Professional Documents
Culture Documents
0 ratings0% found this document useful (0 votes)
5 viewsCBSE-Class-X Science CHP 01
CBSE-Class-X Science CHP 01
Uploaded by
hem mishraCarbon forms covalent bonds with other carbon atoms, with a maximum of four bonds per carbon atom. Nitrogen forms triple bonds between nitrogen atoms in N2 molecules. Carbon has an atomic mass of 12 and buckminster fullerene, an allotrope of carbon, has the formula C60. Carbon atoms form four covalent bonds to satisfy the octet rule, making carbon tetravalent. Propene and propyne are unsaturated hydrocarbons that can form isomers. Methane has the molecular formula CH4 and its electron dot structure shows the covalent bonds formed between carbon and hydrogen atoms.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You might also like
- Chemistry in Focus A Molecular View of Our World 6th Edition Tro Solutions Manual 1Document12 pagesChemistry in Focus A Molecular View of Our World 6th Edition Tro Solutions Manual 1alison100% (48)
- Schaum's Easy Outline of Organic Chemistry, Second EditionFrom EverandSchaum's Easy Outline of Organic Chemistry, Second EditionRating: 3.5 out of 5 stars3.5/5 (2)
- Fixed OilsDocument11 pagesFixed OilsObbrei M. De Ocampo100% (1)
- Chapter1 and 2 HandoutDocument20 pagesChapter1 and 2 HandoutsugNo ratings yet
- KEg JYVKcfp 6 PH 6 Gs FS5 DDocument12 pagesKEg JYVKcfp 6 PH 6 Gs FS5 DNadim BashirNo ratings yet
- Solution 466049Document9 pagesSolution 466049S.N. RagulNo ratings yet
- Bonding 08Document1 pageBonding 08AshrafNo ratings yet
- Xi Chem CH 4Document24 pagesXi Chem CH 4LUCKY BHARADWAJNo ratings yet
- Chapter 12 Organic - ChemistryDocument86 pagesChapter 12 Organic - Chemistry9415ushabhargavaNo ratings yet
- Chemistry 10006Document27 pagesChemistry 10006Shai Shazza GrossNo ratings yet
- Covalent BondingDocument23 pagesCovalent BondingJames BorgNo ratings yet
- Consider The Following Anion CH CH CHCHDocument13 pagesConsider The Following Anion CH CH CHCHbobNo ratings yet
- Chemistry in Focus A Molecular View of Our World 6Th Edition Tro Solutions Manual Full Chapter PDFDocument33 pagesChemistry in Focus A Molecular View of Our World 6Th Edition Tro Solutions Manual Full Chapter PDFjulianna.washington847100% (14)
- ORGANIC ChemistryDocument150 pagesORGANIC ChemistryAhmad AlShahrourNo ratings yet
- Chemistry Reading Material Part-2Document12 pagesChemistry Reading Material Part-2RashpreetNo ratings yet
- L8,9 - Covalent BondingDocument21 pagesL8,9 - Covalent BondingKashifNo ratings yet
- Lab 01 Structure and BondingDocument19 pagesLab 01 Structure and BondingynottripNo ratings yet
- Organic Chemistry - 1Document14 pagesOrganic Chemistry - 1Habiba N. ElfrashNo ratings yet
- Drawing Lewis StructureDocument23 pagesDrawing Lewis StructureJose Gilberto De LeonNo ratings yet
- Compounds: Carbon andDocument17 pagesCompounds: Carbon andankitrathoreagentNo ratings yet
- 02 The Chemistry of Milk PDFDocument26 pages02 The Chemistry of Milk PDFTintin Brusola SalenNo ratings yet
- Organic ChemistryDocument40 pagesOrganic ChemistryGlenda ResultayNo ratings yet
- What Is Chemistry?: The Science That Studies The Interactions of MatterDocument37 pagesWhat Is Chemistry?: The Science That Studies The Interactions of Matterabd1usNo ratings yet
- Fundamentals of Mechanism and Stereochemistry: CHEM0005 Chemical Foundations: Section CDocument174 pagesFundamentals of Mechanism and Stereochemistry: CHEM0005 Chemical Foundations: Section Cssayed1No ratings yet
- CH1O3 Questions PDFDocument52 pagesCH1O3 Questions PDFPrince T MashandaNo ratings yet
- Bonding 07Document1 pageBonding 07AshrafNo ratings yet
- 1.5 Introduction To Organic ChemistryDocument14 pages1.5 Introduction To Organic Chemistrymaya 1DNo ratings yet
