fly Proportion ChartDocument3 pagesSkip Morris Trout Fly Proportion ChartScottDT1100% (1)
- Important NoticeDocument219 pagesImportant Noticeapi-210904824No ratings yet
- Metaphysics of AristotleDocument60 pagesMetaphysics of AristotleJaimon Thadathil100% (4)
- 02 - The Concept of Professionalism in Relation To ItqanDocument40 pages02 - The Concept of Professionalism in Relation To Itqanhappy100% (1)
- CTI VCS CONTRL K9 - DatasheetDocument7 pagesCTI VCS CONTRL K9 - Datasheetnihadabed77No ratings yet
- Neuropsychological Assessment in Illiterates: II. Language and Praxic AbilitiesDocument16 pagesNeuropsychological Assessment in Illiterates: II. Language and Praxic AbilitiesroxanaNo ratings yet
Article 1685528017
Article 1685528017
Uploaded by
mohdamer161616Original Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Article 1685528017
Article 1685528017
Uploaded by
mohdamer161616Copyright:
Available Formats
wjpmr, 2023,9(6), 01-03 SJIF Impact Factor: 5.
922
Case Report
Wissal et al. WORLD JOURNAL OF PHARMACEUTICAL
World Journal of Pharmaceutical and Medical Research
ISSN 2455-3301
AND MEDICAL RESEARCH
www.wjpmr.com Wjpmr
EXTRA-UTERINE PREGNANCY ON INTRA-UTERINE DEVICE: A CASE REPORT
*Wissal Zahir, Chaimaa Nadim, Nehad Mohammed Ali, Anass Ansari, Samir Bargach
Department of Obstetrics and Gynecology, Cancerology and High Risk Pregnancy, Souissi Maternity Hospital Rabat,
Morocco.
*Corresponding Author: Wissal Zahir
Department of Obstetrics and Gynecology, Cancerology and High Risk Pregnancy, Souissi Maternity Hospital Rabat, Morocco.
Article Received on 29/03/2023 Article Revised on 19/04/2023 Article Accepted on 09/05/2023
ABSTRACT
We present the observation of a patient with an intrauterine device consulting for left pelvic pain with minimal
blackish metrorrhagia on amenorrhea of 10 weeks. The pelvic ultrasound was in favour of a left ampullary
pregnancy. Diagnosis confirmed at exploratory laparotomy.
KEYWORDS: Intrauterine device, Ectopic pregnancy, Risk.
INTRODUCTION pelvic pain lateralized to the left with a small amount of
bleeding over an amenorrhea of 10 weeks.
The occurrence of pregnancy in a woman with an
intrauterine device as contraceptive is rare. Expressed by
The admission examination noted
the PEARL index, the failure rate of this contraceptive
BP = 100/60 mm Hg, Heart rate = 98 bpm, temperature
method varies between 0.3% and 3% depending on the
at 37 ° C.
performance of the device, regardless of the location of
egg implantation.[1,2,3] The most serious but exceptional
A soft abdomen.
location of implantation is ectopic. Users of the
At the speculum: a macroscopically healthy cervix;
intrauterine device are 50% less likely to have an ectopic
minimal metrorrhagia of endo uterine origin made of
pregnancy than women who do not use contraception.[3]
blackish blood. The marking wires of the intrauterine
But the probability of an intrauterine device wearer
device were visible.
having an ectopic pregnancy is 88%.[4] Histological tubal
changes favorable to ectopic pregnancy are mostly
On vaginal examination: a long posterior cervix softened
encountered with copper IUDs.[5]
and closed; a uterus of subnormal size and sensitive; a
very sensitive left adnexal mass, more or less soft, filling
OBSERVATION
the left lateral cul de sac.
This is a 36-year-old patient, G3P2 (2 live children
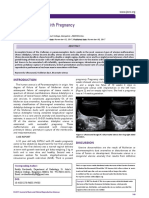
delivered vaginally), married, with a copper IUD for 2 A pelvic ultrasound (Figure 1) showed a left latero-
years, who consulted the obstetric emergency room for uterine mass measuring 49.8 mm × 33.4 mm with a
positive cardiac activity embryo.
