Professional Documents
Culture Documents
65601a476c0a0100185f2bbc ## Dual Nature of Radiation & Matter Mind
65601a476c0a0100185f2bbc ## Dual Nature of Radiation & Matter Mind
Uploaded by
principaltamannaOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
65601a476c0a0100185f2bbc ## Dual Nature of Radiation & Matter Mind
65601a476c0a0100185f2bbc ## Dual Nature of Radiation & Matter Mind
Uploaded by
principaltamannaCopyright:
Available Formats
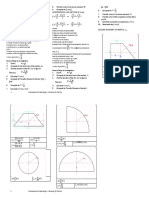
Photon Theory DUAL NATURE OF RADIATION & MATTER PHYSICS
WALLAH Thermal Neutron
Power E h 30.83
Intensity (I) = = Perfectly Reflecting at an angle Nature of material λ= = A
Area
E/
tA C
3mkBT T
= energy per unit area per unit time 2E
∆p= _ COS θ (
m1 m2
Electron λ= 12.27 A
θ
K.E
P 1 C (
Point source I= = I
θ
4π r2 r2 2P V
F = _ COS θ
C
1 Proton λ= 0.286 A
i
Line source I= P E/
V0
I
C
2
_I
2π rl r Rad. Pressure = COS θ -ve +ve
01 02
V
C for same energy E
0.202
PHOTOELECTRIC EFFECT
Voltage (V)
02 > 01
Deutron (λ)= A
1 c -c ) V
1 no.of Photons
2
Energy of photon E = h (K.E)max= eV0= 2 mVmax = h( - 0) =h ( w02 > w01
λ 0.101
n= E = E
0
c
E= n h = Frequency of incident light in Hz = K.E2 K.E1 -Particle (λ)= A
>
h hC Max.kinetic energy of emitted photoelectron V02 V01
> V
2 no.of Photons per unit time 1 2
(K.E)max = E-w = mVmax
2
Factors affecting stopping potential 1
H (Proton) 1 Proton m,q
n = E = P = IA Intensity (I) 2m,q
= IA λ
2
H (Deuteron) 1 Proton + 1Neutron
Conceptual question
t th h h hc Intensity ,K.E Remains same 4
He ( -Particle) 2 Proton+2 Neutron
Work function (w) Stopping potential remains same If green color have just sufficient energy 2
no.of Photons per area per unit time 4m,2q
3 Minimum energy required for photoelectric
for photoelectric effect
increasing
n = E = P = I Iλ effect to occur I3>I2>I1 E no photo electric effect
At tAh Ah h = c I3 order of λ
hc
{
w=h 0 =h h=6.63x10 -34
Js i Relationship b/w wavelength of photon
0 V I B G Y O R
I2 & that of electron
=Threshold frequency in Hz
{
0
Power of incident radiation (P) I1 sure photo electric effect Ratio of wavelength of photon
0 =Threshold wavelength in m
λ to that of electron with same
(λ
E
n
P= nthc
( Work function only deponds on nature of energy E
P metal
λ
source1 P1 λ1 n1 V0 Voltage (V) Useful conversions photon
=c 2m λphoton λ2 e
Frequency Wave Length (nm) K.E (eV)
e E
source1 P2 λ2 n2 Factors affecting photoelectric effect Frequency ,Energy , K.E ,V0 K.E= 1240 (eV) Ratio of K.E of electron to that of
λ
P1 n1 Intensity
= + λ2 Intensity ,photoelectrons ,photocurrent
Wave Length (A ) K.E (eV) photon with same wavelength
P2 n2 λ1 (K.E Remains same)
i K.E= 12400 (eV)
λ K.Ee = V
for Same
K.E photon 2C
Dual Nature of Radiation
photocurrent (i) Intensity (I) 3 Two Identical photo cathode recieve
Frequency 2 light of frequencies f1& f2. If velocity
Frequency ,Energy , K.E
1 of photo elecrons are v1 & v2
i -ve +ve A particles formed due to completely
(Work function Remains same) then v 2-v 2= 2h [f -f ]
E h V03 V02 V01 m inelastic collision of particle ‘x‛ and ‘y‛
Momentum of photon (p) = c =
1 2 1 2
Voltage (V)
λ I having debroglie wave length λx and λy
∆p > > V03> V02 >V01 respectively.