- Organic Chemistry 1&2Document142 pagesOrganic Chemistry 1&2Kennedy ChitayiNo ratings yet
- Exercise #4 - Covalent BondsDocument2 pagesExercise #4 - Covalent BondsFucboi CarloNo ratings yet
- Introduction To Organic Chemistry 2019Document127 pagesIntroduction To Organic Chemistry 2019Nonhlanhla NdlovuNo ratings yet
- Carbonanditscompounds 140323010050 Phpapp01Document22 pagesCarbonanditscompounds 140323010050 Phpapp01Rakesh KumarNo ratings yet
- Carbon and Its Compound NotesDocument30 pagesCarbon and Its Compound Noteskrishna industries100% (1)
- Organic Chemistry CCDocument77 pagesOrganic Chemistry CCmunthalipeggy4No ratings yet
- 6 Chemical Bonding and Structures of Environmental PollutantsDocument20 pages6 Chemical Bonding and Structures of Environmental PollutantsMaaz WaseemNo ratings yet
- Kimia Organik 1 Stuktur Atom: - Jurusan/Program Studi: Kimia/Pendidikan KimiaDocument50 pagesKimia Organik 1 Stuktur Atom: - Jurusan/Program Studi: Kimia/Pendidikan Kimiatimbul wibowoNo ratings yet
- Chapter 1Document43 pagesChapter 1zztoppsNo ratings yet
- Basic Principles of Organic ChemistryDocument25 pagesBasic Principles of Organic ChemistryjagannivasNo ratings yet
- Organic Chemistry 1 - Chem - f3 - v1 1Document77 pagesOrganic Chemistry 1 - Chem - f3 - v1 1Lubanga N JamesNo ratings yet
- Kami Export - Free Response Questions For Surface Tension and Intermolecular ForcesDocument5 pagesKami Export - Free Response Questions For Surface Tension and Intermolecular ForcesVineet ErellaNo ratings yet
- Aldehyde Andd KetonsDocument33 pagesAldehyde Andd KetonssandipNo ratings yet
- Chapter 11 Carbon CompoundDocument50 pagesChapter 11 Carbon CompoundvaogerNo ratings yet
- Alkanes Lecture - 1Document54 pagesAlkanes Lecture - 1hagshhsiauhagah516525No ratings yet
- Unit 11 Fundamentals Org ChemDocument35 pagesUnit 11 Fundamentals Org ChemKavisha AshaNo ratings yet
- Carbon and Its Compounds 1Document25 pagesCarbon and Its Compounds 1dubstepoNo ratings yet
- Carbon and Its CompoundsDocument25 pagesCarbon and Its CompoundsdubstepoNo ratings yet
- 1-Carbon and Its CompoundsDocument3 pages1-Carbon and Its CompoundsPrasan NandaNo ratings yet
- Conjugation and HyperconjugationDocument3 pagesConjugation and Hyperconjugationmschlecht100% (1)
- Aldehyde Jeemain - GuruDocument33 pagesAldehyde Jeemain - GuruanshulNo ratings yet
- Organic Revision GuideDocument142 pagesOrganic Revision Guidetrangvi.hcmNo ratings yet
- Lecture 5 Chemical Bonding and StructureDocument33 pagesLecture 5 Chemical Bonding and StructurekedirNo ratings yet
- مقدمة عن الكيمياء العضويةDocument4 pagesمقدمة عن الكيمياء العضويةHesham Al Saide0% (1)
- Carbon Its CompoundsDocument54 pagesCarbon Its CompoundsthinkiitNo ratings yet
- Tutorial Letter 203/1/2018: General Chemistry 1BDocument12 pagesTutorial Letter 203/1/2018: General Chemistry 1BLeigh MakanNo ratings yet
- Tutorial Letter 201/1/2016: General Chemistry 1BDocument22 pagesTutorial Letter 201/1/2016: General Chemistry 1BLeigh MakanNo ratings yet
- CN BC Organic - Part1Document28 pagesCN BC Organic - Part1Trisha MarieNo ratings yet
- Carbon and Its CompoundsDocument22 pagesCarbon and Its CompoundsvishalchhatriNo ratings yet
- Introduction To Organic ChemistryDocument92 pagesIntroduction To Organic ChemistryNUR HAZWANI BINTI MOHAMAD SANI / UPMNo ratings yet
- Practice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersFrom EverandPractice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersNo ratings yet
- Chloro BenzeneDocument22 pagesChloro BenzeneAnshumanSrivastavaNo ratings yet
- Merck AR1 2011Document39 pagesMerck AR1 2011rekatani indonesiaNo ratings yet
- TEST-1 ALCOHOL Physical PropertiesDocument5 pagesTEST-1 ALCOHOL Physical PropertiesFableNo ratings yet