Figure 1.
www.wjpmr.com │ Vol 9, Issue 6, 2023. │ ISO 9001:2015 Certified Journal │ 1
Wissal et al. World Journal of Pharmaceutical and Medical Research
The diagnosis of an extra uterine pregnancy was posterior uterine adhesions and a left ampullary
suspected, hence the indication for an emergency pregnancy of 5 cm long axis (Figure 2).
exploratory laparotomy, which showed periannexal and
Figure 2.
An adhesiolysis was performed with a left uterine cavity and the changes in the histological
salpingectomy. structure of the endometrium would be predisposing
factors for ectopic pregnancy.
The postoperative course was simple.
For FERNANDEZ.[4] wearing an IUD at the time of
DISCUSSION conception is responsible for 2.5% of ectopic
pregnancies. ORY[8] found that among women on
Amenorrhea on the intrauterine device is a rare
contraception, those with an IUD have 3 times the risk of
phenomenon. However, episodes of bleeding and pelvic
an ectopic pregnancy than those on the pill. The
pain are frequent.[5,1,3] They make the differential clinical
intrauterine device (IUD) is not a risk factor for ectopic
diagnosis of ectopic pregnancy complex with the other
pregnancy (EP), but it is less effective in preventing EP
complications related to the intrauterine device,
than intrauterine pregnancy. And the risk of ectopic
including infection, which is, moreover, the main factor
pregnancy does not depend on the type of intrauterine
promoting ectopic pregnancy.[5,1,6]
device. Regardless, the possibility of ectopic pregnancy
should be considered in any woman who becomes
The infectious process is aggravated by functional and
pregnant with an intrauterine device.
dynamic alterations of the tube, such as the phenomena
of cellular deciliation generated by intrauterine
CONCLUSION
devices.[5,7,4,6] The deciliation of the tubal epithelium
would favour the squamous localisation of tubal Amenorrhea on an intrauterine device in a previously
pregnancies on IUDs.[6] well-adjusted woman should act as a warning sign to
which users' attention should be drawn. Thus, correctly
Also, women who use the intrauterine device are about informing an IUD wearer must allow early diagnosis of
twice as likely to have pelvic inflammatory disease as ectopic pregnancy before rupture, which is not without
women who do not use contraception.[3] This pelvic vital and therapeutic consequences.
inflammatory disease is more common during the first 3
months after insertion, which can lead to other Aseptic precautions during insertion of the intrauterine
complications such as ectopic pregnancy.[2] Thus ectopic device are a preventive measure.
pregnancy, due to the delay in the transport of the egg
from the place of fertilization to the uterus, is in the REFERENCES
majority of cases the consequence of the infection.[2]
1. ERNY R. - ERNY N. - NOIZET A. Régulation des
This notion illustrates our observation in view of the
naissances. Encycl. Méd. Chir. (Paris), Gynécologie,
multiple adhesions identified, witnessing episodes of
1984; 738A: 10, 12, 26.
pelvic inflammatory disease.
2. FAMILY HEALTH INTERNATIONAL. Network
[5] [7] en français, Oct. 1987; 6(3).
AUDEBERT. and DUBUISSON. concluded that the
antinidatory action of the intra-uterine device in the
www.wjpmr.com │ Vol 9, Issue 6, 2023. │ ISO 9001:2015 Certified Journal │ 2
Wissal et al. World Journal of Pharmaceutical and Medical Research
3. Population report: Nouveau regard sur le D. I. U. B-
5. Population Information Program, Center for
Communication Programs. The Johns Hopkins
University, Baltimore, 1988.
4. FERNANDEZ H. - COSTE J. - JOB-SPIRA A. -
PAPIERNIK E. Facteurs de risque de la grossesse
extra-utérine. Etude de cas - témoins dans 7
maternités de la région parisienne.
5. AUDEBERT A. J. M. - COHEN J. La contraception
endo-utérine en. Données pratiques. Mises à jour en
Gynécol. Obstét. - Collège Nat. des Gynécol. Obstét.
Fr., 1985; 9: 331 - 381.
6. POULY J. L. - CHAPRON C. - CANIS M. - MAGE
G. - WATTIEZ A. - MANHES H. - BRUHAT M.
A. Grossesses extra-utérines sur stérilet.
Caractéristiques et fertilité ultérieure. J.
