Professional Documents
Culture Documents
Muhammad Alwi Sutomi Procedure TOEFL
Muhammad Alwi Sutomi Procedure TOEFL
Uploaded by
Reskia Nabila0 ratings0% found this document useful (0 votes)
8 views4 pages1. The document outlines the procedure for conducting a GeneXpert test, which is a molecular examination that detects Mycobacterium tuberculosis and resistance to rifampicin.
2. The procedure involves preparing sputum samples by mixing them with buffer solution, inserting the samples into cartridges, running the test on the GeneXpert machine, and interpreting the results.
3. Results are reported as detected or not detected for MTB, and rifampicin resistance or susceptibility based on cutoff thresholds for cycle threshold values between probes.
Original Description:
Original Title
1699356495086 5120025 Muhammad Alwi Sutomi Procedure TOEFL
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Document1. The document outlines the procedure for conducting a GeneXpert test, which is a molecular examination that detects Mycobacterium tuberculosis and resistance to rifampicin.
2. The procedure involves preparing sputum samples by mixing them with buffer solution, inserting the samples into cartridges, running the test on the GeneXpert machine, and interpreting the results.
3. Results are reported as detected or not detected for MTB, and rifampicin resistance or susceptibility based on cutoff thresholds for cycle threshold values between probes.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
0 ratings0% found this document useful (0 votes)
8 views4 pagesMuhammad Alwi Sutomi Procedure TOEFL
Muhammad Alwi Sutomi Procedure TOEFL
Uploaded by
Reskia Nabila1. The document outlines the procedure for conducting a GeneXpert test, which is a molecular examination that detects Mycobacterium tuberculosis and resistance to rifampicin.
2. The procedure involves preparing sputum samples by mixing them with buffer solution, inserting the samples into cartridges, running the test on the GeneXpert machine, and interpreting the results.
3. Results are reported as detected or not detected for MTB, and rifampicin resistance or susceptibility based on cutoff thresholds for cycle threshold values between probes.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
You are on page 1of 4
“Procedure“
Disusun Untuk Memenuhi Tugas Toefl and Academic Writing
Dosen Pengampu : Muhammad Muayyad Billah, S.Tr. Gz, M. Gz, Dietisien
Disusun Oleh:
Muhammad Alwi Sutomi 5120025
Riswan Bintang Pratama 5120042
FAKULTAS KESEHATAN
PROGRAM STUDI D-IV TEKNOLOGI LABORATORIUM MEDIK
INSTITUT KESEHATAN RAJAWALI
TAHUN AJARAN 2023/2024
PROCEDURE BTA/TCM GeneXpert
Page : 3
STANDARD OPERATIONAL PROCEDURES
Definition GeneXpert is an automatic molecular examination to detect
Mycobacterium tuberculosis. This test uses catridge based on the
Nucleic Acid Amplification Test (NAAT) to detect cases of TB and
resistance to the antibiotic rifampicin.
Objective As a basis for the implementation of measures to conduct a quick
molecular test Gen Expert.
Procedure A. Tools
1. Biosafety Cabinet
2. Catridge
3. GeneXpert System
4. Gloves
5. Label
6. Pippete sterile transfer
7. Tissue
8. Vortex
B. Materials
1. Buffer GeneXpert
2. Disinfectant, 10% hypochlorite solution
3. Sputum samples in pots
C. Procedures
Preparations Samples
1. Each catridge must be labeled with an identity. The
identity of the specimen can be sticky or written on the
side of the catrid.
2. Open the pot cover and add the sample buffer with a
comparison of 1 part sample volume and 2 parts sample
tamper volume available. Two samples should not use the
same buffer.
3. If the sputum volume is 4 ml, then it is recommended to
divide the specimen into 2 parts. One part is used for
GeneXpert MTB/RIF examination, and the other one is
stored in a new sputum pot as a reserve.
4. Close the sputum pot again, then rub it until the mixture of
sputum and the buffer sample becomes homogeneous.
5. Leave for 10 minutes at room temperature.
6. Shake the mixture again and leave it standing for 5
minutes.
7. If there is still a clot, rub it again to make the mixture of
sputum and the buffer sample perfectly homogeneous, and
leave for 5 minutes at room temperature.