- Resonance DPP 1Document6 pagesResonance DPP 1Subham YadavNo ratings yet
- Class 12 Chemistry Set 1Document15 pagesClass 12 Chemistry Set 1latestdaaNo ratings yet
- Protein FoldingDocument32 pagesProtein Folding9374 DIVYATA PATILNo ratings yet
- Phosphorus, Acid Hydrolyzable, TNT, Method 8180, 02-2009, 9th EdDocument7 pagesPhosphorus, Acid Hydrolyzable, TNT, Method 8180, 02-2009, 9th Edb wrNo ratings yet
- Chemistry s5 Theory and Pract.Document29 pagesChemistry s5 Theory and Pract.ngabonzizayusuf9No ratings yet
- Cymel 1158Document2 pagesCymel 1158sidneidecarvalho100% (1)
- PPDDocument79 pagesPPDEddie ChangNo ratings yet
- 1st Final Examination of OcDocument2 pages1st Final Examination of OcRahmanida SusianaNo ratings yet
- AkzoNobel MEA PIS Tcm53-24552Document1 pageAkzoNobel MEA PIS Tcm53-24552Desi Apriyanti RahayuNo ratings yet
- Buffer in Biological & Pharmaceutical SystemsDocument28 pagesBuffer in Biological & Pharmaceutical Systemshamam salih badriNo ratings yet
- Syllabus IIT JEE Main Chemistry 20181031165822Document2 pagesSyllabus IIT JEE Main Chemistry 20181031165822Aditi ShuklaNo ratings yet
- January 2014 - Question Paper - Chemistry U2Document20 pagesJanuary 2014 - Question Paper - Chemistry U2lolomg90No ratings yet
- AFCONA - 7500 TDS EngDocument1 pageAFCONA - 7500 TDS EngHamood AbdoNo ratings yet
- Highly Acidic BINOL-Derived Phosphoramidimidates and Their ApplicationDocument3 pagesHighly Acidic BINOL-Derived Phosphoramidimidates and Their Applicationxuyijing2007comNo ratings yet
- Answers Equilibrium Solutions and KSPDocument10 pagesAnswers Equilibrium Solutions and KSPrajNo ratings yet
- Olympiad 2012 r1 Mark Scheme PDFDocument8 pagesOlympiad 2012 r1 Mark Scheme PDFJackieWilsonNo ratings yet
- Transition Metal Chem Part 2-NomenclatureDocument3 pagesTransition Metal Chem Part 2-NomenclatureAakash VermaNo ratings yet
- Tit Rime TryDocument40 pagesTit Rime TryGab TrinillaNo ratings yet
- Flavor Potentiators Maga 1983Document83 pagesFlavor Potentiators Maga 1983FAS AAMU100% (1)
- Naming Hydrocarbons Worksheet1 KDocument2 pagesNaming Hydrocarbons Worksheet1 KRare RootNo ratings yet
- Canadian Business English Canadian 7th Edition Guffey Solutions ManualDocument35 pagesCanadian Business English Canadian 7th Edition Guffey Solutions Manualpeanutsofteniscd1n100% (31)
- Friedel-Crafts Acylation of TolueneDocument6 pagesFriedel-Crafts Acylation of TolueneKybernetikumNo ratings yet
- Carboxylic Acids and Ester WS-2Document5 pagesCarboxylic Acids and Ester WS-2Me and the boysNo ratings yet
- Weight Loss Corrosion Study of Some Metals in AcidDocument8 pagesWeight Loss Corrosion Study of Some Metals in AcidAdisya Yuliasari RohimanNo ratings yet
- Catalytic Decarboxylation of Naphthenic Acids in Crude OilsDocument9 pagesCatalytic Decarboxylation of Naphthenic Acids in Crude Oilsjuan9601No ratings yet
- (A-B) Mini-Encyclopedia of Papermaking Wet-End ChemistryDocument5 pages(A-B) Mini-Encyclopedia of Papermaking Wet-End ChemistryAhmed AsforaNo ratings yet
CBSE-Class-X Science CHP 01
CBSE-Class-X Science CHP 01
Uploaded by
hem mishra0 ratings0% found this document useful (0 votes)
5 views1 pageCarbon forms covalent bonds with other carbon atoms, with a maximum of four bonds per carbon atom. Nitrogen forms triple bonds between nitrogen atoms in N2 molecules. Carbon has an atomic mass of 12 and buckminster fullerene, an allotrope of carbon, has the formula C60. Carbon atoms form four covalent bonds to satisfy the octet rule, making carbon tetravalent. Propene and propyne are unsaturated hydrocarbons that can form isomers. Methane has the molecular formula CH4 and its electron dot structure shows the covalent bonds formed between carbon and hydrogen atoms.