Gynécol.Obstét. Biol. Reprod, 1991; 20: 1069 -
1072.
7. DUBUISSON J. B. La grossesse tubaire. Mises à
jour en Gynécol. Obstét. Collège Nat. des Gynécol.
Obstét. Fr., 1981; 5: 95 - 117.
8. ORY H. W. Ectopic pregnancy and intra-uterine
contraceptive device : new perspectives. J. Obstét.
Gynécol, 1981; 57: 137 - 144.
www.wjpmr.com │ Vol 9, Issue 6, 2023. │ ISO 9001:2015 Certified Journal │ 3
You might also like
- Narrative Report-Virtual Parent's OrientationnDocument3 pagesNarrative Report-Virtual Parent's OrientationnApril Ramos Dimayuga95% (21)
- 50 Tapping Scripts Gene MonterastelliDocument27 pages50 Tapping Scripts Gene Monterastellilalek00100% (5)
- Rare Case of Bicornuate Uterus With Fetal Death in A Multipara WomanDocument2 pagesRare Case of Bicornuate Uterus With Fetal Death in A Multipara WomanInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Uterine Rupture in Healthy Uterus Complication of Misoprostol (About Two Cases and Review of The Literature)Document3 pagesUterine Rupture in Healthy Uterus Complication of Misoprostol (About Two Cases and Review of The Literature)International Journal of Innovative Science and Research TechnologyNo ratings yet
- Ovarian Pregnancy: Two Case Reports.: Jayeeta Roy, Anindita Sinha BabuDocument5 pagesOvarian Pregnancy: Two Case Reports.: Jayeeta Roy, Anindita Sinha BabuAndriyani MuslimNo ratings yet
- Interstitial Ectopic Pregnancy A Case ReportDocument4 pagesInterstitial Ectopic Pregnancy A Case ReportRina Auliya WahdahNo ratings yet
- Ovarian Ectopic Pregnancy: A Rare Case: Iran J Reprod Med Vol. 12. No. 4. PP: 281-284, April 2014Document4 pagesOvarian Ectopic Pregnancy: A Rare Case: Iran J Reprod Med Vol. 12. No. 4. PP: 281-284, April 2014alif bagusNo ratings yet
- Ruptured Ectopic Pregnancy in The Presence of An Intrauterine DeviceDocument5 pagesRuptured Ectopic Pregnancy in The Presence of An Intrauterine DevicePatrick NunsioNo ratings yet
- Cervical Tear: A Rare Route of Delivery: Case Report: Bhaktii Kohli and Madhu NagpalDocument2 pagesCervical Tear: A Rare Route of Delivery: Case Report: Bhaktii Kohli and Madhu NagpalAisyah MajidNo ratings yet
- 3930 14694 1 PBDocument3 pages3930 14694 1 PBSilvia RNo ratings yet
- Acute Uterine InversionDocument6 pagesAcute Uterine InversionBima GhovaroliyNo ratings yet
- Spontaneous Rupture of An Unscarred GravidDocument4 pagesSpontaneous Rupture of An Unscarred GraviddelaNo ratings yet
- Case Report Ghazanfar Page 130-132Document3 pagesCase Report Ghazanfar Page 130-132sohailurologistNo ratings yet
- Chronic Non-Puerperal Uterine Inversion Mimicking Complete Uterine ProlapsedDocument6 pagesChronic Non-Puerperal Uterine Inversion Mimicking Complete Uterine ProlapsedCentral Asian StudiesNo ratings yet
- Myoma Praevia in A Pregnancy Carried To Term: A Case Report and Updated Review of The LiteratureDocument3 pagesMyoma Praevia in A Pregnancy Carried To Term: A Case Report and Updated Review of The LiteratureInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Artigo Gravidez EctópicaDocument2 pagesArtigo Gravidez EctópicaKevin UchoaNo ratings yet
- Trial of Labor at The Maternity Department of The Sourosanou University Hospital (Chuss) : Clinical Aspects, The Maternal and Perinatal PrognosticsDocument8 pagesTrial of Labor at The Maternity Department of The Sourosanou University Hospital (Chuss) : Clinical Aspects, The Maternal and Perinatal PrognosticsIJAR JOURNALNo ratings yet
