Professional Documents
Culture Documents
0 NC Q NC 0 P V NR T NC Q+W NC Q NC P T NRTLN NRTLN 0 0
0 NC Q NC 0 P V NR T NC Q+W NC Q NC P T NRTLN NRTLN 0 0
Uploaded by
John Andrew Gonzales0 ratings0% found this document useful (0 votes)
5 views1 pageThe document summarizes key equations for ideal gas conditions during common thermodynamic processes:
1) Isochoric (constant volume) processes have no work done, heat equals the change in internal energy, and enthalpy equals the heat capacity times the change in temperature.
2) Isobaric (constant pressure) processes have work that equals the negative change in pressure times volume, heat equals the heat capacity times the change in temperature, and enthalpy equals the heat plus work.
3) Isothermal (constant temperature) processes have work that equals negative pressure times the change in volume or the gas constant times temperature times the natural log of the final over initial pressure or volume, with no change
Original Description:
Original Title
2
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document summarizes key equations for ideal gas conditions during common thermodynamic processes:
1) Isochoric (constant volume) processes have no work done, heat equals the change in internal energy, and enthalpy equals the heat capacity times the change in temperature.
2) Isobaric (constant pressure) processes have work that equals the negative change in pressure times volume, heat equals the heat capacity times the change in temperature, and enthalpy equals the heat plus work.
3) Isothermal (constant temperature) processes have work that equals negative pressure times the change in volume or the gas constant times temperature times the natural log of the final over initial pressure or volume, with no change
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
0 ratings0% found this document useful (0 votes)
5 views1 page0 NC Q NC 0 P V NR T NC Q+W NC Q NC P T NRTLN NRTLN 0 0
0 NC Q NC 0 P V NR T NC Q+W NC Q NC P T NRTLN NRTLN 0 0
Uploaded by
John Andrew GonzalesThe document summarizes key equations for ideal gas conditions during common thermodynamic processes:
1) Isochoric (constant volume) processes have no work done, heat equals the change in internal energy, and enthalpy equals the heat capacity times the change in temperature.
2) Isobaric (constant pressure) processes have work that equals the negative change in pressure times volume, heat equals the heat capacity times the change in temperature, and enthalpy equals the heat plus work.
3) Isothermal (constant temperature) processes have work that equals negative pressure times the change in volume or the gas constant times temperature times the natural log of the final over initial pressure or volume, with no change
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
You are on page 1of 1
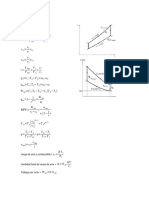
SUMMARY OF EQUATIONS FOR IDEAL GAS CONDITIONS
IDEAL GAS INTERNAL
PROCESS WORK HEAT ENTHALPY
LAW ENERGY
ISOCHORIC
P1T2 = P2T1 W=0 Q=nC v dT ∆ U =Q ∆ H =n C p dT
∆ V =0
ISOBARIC W =−P ∆ V ∆ U =Q+W ∆ H =Q
V1T2 = V2T1 Q=nC p dT
∆ P=0 Q=nR ∆ T ∆ U =n C v dT ∆ H =n C p dT
W =−P ∆ T
V1
ISOTHERMAL W =nRTln