Original Description:
Original Title
CBSE-Class-X Science Chp 01(1)
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCarbon forms covalent bonds with other carbon atoms, with a maximum of four bonds per carbon atom. Nitrogen forms triple bonds between nitrogen atoms in N2 molecules. Carbon has an atomic mass of 12 and buckminster fullerene, an allotrope of carbon, has the formula C60. Carbon atoms form four covalent bonds to satisfy the octet rule, making carbon tetravalent. Propene and propyne are unsaturated hydrocarbons that can form isomers. Methane has the molecular formula CH4 and its electron dot structure shows the covalent bonds formed between carbon and hydrogen atoms.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
5 views1 pageCBSE-Class-X Science CHP 01
CBSE-Class-X Science CHP 01
Uploaded by
hem mishraCarbon forms covalent bonds with other carbon atoms, with a maximum of four bonds per carbon atom. Nitrogen forms triple bonds between nitrogen atoms in N2 molecules. Carbon has an atomic mass of 12 and buckminster fullerene, an allotrope of carbon, has the formula C60. Carbon atoms form four covalent bonds to satisfy the octet rule, making carbon tetravalent. Propene and propyne are unsaturated hydrocarbons that can form isomers. Methane has the molecular formula CH4 and its electron dot structure shows the covalent bonds formed between carbon and hydrogen atoms.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 1
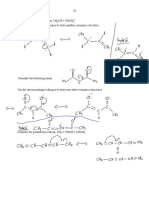
1 Carbon and its Compounds
1. Maximum angle strain is obtained when the two H H OH H
carbon atoms are linked by more than three covalent
or H¾C¾C¾C¾C¾H
bonds, thus it is not formed.
2. (i) H H H H
12. Covalent compounds like urea, do not conduct
N N ÞN
electricity in aqueous solution. In contrast ionic
N
compounds like potassium chloride can conduct
electricity in aqueous solution.
Thus, N 2 contains triple bond.
13. Oxygen (8) has 6 electrons in its outermost shell. It
(ii) Among the given compound Cl 2 and N 2 are shares two electrons with another atom of oxygen,
covalent compound. While NaCl is ionic making a molecule of oxygen.
compound.
As a result of which oxygen gets 8 electrons in its
3. Atomic mass of C = 12 outermost shell and a double bond is formed.
Molecular mass of allotrope of C = 720
720 O O O O ÞO O
\ Number of C-atoms = = 60
12 Oxygen Atoms
Oxygen molecule
Thus, C 60 is the formula of given allotrope and its
name is Buckminster fullerene. 14. (i) I. C 3H 8O contains alcoholic functional groups.
4. Carbon atom has 4 electrons in its outermost shell. (ii) II. C 3H 6O 2 contains carboxylic acid functional
It needs 4 more electrons to complete its octet. group.
Therefore, carbon is tetravalent. Their structures are as follows
H H H
5. They are generally covalent compounds.
½ ½ ½
They show isomerism. H ¾ C ¾ C ¾ C ¾ OH
6. Propene and propyne are unsaturated hydrocarbons. ½ ½ ½
H H H
7. Three hydrocarbons are possible by joining two (An alcohol)
carbon atoms. These are ethane, ethene, ethyne. H H O
8. In the given compound (i) ¾ COOH (carboxylic ½ ½ ½½
H¾ C¾ C¾ C ¾ O¾ H
group) is the functional group and in ½ ½
O
H H
½½ (A carboxylic acid)
(ii) ¾ C ¾ (ketone) is the functional group.