- Ectopic Pregnancy: Clinical PracticeDocument9 pagesEctopic Pregnancy: Clinical PracticeNurul WulandariNo ratings yet
- Case Report: Retained Intrauterine Device (IUD) : Triple Case Report and Review of The LiteratureDocument9 pagesCase Report: Retained Intrauterine Device (IUD) : Triple Case Report and Review of The LiteratureYosie Yulanda PutraNo ratings yet
- Acute Inversion of The Uterus PDFDocument6 pagesAcute Inversion of The Uterus PDFEvelyn TingNo ratings yet
- Diagnosis and Management of Ectopic Pregnancy-A Basic View Through LiteratureDocument2 pagesDiagnosis and Management of Ectopic Pregnancy-A Basic View Through LiteraturefprasasyaaNo ratings yet
- Case Report: Ruptured Ectopic Pregnancy in Caesarean Section Scar: A Case ReportDocument4 pagesCase Report: Ruptured Ectopic Pregnancy in Caesarean Section Scar: A Case ReportwennyNo ratings yet
- Total and Acute Uterine Inversion After Delivery A Case Report PDFDocument4 pagesTotal and Acute Uterine Inversion After Delivery A Case Report PDFatika sgrtNo ratings yet
- CessarDocument4 pagesCessarkknsNo ratings yet
- Cervical Ectopic Pregnancy After in Vitro Fertilization: Case Report Successfully Treated With Cervical Electric AspirationDocument5 pagesCervical Ectopic Pregnancy After in Vitro Fertilization: Case Report Successfully Treated With Cervical Electric AspirationMatias Alarcon ValdesNo ratings yet
- Primary Abdominal Ectopic Pregnancy: A Case ReportDocument4 pagesPrimary Abdominal Ectopic Pregnancy: A Case ReportYared TJNo ratings yet
- Merced 2019Document14 pagesMerced 2019Vanessa CarinoNo ratings yet
- UC Irvine: Clinical Practice and Cases in Emergency MedicineDocument6 pagesUC Irvine: Clinical Practice and Cases in Emergency MedicinePatrick NunsioNo ratings yet
- Ectopic Pregnancy: A ReviewDocument13 pagesEctopic Pregnancy: A ReviewDinorah MarcelaNo ratings yet
- Print 3 FixDocument4 pagesPrint 3 Fixafriskha bulawanNo ratings yet
- Primary Ovarian Abscess in Pregnancy: Case ReportDocument3 pagesPrimary Ovarian Abscess in Pregnancy: Case ReportVinnyRevinaAdrianiNo ratings yet
- Ovarian Torsion in Pregnancy A Case ReportDocument3 pagesOvarian Torsion in Pregnancy A Case ReportCakraEkkyNo ratings yet
- Jurnal Internasional 1Document5 pagesJurnal Internasional 1Ifni AngraeniNo ratings yet
- Complications After Interval Postpartum IntrauterineDocument8 pagesComplications After Interval Postpartum IntrauterinekarlinNo ratings yet
- Insertion and Removal of Intrauterine Devices-AAFPDocument8 pagesInsertion and Removal of Intrauterine Devices-AAFPnouval_iqbalNo ratings yet
- Criog2015 596826Document3 pagesCriog2015 596826charles limbuNo ratings yet
- Spontaneous Heterotopic Pregnancy With Intrauterine Pregnancy Stopped Case Report and Review of LiteratureDocument3 pagesSpontaneous Heterotopic Pregnancy With Intrauterine Pregnancy Stopped Case Report and Review of LiteratureInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Effectiveness of Delayed Absorbable Monofila - 2014 - Taiwanese Journal of ObsteDocument3 pagesEffectiveness of Delayed Absorbable Monofila - 2014 - Taiwanese Journal of ObsteSami KahtaniNo ratings yet
- Bicornuate Uterus With PregnancyDocument2 pagesBicornuate Uterus With Pregnancyain trophisNo ratings yet
- Cases Journal: Torsion of Ovarian Cyst During Pregnancy: A Case ReportDocument3 pagesCases Journal: Torsion of Ovarian Cyst During Pregnancy: A Case ReportAde Gustina SiahaanNo ratings yet
- DTSCH Arztebl Int-112-0693 PDFDocument13 pagesDTSCH Arztebl Int-112-0693 PDFKhoirunnisaSPazkaNo ratings yet