Force (F) = ∆t
K.E
3 2 1
Dual Nature of If they are moving in opposite directions
Radiation Pressure = F
Stopping potential V0 vs frequency graph
Matter
A p=pX-pY PX PY
For perfectly reflecting V0
Wave nature of Matter
then λx λy
surface E/
h = h h λ=
C 0
Debroglie waves - λx- λy
2E 2P fast moving particles like electron with λ λx λy
∆p= F=
c c E/
(K.E)max = h -w much smaller mass behaves like a wave If they are moving at right angle to
2I C ie., Circular stationary waves
each other
Rad. Pressure = -w Slope = h 0
C Y Intercept = -w E=mc2 =
hc h = h2 + h2
x Intercept = 0 = p= p X2+pY2
For Perfectly Absorbing eVO = h -h O h
= mc =h h h h h λ λ2x λy2
p = mv = = =
Surface
2m(K.E) 3kmT 2mqV
- he O VO = h h
e -e O K.E = qV (for charged particle)
1= 1 + 1
Anode potential
E P Opposes K.E of electron h V = accelerating potential in Volt
λ λx2 λy2 λx
∆p=
c F= c Max Negative anode potential
Slope = e 3 PX
I Y Intercept =- h
K.E =
2 B
K T (thermal neutron )
Rad. Pressure(PR) = = Stopping potential (V0) e O
C for which Photocurrent (i) = 0
KB = Boltzmann‛s constant λy
x Intercept = 0 T = Temperature in Kelvin
PY
You might also like
- BJT Small Signal Configurations Cheat SheetDocument1 pageBJT Small Signal Configurations Cheat SheetAdi SaggarNo ratings yet
- Dual Nature of Matter & Radiation - Mind Maps - Lakshya JEE 2024Document1 pageDual Nature of Matter & Radiation - Mind Maps - Lakshya JEE 2024xoranek474No ratings yet
- PPPTHĐDocument12 pagesPPPTHĐNHI NGUYỄN TRẦN THẢONo ratings yet
- Biothermo Cheatsheet Copy DESKTOP BLE90C7Document5 pagesBiothermo Cheatsheet Copy DESKTOP BLE90C7Pay XinniNo ratings yet
- Emanating: BroglieDocument5 pagesEmanating: BroglieGourav SinghNo ratings yet
- Beam Deflection PDFDocument7 pagesBeam Deflection PDFHaikal HakimNo ratings yet
- From Last Time Mathematical Description: Inductors in Circuits +Document5 pagesFrom Last Time Mathematical Description: Inductors in Circuits +mail2sgarg_841221144No ratings yet
- ENGR-244 Formula Sheet Midterm W2018 PDFDocument1 pageENGR-244 Formula Sheet Midterm W2018 PDFPhilNo ratings yet
- Final Formula Sht2Document1 pageFinal Formula Sht2maxdweeks0% (1)
- Quantum Mechanics Notes - Ii: Amit Kumar Jha (Iitian)Document15 pagesQuantum Mechanics Notes - Ii: Amit Kumar Jha (Iitian)Tiasha DevNo ratings yet
- DC Motors FormulasDocument3 pagesDC Motors FormulasSaabierah SalieNo ratings yet
- Ce226 Q6 Corpuz J 3cegDocument12 pagesCe226 Q6 Corpuz J 3cegJermaine CorpuzNo ratings yet
- Tabela de IgbtsDocument16 pagesTabela de IgbtsEdmilson Mendes PimentelNo ratings yet
- 11ไฟฟ้าสถิตDocument10 pages11ไฟฟ้าสถิตtim846gNo ratings yet
- TD Introduction To Quantum PhysicsDocument11 pagesTD Introduction To Quantum PhysicsusaroufNo ratings yet
- Formula Lab Sheet - 1p22 PDFDocument2 pagesFormula Lab Sheet - 1p22 PDFRoy VeseyNo ratings yet
- Physical Chemistry Formula (2) 3Document6 pagesPhysical Chemistry Formula (2) 3Anand RockyNo ratings yet
- Tarea 3.1Document3 pagesTarea 3.1maria pazNo ratings yet
- LawsDocument2 pagesLawsMohamed AbdallahNo ratings yet
- P2 CapacitorDocument1 pageP2 CapacitorAadya BajpaiNo ratings yet
- Lecture 17Document9 pagesLecture 17Abhideep KhareNo ratings yet
- Department of Electrical and Electronics Engineering Energy Conversion Laboratory Course Code: EEE206 Experiment No. 1Document10 pagesDepartment of Electrical and Electronics Engineering Energy Conversion Laboratory Course Code: EEE206 Experiment No. 1mahmudulNo ratings yet