8. Open the caterpillar cover. Insert the sample mixture into
the cathride using a pipette of 2 ml by flowing through the
wall of the cathride to prevent the passage of bubbles that
may cause errors.
9. Slowly close the catheter and insert it into the GeneXpert
engine.
10. Operate GeneXpert.
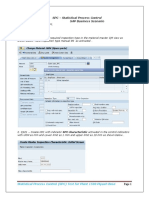
Turn on the machine and operate the software
1. Turn on the computer and the machine. Run the
GeneXpert DX software.
2. Click "Create Test".
3. Scan the barcode on the katrid or select "Manual Entry" if
the scanner cannot be used.
4. In the "Create Test" tab "Patient ID", "Sample 1". The
module section will be automatically loaded.
5. Click "Start Test".
6. The light will blink on the selected module. Insert the
katrid and close the module door until it is perfect and
marked "Click".
7. The inspection will take two hours, and when it's done, the
lights will automatically turn off.
8. Open the module door, remove the catridge and throw it
into the infectious trash.
Read Results
1. Click "View Result".
2. Click "View Test".
3. Click "Select Test to Be Viewed" → "OK".
4. To create a review report, click "Report".
5. The results can be interpreted as follows:
a) Detected MTB' when there are two probes gives Ct
values within the valid limit and delta Ct min (the
smallest Ct difference between probing pairs) <2.0.
b) Rifampicin Resistant is not detected when the max Ct
delta (difference between the earliest and most recent
probes) is ≤ 4.0.
c) Rifampicin Resistant Detected' when delta Ct max>
4.0.
6. Rifampicin Resistant Indeterminate' when two conditions
are found as follows:
a) The Ct value on the probe exceeds the maximum valid
value (or value 0).
b) The Ct value on the earliest probe appears (valid Ct
values max delta Ct max cut-off 4.0).
c) No MTB' detected when there is only one or no
positive probe.
You might also like
- Bio411 Lab Report 2Document25 pagesBio411 Lab Report 2Nur Aqillah100% (1)
- SOP For Parasitology Sample Collection and ExaminationDocument11 pagesSOP For Parasitology Sample Collection and ExaminationDr.Kedar Karki ,M.V.Sc.Preventive Vet.Medicine CLSU Philippines88% (8)
- Introduction and Discussion1Document28 pagesIntroduction and Discussion1Vy NguyễnNo ratings yet
- Core Practical 9 Antimicrobial Properties of Plants Writing FrameDocument6 pagesCore Practical 9 Antimicrobial Properties of Plants Writing FrametiaNo ratings yet
- Gene Xpert FinalDocument10 pagesGene Xpert FinalQaiser ZamanNo ratings yet
- Hayat cp3Document5 pagesHayat cp3hayatkuwaityNo ratings yet
- Tuberculosis: Protocol Book June 12 - 23, 2017Document14 pagesTuberculosis: Protocol Book June 12 - 23, 2017Revathi KNo ratings yet
- Genexpert Sars-Cov-2 (Covid-19) Vs Genexpert Mdr/Rif (TB) Test. Keep Moving Forward!Document4 pagesGenexpert Sars-Cov-2 (Covid-19) Vs Genexpert Mdr/Rif (TB) Test. Keep Moving Forward!Bashir MtwaklNo ratings yet
- Sample Processing 1Document6 pagesSample Processing 1rupalraibplNo ratings yet
- XBMB3104 (Microbiology) - Lab Sheet Sept 2022Document17 pagesXBMB3104 (Microbiology) - Lab Sheet Sept 2022Muhammad Sufri SalimunNo ratings yet
- Lab Policies Culture Wounds Lab 3115Document7 pagesLab Policies Culture Wounds Lab 3115Marj MendezNo ratings yet