P1V1 = P2V2 V2 Q = -W ∆ U =0 ∆ H =0
∆ P=0
P2
W =nRTln
P1
P1V1k = P2V2k
( )
k−1
T2 V 1
= nR(T 2−T 1) ∆ U =W
ADIABATIC T1 V 2 W= Q=0 ∆ H =n C p dT
K−1 ∆ U =n C v dT
( )
k −1
T2 P 2 1
=
T 1 P1
You might also like
- Thermodynamics FormulaDocument9 pagesThermodynamics FormulaJayvie TumangNo ratings yet
- ThermodynamicsDocument13 pagesThermodynamicsMonique OrugaNo ratings yet
- Djj2073 - Thermodynamics: 1. Properties of Pure Substance SteamDocument2 pagesDjj2073 - Thermodynamics: 1. Properties of Pure Substance SteamDkm3a GeraldmosesNo ratings yet
- Formula DJJ20063Document2 pagesFormula DJJ20063NurHiday2010No ratings yet
- COP COP P=P P m m p p ω= m m w=∅ p p: Mezclas gas-vaporDocument2 pagesCOP COP P=P P m m p p ω= m m w=∅ p p: Mezclas gas-vaporTania MarisolNo ratings yet
- Section A: Straight Objective Type: (Only One Correct Answer)Document12 pagesSection A: Straight Objective Type: (Only One Correct Answer)punitr2007No ratings yet
- Formulario Termodinamica 1Document2 pagesFormulario Termodinamica 1Jesus LyroyNo ratings yet
- Thermodynamic ProcessDocument12 pagesThermodynamic ProcessSatoru FujinumaNo ratings yet
- Kinematics Modern PhysicsDocument1 pageKinematics Modern PhysicsBoldie LutwigNo ratings yet
- Bab 7 Entropi: NugrahaDocument33 pagesBab 7 Entropi: NugrahaGilbert SihombingNo ratings yet
- Physics 40S Formula SheetDocument1 pagePhysics 40S Formula SheetWaleedSubhanNo ratings yet
- 7B Final Equation SheetDocument3 pages7B Final Equation SheetFangZiWenNo ratings yet
- FormulasDocument2 pagesFormulasRogerNo ratings yet
- Q W U H S: Isotérmico DT 0 Isobárico DP 0 Isocórico DV 0 Adiabático DQ 0Document2 pagesQ W U H S: Isotérmico DT 0 Isobárico DP 0 Isocórico DV 0 Adiabático DQ 0Aldasaurio SPNo ratings yet
- Physics FormulaeDocument1 pagePhysics FormulaeAlmasNo ratings yet
- NCSU Py208n Cheat SheetDocument3 pagesNCSU Py208n Cheat SheetMark FernandezNo ratings yet
- P T P T mC ΔT mC ΔT V Δ P mC ΔT mC T T T T P P V V mC ΔT mC ΔT P V P V kWDocument1 pageP T P T mC ΔT mC ΔT V Δ P mC ΔT mC T T T T P P V V mC ΔT mC ΔT P V P V kWJohn Paul BersabeNo ratings yet
- Formeert PDFDocument1 pageFormeert PDFJuraj BrozovicNo ratings yet
- L24 PDFDocument11 pagesL24 PDFManchimsetty Sri NidhiNo ratings yet
- Formula Rio 1Document1 pageFormula Rio 1Brandon ChavesNo ratings yet
- Kompresible - 1Document11 pagesKompresible - 1isudirmanNo ratings yet
- Fizikos Vbe Pagrindinės FormulėsDocument2 pagesFizikos Vbe Pagrindinės FormulėsxGytis3xNo ratings yet
- EquationDocument2 pagesEquationDarrel HindoyNo ratings yet
- FormularioDocument1 pageFormularioLiliana GuerraNo ratings yet
- Physics FormulasDocument2 pagesPhysics FormulasKristine BalansagNo ratings yet
- Thermodynamics Problems1-100Document5 pagesThermodynamics Problems1-100Monique OrugaNo ratings yet
- Can IdealDocument3 pagesCan IdealHERRERA GAVINO LORIAN GERMANNo ratings yet
- FormulasDocument8 pagesFormulasTecnoLife BaltoNo ratings yet
- Thermodynamics Formulae BookletDocument2 pagesThermodynamics Formulae BookletwardeqNo ratings yet
- Isothermal Process: Van Der Waals Eos (VDW)Document3 pagesIsothermal Process: Van Der Waals Eos (VDW)Keith Danae SuquibNo ratings yet
- MIT16 121F17 Lec04Document13 pagesMIT16 121F17 Lec04nophdNo ratings yet