- Myoma in Necrobiosis and PregnancyDocument2 pagesMyoma in Necrobiosis and PregnancyInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Scar PregnancyDocument11 pagesScar Pregnancyasshagab04No ratings yet
- Case Report Large Uterine Fibroids in Pregnancy With Successful Caesarean MyomectomyDocument4 pagesCase Report Large Uterine Fibroids in Pregnancy With Successful Caesarean MyomectomyChidimma VictoryNo ratings yet
- Case Operation Procedure: Unruptured Ectopic Pregnancy With Risk Factor History of MiscarriageDocument1 pageCase Operation Procedure: Unruptured Ectopic Pregnancy With Risk Factor History of Miscarriageria.janitaNo ratings yet
- Srgype 02 00039 PDFDocument4 pagesSrgype 02 00039 PDFkhusnulNo ratings yet
- Case Report: Ruptured Ectopic Pregnancy in Caesarean Section Scar: A Case ReportDocument3 pagesCase Report: Ruptured Ectopic Pregnancy in Caesarean Section Scar: A Case ReportEga MegawatiNo ratings yet
- Cranney Et Al-2017-Acta Obstetricia Et Gynecologica ScandinavicaDocument11 pagesCranney Et Al-2017-Acta Obstetricia Et Gynecologica ScandinavicaAlejandro Romero LinaresNo ratings yet
- Successful Conservative Treatment of Microinvasive Cervical Cancer During PregnancyDocument3 pagesSuccessful Conservative Treatment of Microinvasive Cervical Cancer During PregnancyEres TriasaNo ratings yet
- Acog Practice Bulletin No 191 Tubal Ectopic.38Document13 pagesAcog Practice Bulletin No 191 Tubal Ectopic.38Uma HamNo ratings yet
- The "Vanishing Twin" Syndrome - A Myth or Clinical Reality in The Obstetric Practice?Document3 pagesThe "Vanishing Twin" Syndrome - A Myth or Clinical Reality in The Obstetric Practice?ariniNo ratings yet
- Toolbox: Ectopic PregnancyDocument4 pagesToolbox: Ectopic PregnancyLuciana LorenzaNo ratings yet
- Atb of Amniotic Fluid SludgeDocument20 pagesAtb of Amniotic Fluid SludgeSantiago RollanNo ratings yet
- Articulo Rotura PrimigestaDocument3 pagesArticulo Rotura PrimigestaYeitzmarNo ratings yet
- Ijmr 136 370Document2 pagesIjmr 136 370Eka MegawatiNo ratings yet
- Safety and Efficacy of Misoprostol in Induction of Labour in Prelabour Rupture of Fetal Membrane in Nigerian Women: A Multicenter StudyDocument6 pagesSafety and Efficacy of Misoprostol in Induction of Labour in Prelabour Rupture of Fetal Membrane in Nigerian Women: A Multicenter StudyAnonymous GssdN5No ratings yet
- Case Report: Puerperal Uterine Inversion Managed by The Uterine Balloon TamponadeDocument4 pagesCase Report: Puerperal Uterine Inversion Managed by The Uterine Balloon TamponadeXubair KiyaniNo ratings yet
- 9.radha Et Al.Document3 pages9.radha Et Al.International Journal of Clinical and Biomedical Research (IJCBR)No ratings yet
- 2016 Article 162 PDFDocument8 pages2016 Article 162 PDFefanNo ratings yet
- Advanced Abdominal Pregnancy: The Challenges of Management: Case ReportDocument3 pagesAdvanced Abdominal Pregnancy: The Challenges of Management: Case ReportaldyNo ratings yet
- A Randomized Trial of Misoprostol Compared With Manual Vacuum Aspiration For Incomplete AbortionDocument8 pagesA Randomized Trial of Misoprostol Compared With Manual Vacuum Aspiration For Incomplete AbortionElena ZepedaNo ratings yet
- Treatment Strategy for Unexplained Infertility and Recurrent MiscarriageFrom EverandTreatment Strategy for Unexplained Infertility and Recurrent MiscarriageKeiji KurodaNo ratings yet
- v3.2 Data Risk Analytics User Guide 3-21-2023Document236 pagesv3.2 Data Risk Analytics User Guide 3-21-2023singhpriyank.psNo ratings yet