- Lab Manual DMT 10023Document32 pagesLab Manual DMT 10023Bradly LaloNo ratings yet
- Standard Procedure For Microbiology Swabbing MethoDocument16 pagesStandard Procedure For Microbiology Swabbing MethoSitara KiranNo ratings yet
- GENEXPERT SOPDocument7 pagesGENEXPERT SOPjustinNo ratings yet
- Jove Protocol Multiplex PCR and Reverse Line Blot Hybridization Assay MPCRRLBDocument5 pagesJove Protocol Multiplex PCR and Reverse Line Blot Hybridization Assay MPCRRLBrajeshkundapur123No ratings yet
- FatmaDocument25 pagesFatmaRodina KhaledNo ratings yet
- Endotoxin Test Protocol PTSDocument5 pagesEndotoxin Test Protocol PTSSebastián SalazarNo ratings yet
- Chapter 6 LABORATORY TESTSDocument3 pagesChapter 6 LABORATORY TESTStinoNo ratings yet
- Food Analysis Lab ManualDocument17 pagesFood Analysis Lab ManualChing YeeNo ratings yet
- Chemistry Practical III - Lab ManualDocument69 pagesChemistry Practical III - Lab ManualVini syiniNo ratings yet
- Sops For Xpert Mtb/Rif Assay I UDocument3 pagesSops For Xpert Mtb/Rif Assay I USajjad AhmadNo ratings yet
- Dna Recombinant Lab 1Document18 pagesDna Recombinant Lab 1Mohd Amirul Firdhaus Mohd RidhwanNo ratings yet
- Labwork 1Document12 pagesLabwork 1niikwabena36No ratings yet
- Stool GeneXpert MTB-Rif Testing SOP - 0Document5 pagesStool GeneXpert MTB-Rif Testing SOP - 0Chris TianNo ratings yet
- Lab Sheet 4 Pour PlateDocument8 pagesLab Sheet 4 Pour PlateMohd ShafiqNo ratings yet
- Food Microbiology (CFD 20203) Unikl Lab Manual Micet: Malaysian Institute of Chemical and Bioengineering TechnologyDocument7 pagesFood Microbiology (CFD 20203) Unikl Lab Manual Micet: Malaysian Institute of Chemical and Bioengineering TechnologyNur AsiahNo ratings yet
- Laboratory Manual: University College Sedaya InternationalDocument16 pagesLaboratory Manual: University College Sedaya InternationalnatalieNo ratings yet
- Instruments Used in Microbiology Lab With Principle and UsesDocument18 pagesInstruments Used in Microbiology Lab With Principle and UsesJuan Alxander BriceñoNo ratings yet
- E COLI swabDocument7 pagesE COLI swabvetrohdes aliNo ratings yet
- Antibacterial Activity of Novel Strains of Bacteriophages: An Experimental ApproachDocument12 pagesAntibacterial Activity of Novel Strains of Bacteriophages: An Experimental ApproachM. Imran QadirNo ratings yet
- NORO LIFERIVEDocument8 pagesNORO LIFERIVEvetrohdes aliNo ratings yet
- FELINE FGF 23 KIT MANUALDocument4 pagesFELINE FGF 23 KIT MANUALanninhabandpotterNo ratings yet
- Experiments No 1 To 9 PDFDocument58 pagesExperiments No 1 To 9 PDFVarun kariyaNo ratings yet
- Chapter IIb - Clinical Immunology UG Med StudentsDocument31 pagesChapter IIb - Clinical Immunology UG Med StudentsAyanayuNo ratings yet
- Title: The Micronucleus Assay: Applies ToDocument6 pagesTitle: The Micronucleus Assay: Applies ToHari BabuNo ratings yet
- GEN 213 Cell Biology Laboratory: Biruni University Istanbul 2020Document6 pagesGEN 213 Cell Biology Laboratory: Biruni University Istanbul 2020ŞEVVAL SARGINNo ratings yet
- Techniques in Cell Biology Manual New1Document226 pagesTechniques in Cell Biology Manual New1kamalNo ratings yet
- Experimental Design and Analysis For MicrobiologyDocument7 pagesExperimental Design and Analysis For MicrobiologyErick TatroNo ratings yet