- Assignment 2 SolutionsDocument13 pagesAssignment 2 SolutionsP34Akshay ChoudharyNo ratings yet
- Discrete Igbts: SemiconductorDocument16 pagesDiscrete Igbts: Semiconductormoacir carlos guandaliniNo ratings yet
- Igbt 30F125Document15 pagesIgbt 30F125Efren CisnerosNo ratings yet
- Analysis of Stresses and StrainsDocument11 pagesAnalysis of Stresses and StrainsdearsaswatNo ratings yet
- Formula Sheet EE-434 Power Electronics: Basic Expressions CapacitorDocument3 pagesFormula Sheet EE-434 Power Electronics: Basic Expressions CapacitorMuhammad Muzammil SaleemNo ratings yet
- 30F122 PDFDocument16 pages30F122 PDFLeonel AntonioNo ratings yet
- TD 4 Experimental Base of Quantum TheoryDocument15 pagesTD 4 Experimental Base of Quantum TheoryusaroufNo ratings yet
- PDF Updated Class 11 Physics Formula Sheet CompressDocument22 pagesPDF Updated Class 11 Physics Formula Sheet CompressdrjbjpNo ratings yet
- Resistance List En-DeDocument9 pagesResistance List En-DeLuis Fernando Becerra JimenezNo ratings yet
- Catalogo Toshiba Transistores IgbtDocument16 pagesCatalogo Toshiba Transistores Igbtluis daniel suarez narvaezNo ratings yet
- p5 Q2 SolvedDocument16 pagesp5 Q2 Solvedbookworm12045No ratings yet
- Electricity (Phy 2)Document10 pagesElectricity (Phy 2)Rafi DaffaNo ratings yet
- Sodapdf-Merged 4Document5 pagesSodapdf-Merged 4harshrajclass9brollno.21No ratings yet
- Assignment 4Document19 pagesAssignment 4Radhe JhaNo ratings yet
- Hydrostatics 2.a ExercisesDocument3 pagesHydrostatics 2.a ExercisesKat DjordjevicNo ratings yet
- Ramraj: SolutionDocument8 pagesRamraj: SolutionRajat Verma X D 39No ratings yet
- TD - tn#fJ9Iidengain: t.dk?nqd-1) ) 9IDocument6 pagesTD - tn#fJ9Iidengain: t.dk?nqd-1) ) 9Ifaisal adhiNo ratings yet
- ELECSDocument37 pagesELECSIra CervoNo ratings yet
- Ejercicios de FísicaDocument25 pagesEjercicios de FísicaVictor MaidanaNo ratings yet
- ProblemasDocument53 pagesProblemasvictor gerardo aguilera aguilarNo ratings yet
- Allen Physics Solutions 05Document27 pagesAllen Physics Solutions 05Dhani SharmaNo ratings yet
- 02 Electricity QuestionsDocument24 pages02 Electricity QuestionsruppertoopersdorffNo ratings yet
- PCD Reviewer PrelimsDocument2 pagesPCD Reviewer PrelimsJeremiah VillanuevaNo ratings yet
- Img 0003Document1 pageImg 0003ovidiu2014No ratings yet
- Freely: BoundaryDocument7 pagesFreely: BoundaryTitu RajputNo ratings yet
- 10 BhattiAcademy - Com Physics 6. Scohlar SeriesDocument21 pages10 BhattiAcademy - Com Physics 6. Scohlar SeriesBadar Mehmood BhattiNo ratings yet
- (2024) SD04 Excess Carriers and DiffusionDocument22 pages(2024) SD04 Excess Carriers and Diffusiontjkyle14No ratings yet
- Incidence Map 100421Document1 pageIncidence Map 100421haeli spearsNo ratings yet
- GT45F122 PDFDocument16 pagesGT45F122 PDFSinkdna AmdNo ratings yet
- Kimia Us AbyanDocument1 pageKimia Us AbyanYoga PratamaNo ratings yet
- B1 ElectrostaticsDocument1 pageB1 Electrostaticsdarkgaemer47No ratings yet
- Problem Sets 4Document1 pageProblem Sets 4Rhea Jane DugadugaNo ratings yet
- Class: GraphsDocument28 pagesClass: GraphsAlifiyah HussainNo ratings yet
- Utumporn Sonmak HW1Document9 pagesUtumporn Sonmak HW1Utumporn SonmakNo ratings yet
- COURS AstroDocument23 pagesCOURS Astro7b98gyyzf8No ratings yet
- Assignment 5 FM Intan Nur Haslinda 18001912Document14 pagesAssignment 5 FM Intan Nur Haslinda 18001912Intan NurhaslindaNo ratings yet