- Soul Winning TractDocument2 pagesSoul Winning TractPaul Moiloa50% (2)
- Canadian Business English Canadian 7th Edition Guffey Test Bank 1Document20 pagesCanadian Business English Canadian 7th Edition Guffey Test Bank 1tedNo ratings yet
- Service Operation Management: Session 8Document59 pagesService Operation Management: Session 8mukul3087_305865623No ratings yet
- INCREASE CARDIAC OUTPUT Related To Altered Heart Rate RythmDocument3 pagesINCREASE CARDIAC OUTPUT Related To Altered Heart Rate RythmSenyorita KHayeNo ratings yet
- Curriculum Vitae: Dawn M. McbrideDocument15 pagesCurriculum Vitae: Dawn M. McbrideAndrea NemenzoNo ratings yet
- Matabuena vs. CervantesDocument1 pageMatabuena vs. CervantesAndrew GallardoNo ratings yet
- If You Liked Selling The 740 Series You'll Love Selling The New 753/754Document8 pagesIf You Liked Selling The 740 Series You'll Love Selling The New 753/754HINANo ratings yet
- Discovery Products (BD) Ltd-ConsDocument1 pageDiscovery Products (BD) Ltd-ConsUnimart DhanmondiNo ratings yet
- A Management Information SystemDocument15 pagesA Management Information SystemFRAkfurdNo ratings yet
- Chapter 8 Bonding Powerpoint AP ChemDocument68 pagesChapter 8 Bonding Powerpoint AP ChemAbdul jan sultaniNo ratings yet
- The Physical Life of Woman:Advice To The Maiden, Wife and Mother by Napheys, George H. (George Henry), 1842-1876Document192 pagesThe Physical Life of Woman:Advice To The Maiden, Wife and Mother by Napheys, George H. (George Henry), 1842-1876Gutenberg.orgNo ratings yet
- Pile CapDocument44 pagesPile Capbhavik modiNo ratings yet
- Compounding Self AssessmentDocument15 pagesCompounding Self AssessmentLara LaiNo ratings yet
- Identification and Prioritisation of Risk Factors in R&D Projects Based On An R&D Process ModelDocument18 pagesIdentification and Prioritisation of Risk Factors in R&D Projects Based On An R&D Process ModelSravani JonnadulaNo ratings yet
- The Moderation Effect of Age On Adopting E-Payment TechnologyDocument8 pagesThe Moderation Effect of Age On Adopting E-Payment TechnologypirehNo ratings yet
- Purser - Review of Bellanger's OrientiusDocument35 pagesPurser - Review of Bellanger's OrientiusRaphael SoaresNo ratings yet
- At 01 - Introduction To Assurance and Related Services (Incl. Intro To Audit) - QuizzerDocument6 pagesAt 01 - Introduction To Assurance and Related Services (Incl. Intro To Audit) - QuizzerRei-Anne Rea100% (1)
- Week 9 - STS The Good LifeDocument4 pagesWeek 9 - STS The Good LifeGilda Genive Ariola100% (4)
- BattleNight - Red HalidomsDocument47 pagesBattleNight - Red HalidomsWinner ElbertNo ratings yet
- Potassium FormateDocument4 pagesPotassium FormateHeris SitompulNo ratings yet
- Skip Morris Trout Fly Proportion ChartDocument3 pagesSkip Morris Trout Fly Proportion ChartScottDT1100% (1)
- Important NoticeDocument219 pagesImportant Noticeapi-210904824No ratings yet
- Metaphysics of AristotleDocument60 pagesMetaphysics of AristotleJaimon Thadathil100% (4)
- 02 - The Concept of Professionalism in Relation To ItqanDocument40 pages02 - The Concept of Professionalism in Relation To Itqanhappy100% (1)
- CTI VCS CONTRL K9 - DatasheetDocument7 pagesCTI VCS CONTRL K9 - Datasheetnihadabed77No ratings yet
- Neuropsychological Assessment in Illiterates: II. Language and Praxic AbilitiesDocument16 pagesNeuropsychological Assessment in Illiterates: II. Language and Praxic AbilitiesroxanaNo ratings yet