- Thermo Cheat SheetDocument1 pageThermo Cheat Sheetpoly WannaNo ratings yet
- Thermo Cheat SheetDocument1 pageThermo Cheat Sheetpoly WannaNo ratings yet
- Formule OETDocument1 pageFormule OETLeon TrohaNo ratings yet
- Old Thermo Exam Formula SheetDocument1 pageOld Thermo Exam Formula SheetjonahNo ratings yet
- Boardgamedisplay14671 140524165124 Phpapp01Document1 pageBoardgamedisplay14671 140524165124 Phpapp01KitkatNo ratings yet
- FORMULASDocument26 pagesFORMULASRaymart LaysonNo ratings yet
- FORMULAS XNXNDocument23 pagesFORMULAS XNXNRaymart Layson0% (1)
- Correction of Final January 2022Document3 pagesCorrection of Final January 2022s2ne228No ratings yet
- One Dimensional ElementDocument51 pagesOne Dimensional ElementAbdullah RamziNo ratings yet
- Physics 112 Formulae IIIDocument3 pagesPhysics 112 Formulae IIISaied RajehaNo ratings yet
- Phys702 Exam 2015Document10 pagesPhys702 Exam 2015Tash MarshallNo ratings yet
- Equations - Structural DynamicsDocument11 pagesEquations - Structural Dynamicsnurul adilahNo ratings yet
- Ideal GasDocument1 pageIdeal GasMike Raphy T. VerdonNo ratings yet
- Processes Involving Ideal Gas FormulasDocument2 pagesProcesses Involving Ideal Gas FormulasYenjie SyNo ratings yet
- Chapter 2 FormulasDocument6 pagesChapter 2 FormulasShellyNo ratings yet
- Termodinamica ProblemDocument6 pagesTermodinamica ProblemJuandearco JuanarcoNo ratings yet
- Exam Grade12 2010 Phy DsDocument1 pageExam Grade12 2010 Phy DsIsrael PopeNo ratings yet
- P ω= dB PT BP H dT dB PT U dT dB P S BP G: - r - = T sat rDocument1 pageP ω= dB PT BP H dT dB PT U dT dB P S BP G: - r - = T sat rDhwani ShahNo ratings yet
- University of Technology, JamaicaDocument7 pagesUniversity of Technology, JamaicaAthinaNo ratings yet
- Scroll of Seals 1Document25 pagesScroll of Seals 1Anthony MacalindongNo ratings yet
- Chemistry Cheat Sheet-1Document1 pageChemistry Cheat Sheet-1r3birthvalNo ratings yet
- PHY 152 Equation List: A=π r A=4 π rDocument4 pagesPHY 152 Equation List: A=π r A=4 π rxcxcvxcvxNo ratings yet
- FoundationsDocument1 pageFoundations이소연[ 학부재학 / 화공생명공학과 ]No ratings yet
- 11.7 Thermodynamics Solution - PremiumDocument24 pages11.7 Thermodynamics Solution - PremiumJonathan ParkerNo ratings yet
- Evidence Consolidation ActivityDocument60 pagesEvidence Consolidation ActivityAlonso GalvisNo ratings yet
- Formulario I ParcialDocument1 pageFormulario I Parcialseca cacaNo ratings yet
- Power Cycles 1 - 1 PDFDocument6 pagesPower Cycles 1 - 1 PDFclarkmaxNo ratings yet
- Lecture Notes On Compressor 2019 PDFDocument21 pagesLecture Notes On Compressor 2019 PDFJerome MaldaNo ratings yet
- 1Document2 pages1John Andrew GonzalesNo ratings yet
- MATERIALSDocument2 pagesMATERIALSJohn Andrew GonzalesNo ratings yet
- BiocheDocument12 pagesBiocheJohn Andrew GonzalesNo ratings yet
- Solvents Used in Liquid-Liquid ExtractionDocument3 pagesSolvents Used in Liquid-Liquid ExtractionJohn Andrew GonzalesNo ratings yet
- Process Equipment and Plant Design: Sections 1 To 8Document17 pagesProcess Equipment and Plant Design: Sections 1 To 8John Andrew GonzalesNo ratings yet
- EnggmechDocument63 pagesEnggmechJohn Andrew GonzalesNo ratings yet