- 1908 - Ink and Questioned DocumentsDocument88 pages1908 - Ink and Questioned DocumentsDongelxNo ratings yet
- JunYuan Secondary Prelim 2021 PhysicsDocument49 pagesJunYuan Secondary Prelim 2021 PhysicsChristian AjaNo ratings yet
- Science, Technology & Clocks - Skinner Auction 2555MDocument172 pagesScience, Technology & Clocks - Skinner Auction 2555MSkinnerAuctions100% (2)
- State-Of-The-Art Measurement and Quality Control of Colours With Special Effects Towards Comfort and Aesthetics For End UsersDocument6 pagesState-Of-The-Art Measurement and Quality Control of Colours With Special Effects Towards Comfort and Aesthetics For End UsersKelson AraujoNo ratings yet
- FTIR Instrumentation Short-1Document6 pagesFTIR Instrumentation Short-1ChristianNo ratings yet
- An Introduction To Chinese Co2 Laser Engravers and CuttersDocument62 pagesAn Introduction To Chinese Co2 Laser Engravers and Cuttersdoriefourie1987No ratings yet
- Introduction To Air: All India RadioDocument47 pagesIntroduction To Air: All India RadioSaurabh MittalNo ratings yet
- Microscope - Id IndustryDocument18 pagesMicroscope - Id IndustryReuben ChristiantoNo ratings yet
- WaveguideDocument98 pagesWaveguideDr.Suresh Chavhan -IIITKNo ratings yet
- Chapter 2 Electrical LightingDocument24 pagesChapter 2 Electrical LightingKan Fock-KuiNo ratings yet
- Electron Energy and LightDocument6 pagesElectron Energy and LightYuxin (Janice) ZhuNo ratings yet
- Ers-S60w LM-79Document22 pagesErs-S60w LM-79LukeNo ratings yet
- CBSE Class 10 Science HOTs Question Human Eye and Colourful WorldDocument18 pagesCBSE Class 10 Science HOTs Question Human Eye and Colourful WorldAadi PunjabiNo ratings yet
- Non Linear Effects in FiberDocument25 pagesNon Linear Effects in Fiberthanhtam3819No ratings yet
- Nature and Properties of LightDocument16 pagesNature and Properties of LightCarl Daniel FandiñoNo ratings yet
- JS05 (Practical) - RGP 3133Document10 pagesJS05 (Practical) - RGP 3133Mohamad Amirul AnisNo ratings yet
- Interference of Light Waves PDFDocument29 pagesInterference of Light Waves PDFSGNo ratings yet
- CBSE Class 12 Physics Practical QuestionsDocument6 pagesCBSE Class 12 Physics Practical QuestionsSusheel Gupta50% (2)
- Completion Type Questions Exercise - 4 35mm Film CamerasDocument2 pagesCompletion Type Questions Exercise - 4 35mm Film CamerasHasnainNo ratings yet
- Fiber Laser PDFDocument2 pagesFiber Laser PDFDavid0% (2)
- Problem Chapter 3Document3 pagesProblem Chapter 3Ventri Galuh DikaraNo ratings yet
- Communication SystemsDocument59 pagesCommunication Systemsulul azmiNo ratings yet
- STRUCTURE OF ATOM at RIMS TS SSCDocument24 pagesSTRUCTURE OF ATOM at RIMS TS SSCSAI PRANEETH REDDY DHADINo ratings yet
- QC Solutions For CosmeticsDocument28 pagesQC Solutions For CosmeticsLuis Felipe BenavidesNo ratings yet
- E 1392 - 96 RtezotitotyDocument12 pagesE 1392 - 96 RtezotitotynooralhudNo ratings yet
- Structure Analysis Electron DiffractionDocument22 pagesStructure Analysis Electron Diffraction陳帥睿No ratings yet
- This Study Resource WasDocument9 pagesThis Study Resource WasNurul izzatiNo ratings yet
- NASA CR-1785, Radiation Effects Design HDBKDocument475 pagesNASA CR-1785, Radiation Effects Design HDBKRGK77No ratings yet
- PhotographyDocument47 pagesPhotographysserr436tttNo ratings yet
- Led Par Lamps: Par20, Par30, Par38 SeriesDocument3 pagesLed Par Lamps: Par20, Par30, Par38 SeriesJorge GomezNo ratings yet