(ii) No, (I) and (II) are not isomers. Isomers are the
9. C 3H 6 and C 4H 8 are the 2nd and 3rd member of organic compounds with same molecular
the homologous series where the first member is formula but different chemical and physical
ethene. properties. But, here molecular formula of (I) and
10. (II) are different.
H
15. Methane is a hydrocarbon having molecular formula
H C C H CH 4 .
H
H
Electron dot structure of CH3CHO H C H
H H H
½ ½ ½ H
11. H 3C ¾ C ¾ C ¾ C ¾ OH
½ ½ ½ Electron dot structure of methane
H H H Covalent bonds are formed in this compound.
You might also like
- Chemistry in Focus A Molecular View of Our World 6th Edition Tro Solutions Manual 1Document12 pagesChemistry in Focus A Molecular View of Our World 6th Edition Tro Solutions Manual 1alison100% (48)
- Schaum's Easy Outline of Organic Chemistry, Second EditionFrom EverandSchaum's Easy Outline of Organic Chemistry, Second EditionRating: 3.5 out of 5 stars3.5/5 (2)
- Fixed OilsDocument11 pagesFixed OilsObbrei M. De Ocampo100% (1)
- Chapter1 and 2 HandoutDocument20 pagesChapter1 and 2 HandoutsugNo ratings yet
- KEg JYVKcfp 6 PH 6 Gs FS5 DDocument12 pagesKEg JYVKcfp 6 PH 6 Gs FS5 DNadim BashirNo ratings yet
- Solution 466049Document9 pagesSolution 466049S.N. RagulNo ratings yet
- Bonding 08Document1 pageBonding 08AshrafNo ratings yet
- Xi Chem CH 4Document24 pagesXi Chem CH 4LUCKY BHARADWAJNo ratings yet
- Chapter 12 Organic - ChemistryDocument86 pagesChapter 12 Organic - Chemistry9415ushabhargavaNo ratings yet
- Chemistry 10006Document27 pagesChemistry 10006Shai Shazza GrossNo ratings yet
- Covalent BondingDocument23 pagesCovalent BondingJames BorgNo ratings yet
- Consider The Following Anion CH CH CHCHDocument13 pagesConsider The Following Anion CH CH CHCHbobNo ratings yet
- Chemistry in Focus A Molecular View of Our World 6Th Edition Tro Solutions Manual Full Chapter PDFDocument33 pagesChemistry in Focus A Molecular View of Our World 6Th Edition Tro Solutions Manual Full Chapter PDFjulianna.washington847100% (14)
- ORGANIC ChemistryDocument150 pagesORGANIC ChemistryAhmad AlShahrourNo ratings yet
- Chemistry Reading Material Part-2Document12 pagesChemistry Reading Material Part-2RashpreetNo ratings yet
- L8,9 - Covalent BondingDocument21 pagesL8,9 - Covalent BondingKashifNo ratings yet
- Lab 01 Structure and BondingDocument19 pagesLab 01 Structure and BondingynottripNo ratings yet
- Organic Chemistry - 1Document14 pagesOrganic Chemistry - 1Habiba N. ElfrashNo ratings yet
- Drawing Lewis StructureDocument23 pagesDrawing Lewis StructureJose Gilberto De LeonNo ratings yet
- Compounds: Carbon andDocument17 pagesCompounds: Carbon andankitrathoreagentNo ratings yet
- 02 The Chemistry of Milk PDFDocument26 pages02 The Chemistry of Milk PDFTintin Brusola SalenNo ratings yet
- Organic ChemistryDocument40 pagesOrganic ChemistryGlenda ResultayNo ratings yet
- What Is Chemistry?: The Science That Studies The Interactions of MatterDocument37 pagesWhat Is Chemistry?: The Science That Studies The Interactions of Matterabd1usNo ratings yet
- Fundamentals of Mechanism and Stereochemistry: CHEM0005 Chemical Foundations: Section CDocument174 pagesFundamentals of Mechanism and Stereochemistry: CHEM0005 Chemical Foundations: Section Cssayed1No ratings yet
- CH1O3 Questions PDFDocument52 pagesCH1O3 Questions PDFPrince T MashandaNo ratings yet
- Bonding 07Document1 pageBonding 07AshrafNo ratings yet