- Preparing A Wet Mount - 508Document1 pagePreparing A Wet Mount - 508NAMPEWO ELIZABETHNo ratings yet
- Test method plate count swabDocument9 pagesTest method plate count swabvetrohdes aliNo ratings yet
- 12 Catheter TipDocument6 pages12 Catheter Tipselvakumar selvarajNo ratings yet
- Varsha Report Industrial EvaluationDocument21 pagesVarsha Report Industrial EvaluationRohit RamchandaniNo ratings yet
- LAB - 1 - ANALYTICAL - CHEMISTRY - Docx SampleDocument16 pagesLAB - 1 - ANALYTICAL - CHEMISTRY - Docx SampleIZWANA AINA NAZIRA BINTI SHUHAIMI BN20110125No ratings yet
- Industrieal Bio Technologyject ReportDocument46 pagesIndustrieal Bio Technologyject ReportAshok KumarNo ratings yet
- 4 - SBT 1102 Cell Biology Lab ManualDocument8 pages4 - SBT 1102 Cell Biology Lab ManualgrahammbanganiNo ratings yet
- MA26 Purification of Bacterial DNA From Primary Samples Using The MagAttract DNA Mini M48 KitDocument5 pagesMA26 Purification of Bacterial DNA From Primary Samples Using The MagAttract DNA Mini M48 Kitt-jala.mohamedNo ratings yet
- XBMB3104 (Microbiology) - Lab Questions Jan 2022 WSPDocument18 pagesXBMB3104 (Microbiology) - Lab Questions Jan 2022 WSPHO SI KING Moe100% (1)
- Microbiology Lab ManualDocument52 pagesMicrobiology Lab ManualHà Anh Minh Lê100% (1)
- Sterlity Validation (Membrane Filtration Method) in Pharmaceuticals - Pharmaceutical GuidelinesDocument4 pagesSterlity Validation (Membrane Filtration Method) in Pharmaceuticals - Pharmaceutical GuidelinesDucNo ratings yet
- PL-Microbiology Specimen Collection Guidelines (2020.07.22)Document14 pagesPL-Microbiology Specimen Collection Guidelines (2020.07.22)Anonymous fgRHAEIMrHNo ratings yet
- ProtocolsDocument12 pagesProtocolsapi-462384159No ratings yet
- Universiti Kuala Lumpur Malaysian Institute of Chemical & Bioengineering TechnologyDocument9 pagesUniversiti Kuala Lumpur Malaysian Institute of Chemical & Bioengineering TechnologyMahainiIm RuzailyNo ratings yet
- Biochemical Tests ReportDocument12 pagesBiochemical Tests ReportExtra AccountNo ratings yet
- Quality Control in Microbiology and SerologyDocument10 pagesQuality Control in Microbiology and SerologyW.F KareemNo ratings yet
- Lesson AssignmentDocument19 pagesLesson AssignmentDjamel AhmedNo ratings yet
- hepatitis A LIFERIVEDocument9 pageshepatitis A LIFERIVEvetrohdes aliNo ratings yet
- Bio Reactor 2016Document7 pagesBio Reactor 2016asim zeshanNo ratings yet
- Lab 2B OffDocument16 pagesLab 2B Offbuithinhatlinh2004No ratings yet
- CLCY-4-03 Cytocentrifugation (1)Document3 pagesCLCY-4-03 Cytocentrifugation (1)amysun0474No ratings yet
- LABORATORY MANUAL FOR A MINI PROJECT: MSCB 1113 BIOCHEMISTRY & MICROBIAL PHYSIOLOGYFrom EverandLABORATORY MANUAL FOR A MINI PROJECT: MSCB 1113 BIOCHEMISTRY & MICROBIAL PHYSIOLOGYNo ratings yet
- Internal Audit Checklist Food Safety-MRDocument5 pagesInternal Audit Checklist Food Safety-MRRavi BaghelNo ratings yet
- Spectrophotometer UseDocument4 pagesSpectrophotometer UseEsperanza Fernández MuñozNo ratings yet
- Envirocare Engineers & Consultant, Surat T J AGRO VADODARA EIA PART 2Document133 pagesEnvirocare Engineers & Consultant, Surat T J AGRO VADODARA EIA PART 2Khánh ĐỗNo ratings yet