- 1.5 Introduction To Organic ChemistryDocument14 pages1.5 Introduction To Organic Chemistrymaya 1DNo ratings yet
- Organic Chemistry 1&2Document142 pagesOrganic Chemistry 1&2Kennedy ChitayiNo ratings yet
- Exercise #4 - Covalent BondsDocument2 pagesExercise #4 - Covalent BondsFucboi CarloNo ratings yet
- Introduction To Organic Chemistry 2019Document127 pagesIntroduction To Organic Chemistry 2019Nonhlanhla NdlovuNo ratings yet
- Carbonanditscompounds 140323010050 Phpapp01Document22 pagesCarbonanditscompounds 140323010050 Phpapp01Rakesh KumarNo ratings yet
- Carbon and Its Compound NotesDocument30 pagesCarbon and Its Compound Noteskrishna industries100% (1)
- Organic Chemistry CCDocument77 pagesOrganic Chemistry CCmunthalipeggy4No ratings yet
- 6 Chemical Bonding and Structures of Environmental PollutantsDocument20 pages6 Chemical Bonding and Structures of Environmental PollutantsMaaz WaseemNo ratings yet
- Kimia Organik 1 Stuktur Atom: - Jurusan/Program Studi: Kimia/Pendidikan KimiaDocument50 pagesKimia Organik 1 Stuktur Atom: - Jurusan/Program Studi: Kimia/Pendidikan Kimiatimbul wibowoNo ratings yet
- Chapter 1Document43 pagesChapter 1zztoppsNo ratings yet
- Basic Principles of Organic ChemistryDocument25 pagesBasic Principles of Organic ChemistryjagannivasNo ratings yet
- Organic Chemistry 1 - Chem - f3 - v1 1Document77 pagesOrganic Chemistry 1 - Chem - f3 - v1 1Lubanga N JamesNo ratings yet
- Kami Export - Free Response Questions For Surface Tension and Intermolecular ForcesDocument5 pagesKami Export - Free Response Questions For Surface Tension and Intermolecular ForcesVineet ErellaNo ratings yet
- Aldehyde Andd KetonsDocument33 pagesAldehyde Andd KetonssandipNo ratings yet
- Chapter 11 Carbon CompoundDocument50 pagesChapter 11 Carbon CompoundvaogerNo ratings yet
- Alkanes Lecture - 1Document54 pagesAlkanes Lecture - 1hagshhsiauhagah516525No ratings yet
- Unit 11 Fundamentals Org ChemDocument35 pagesUnit 11 Fundamentals Org ChemKavisha AshaNo ratings yet
- Carbon and Its Compounds 1Document25 pagesCarbon and Its Compounds 1dubstepoNo ratings yet
- Carbon and Its CompoundsDocument25 pagesCarbon and Its CompoundsdubstepoNo ratings yet
- 1-Carbon and Its CompoundsDocument3 pages1-Carbon and Its CompoundsPrasan NandaNo ratings yet
- Conjugation and HyperconjugationDocument3 pagesConjugation and Hyperconjugationmschlecht100% (1)
- Aldehyde Jeemain - GuruDocument33 pagesAldehyde Jeemain - GuruanshulNo ratings yet
- Organic Revision GuideDocument142 pagesOrganic Revision Guidetrangvi.hcmNo ratings yet
- Lecture 5 Chemical Bonding and StructureDocument33 pagesLecture 5 Chemical Bonding and StructurekedirNo ratings yet
- مقدمة عن الكيمياء العضويةDocument4 pagesمقدمة عن الكيمياء العضويةHesham Al Saide0% (1)
- Carbon Its CompoundsDocument54 pagesCarbon Its CompoundsthinkiitNo ratings yet
- Tutorial Letter 203/1/2018: General Chemistry 1BDocument12 pagesTutorial Letter 203/1/2018: General Chemistry 1BLeigh MakanNo ratings yet
- Tutorial Letter 201/1/2016: General Chemistry 1BDocument22 pagesTutorial Letter 201/1/2016: General Chemistry 1BLeigh MakanNo ratings yet
- CN BC Organic - Part1Document28 pagesCN BC Organic - Part1Trisha MarieNo ratings yet
- Carbon and Its CompoundsDocument22 pagesCarbon and Its CompoundsvishalchhatriNo ratings yet
- Introduction To Organic ChemistryDocument92 pagesIntroduction To Organic ChemistryNUR HAZWANI BINTI MOHAMAD SANI / UPMNo ratings yet
- Practice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersFrom EverandPractice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersNo ratings yet
- Chloro BenzeneDocument22 pagesChloro BenzeneAnshumanSrivastavaNo ratings yet
- Merck AR1 2011Document39 pagesMerck AR1 2011rekatani indonesiaNo ratings yet
- TEST-1 ALCOHOL Physical PropertiesDocument5 pagesTEST-1 ALCOHOL Physical PropertiesFableNo ratings yet
- Resonance DPP 1Document6 pagesResonance DPP 1Subham YadavNo ratings yet
- Class 12 Chemistry Set 1Document15 pagesClass 12 Chemistry Set 1latestdaaNo ratings yet
- Protein FoldingDocument32 pagesProtein Folding9374 DIVYATA PATILNo ratings yet
- Phosphorus, Acid Hydrolyzable, TNT, Method 8180, 02-2009, 9th EdDocument7 pagesPhosphorus, Acid Hydrolyzable, TNT, Method 8180, 02-2009, 9th Edb wrNo ratings yet
- Chemistry s5 Theory and Pract.Document29 pagesChemistry s5 Theory and Pract.ngabonzizayusuf9No ratings yet
- Cymel 1158Document2 pagesCymel 1158sidneidecarvalho100% (1)
- PPDDocument79 pagesPPDEddie ChangNo ratings yet
- 1st Final Examination of OcDocument2 pages1st Final Examination of OcRahmanida SusianaNo ratings yet
- AkzoNobel MEA PIS Tcm53-24552Document1 pageAkzoNobel MEA PIS Tcm53-24552Desi Apriyanti RahayuNo ratings yet
- Buffer in Biological & Pharmaceutical SystemsDocument28 pagesBuffer in Biological & Pharmaceutical Systemshamam salih badriNo ratings yet
- Syllabus IIT JEE Main Chemistry 20181031165822Document2 pagesSyllabus IIT JEE Main Chemistry 20181031165822Aditi ShuklaNo ratings yet
- January 2014 - Question Paper - Chemistry U2Document20 pagesJanuary 2014 - Question Paper - Chemistry U2lolomg90No ratings yet
- AFCONA - 7500 TDS EngDocument1 pageAFCONA - 7500 TDS EngHamood AbdoNo ratings yet
- Highly Acidic BINOL-Derived Phosphoramidimidates and Their ApplicationDocument3 pagesHighly Acidic BINOL-Derived Phosphoramidimidates and Their Applicationxuyijing2007comNo ratings yet
- Answers Equilibrium Solutions and KSPDocument10 pagesAnswers Equilibrium Solutions and KSPrajNo ratings yet
- Olympiad 2012 r1 Mark Scheme PDFDocument8 pagesOlympiad 2012 r1 Mark Scheme PDFJackieWilsonNo ratings yet
- Transition Metal Chem Part 2-NomenclatureDocument3 pagesTransition Metal Chem Part 2-NomenclatureAakash VermaNo ratings yet
- Tit Rime TryDocument40 pagesTit Rime TryGab TrinillaNo ratings yet
- Flavor Potentiators Maga 1983Document83 pagesFlavor Potentiators Maga 1983FAS AAMU100% (1)
- Naming Hydrocarbons Worksheet1 KDocument2 pagesNaming Hydrocarbons Worksheet1 KRare RootNo ratings yet
- Canadian Business English Canadian 7th Edition Guffey Solutions ManualDocument35 pagesCanadian Business English Canadian 7th Edition Guffey Solutions Manualpeanutsofteniscd1n100% (31)
- Friedel-Crafts Acylation of TolueneDocument6 pagesFriedel-Crafts Acylation of TolueneKybernetikumNo ratings yet
- Carboxylic Acids and Ester WS-2Document5 pagesCarboxylic Acids and Ester WS-2Me and the boysNo ratings yet
- Weight Loss Corrosion Study of Some Metals in AcidDocument8 pagesWeight Loss Corrosion Study of Some Metals in AcidAdisya Yuliasari RohimanNo ratings yet
- Catalytic Decarboxylation of Naphthenic Acids in Crude OilsDocument9 pagesCatalytic Decarboxylation of Naphthenic Acids in Crude Oilsjuan9601No ratings yet
- (A-B) Mini-Encyclopedia of Papermaking Wet-End ChemistryDocument5 pages(A-B) Mini-Encyclopedia of Papermaking Wet-End ChemistryAhmed AsforaNo ratings yet