- كيفية التفكير المعماري- د.إبراهيم رزقDocument38 pagesكيفية التفكير المعماري- د.إبراهيم رزقAhmed HusseinNo ratings yet
- Skp-New Price ListDocument26 pagesSkp-New Price ListnuwanwimalNo ratings yet
- For Checking Reso 101Document3 pagesFor Checking Reso 101Cath VillarinNo ratings yet
- Sin & Cos - The Programmer's PalsDocument24 pagesSin & Cos - The Programmer's PalsRicardo De Oliveira AlvesNo ratings yet
- All Rights Reserved. Kim Seng Technical School, 1979Document164 pagesAll Rights Reserved. Kim Seng Technical School, 1979lengyianchua206No ratings yet
- Clay & Shale Industries in OntarioDocument193 pagesClay & Shale Industries in OntarioJohn JohnsonNo ratings yet
- The Dog-And-Rabbit Chase Problem As An Exercise in Introductory KinematicsDocument5 pagesThe Dog-And-Rabbit Chase Problem As An Exercise in Introductory KinematicssilagadzeNo ratings yet
- Tomographic Imaging in Aditya Tokamak: Nitin JainDocument21 pagesTomographic Imaging in Aditya Tokamak: Nitin JainHafiz Luqman SaifiNo ratings yet
- Micom MetrosilDocument3 pagesMicom MetrosilBala ArunNo ratings yet
- Gothra PattikaDocument172 pagesGothra PattikaParimi VeeraVenkata Krishna SarveswarraoNo ratings yet
- Gardening Is Beneficial For Health: A Meta-AnalysisDocument8 pagesGardening Is Beneficial For Health: A Meta-AnalysisHaritha DeviNo ratings yet
- Cooley, Charles - Social Organization. A Study of The Larger Mind (1909) (1910) PDFDocument458 pagesCooley, Charles - Social Organization. A Study of The Larger Mind (1909) (1910) PDFGermán Giupponi100% (1)
- Bokk, Fiber, Naam 1985, BasedDocument210 pagesBokk, Fiber, Naam 1985, BasedDoddy UskonoNo ratings yet
- Sds File-16179386Document7 pagesSds File-16179386omar silimNo ratings yet
- Warning: No Smoking! No Open Flame! While Installing Your Jet KitDocument2 pagesWarning: No Smoking! No Open Flame! While Installing Your Jet KitBrad MonkNo ratings yet
- Mode Emploi Yamaha Cs2xDocument78 pagesMode Emploi Yamaha Cs2xHauserNo ratings yet
- EVS Level A - Workbook 202108041859159269Document50 pagesEVS Level A - Workbook 202108041859159269Nabhasvit HegdeNo ratings yet
- Basic Grammar Unit6 Without AnswersDocument1 pageBasic Grammar Unit6 Without AnswerstitnNo ratings yet
- Buchenwald - Through The Eyes of An ArtistDocument27 pagesBuchenwald - Through The Eyes of An ArtistDennis M. Gilman100% (1)
- Control Charts in SAP QM: Step by StepDocument10 pagesControl Charts in SAP QM: Step by StepPiyush BoseNo ratings yet
- 2010 Lighting and Electrical CatalogDocument90 pages2010 Lighting and Electrical CatalogTravis Erwin100% (1)
- (Revisi) Case Report - Ellita Audreylia (201906010123)Document45 pages(Revisi) Case Report - Ellita Audreylia (201906010123)Jonathan MarkNo ratings yet
- About Barfani DadajiDocument4 pagesAbout Barfani DadajiNaren MukherjeeNo ratings yet
- Dead Phone Testing1Document12 pagesDead Phone Testing1HEM SHRESTHANo ratings yet
- Traumatic Diaphragmatic Hernia: Case Series and Topic ReviewDocument12 pagesTraumatic Diaphragmatic Hernia: Case Series and Topic ReviewMohammad SyahrezkiNo ratings yet
- L T Committee Opinion: Ist of ItlesDocument11 pagesL T Committee Opinion: Ist of ItlesnathanielNo ratings yet
- GMW8 2019 03 (Hot Dip Zinc Coating Sheet Steel)Document8 pagesGMW8 2019 03 (Hot Dip Zinc Coating Sheet Steel)dpfloresNo ratings yet