Professional Documents
Culture Documents
0 ratings0% found this document useful (0 votes)
14 viewsChemicql Effects of Electric Current (Excercise)
Chemicql Effects of Electric Current (Excercise)
Uploaded by
Isha KanojiyaThis document contains 12 exercise questions related to electricity and conductivity. The questions cover topics like conductors and non-conductors, factors that affect conductivity, electroplating, and safety precautions around electricity during rain. Multiple choice and open-ended questions are included and answered in detail explaining concepts like how saltwater is a better conductor than pure water and why it is dangerous to perform electrical repairs outdoors during heavy rain.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You might also like
- DuxebaxemaraviziwizubuzanDocument3 pagesDuxebaxemaraviziwizubuzanIsha KanojiyaNo ratings yet
- Class 12th Chemistry Project On Electrochemical CellsDocument11 pagesClass 12th Chemistry Project On Electrochemical CellsUdit Kanotra57% (37)
- APQP Checklist Report - Version 1.1.Dtd. 13.11.08Document51 pagesAPQP Checklist Report - Version 1.1.Dtd. 13.11.08robbie86290% (10)
- CPR Study Guide 2021: Online CPR Cheat SheetDocument14 pagesCPR Study Guide 2021: Online CPR Cheat SheetHenz Freeman0% (2)
- Family ResourcesDocument33 pagesFamily ResourcesJackie S LaymanNo ratings yet
- Chemical Effects of Electric CurrentDocument6 pagesChemical Effects of Electric CurrentImran ShahidNo ratings yet
- Chemical Effects of Electric CurrentDocument3 pagesChemical Effects of Electric Currentbharat patelNo ratings yet
- Grade 8 Science SyllabusDocument3 pagesGrade 8 Science SyllabusCRYSTAL GODNo ratings yet
- Viii Chapter 14Document6 pagesViii Chapter 14Shubh tiwari TiwariNo ratings yet
- Chemical Effects of Electric Current - Class 8 - NCERT Exercise Questions - PANTOMATHDocument3 pagesChemical Effects of Electric Current - Class 8 - NCERT Exercise Questions - PANTOMATHsourav9823No ratings yet
- NCERT Solutions For Class 8 Science Chapter 14Document4 pagesNCERT Solutions For Class 8 Science Chapter 14Padmalaya paloNo ratings yet
- 3543 93 Textbooksolution PDFDocument4 pages3543 93 Textbooksolution PDFAnushka RaoNo ratings yet
- CBSE NCERT Solutions For Class 8 Science Chapter 14: Back of Chapter QuestionsDocument4 pagesCBSE NCERT Solutions For Class 8 Science Chapter 14: Back of Chapter Questionsprem singhNo ratings yet
- Class 8 - Science - Chemical Effects of Electric CurrentDocument7 pagesClass 8 - Science - Chemical Effects of Electric CurrentSougrakpam SNo ratings yet
- Chemical Effects of ElectricDocument4 pagesChemical Effects of Electricali binNo ratings yet
- Delhi Public School Bangalore - East Chemistry Chemical Effects of Electric Current (Notes) NAME: - Class: Viii SecDocument3 pagesDelhi Public School Bangalore - East Chemistry Chemical Effects of Electric Current (Notes) NAME: - Class: Viii SecSkanda EnterprisesNo ratings yet
- PhysicsDocument9 pagesPhysicsGanesh VNo ratings yet
- Delhi Public School, Bangalore East Chemical Effects of Electric CurrentDocument3 pagesDelhi Public School, Bangalore East Chemical Effects of Electric CurrentSaket TNo ratings yet
- 8 ScienceDocument4 pages8 SciencePratibha GuptaNo ratings yet
- Chemical Effects of Electric CurrentDocument4 pagesChemical Effects of Electric Currentaadithya.v.5502.sssmscNo ratings yet
- Chemical Effect of Electric Current-5 (2021-22)Document24 pagesChemical Effect of Electric Current-5 (2021-22)Avyam SharmaNo ratings yet
- Chemical Effects of Electric CurrentDocument10 pagesChemical Effects of Electric Currentpragunjain2010No ratings yet
- PHYSICS NOTES - CHEMICAL EFFECTS OF ELECTRIC CURRENT - CLASS VIII - FINAL - CombinedDocument20 pagesPHYSICS NOTES - CHEMICAL EFFECTS OF ELECTRIC CURRENT - CLASS VIII - FINAL - CombinedB. Guru PrasadNo ratings yet
- Exemplar - Chemical Effects of CurrentDocument14 pagesExemplar - Chemical Effects of CurrentnitikaNo ratings yet
- Chemical Effects of Electric Current - NotesDocument2 pagesChemical Effects of Electric Current - Notesgmrhfamily2024No ratings yet
- 8 Science NCERT Solutions Chapter 14Document4 pages8 Science NCERT Solutions Chapter 14Raj AnandNo ratings yet
- Chemical Effects of Electric Current C W and H W 08 11 2023 20231108182020326Document3 pagesChemical Effects of Electric Current C W and H W 08 11 2023 20231108182020326dxvybzhcj8No ratings yet
- Chemical Effects NotesDocument3 pagesChemical Effects NotessabreenaapNo ratings yet
- C E O C 14: Hemical Ffects F UrrentDocument7 pagesC E O C 14: Hemical Ffects F UrrentPravat TiadiNo ratings yet
- Answer Key of Previous Worksheet On Chemical Effects of Electric Current (Grade 8 Cbse)Document3 pagesAnswer Key of Previous Worksheet On Chemical Effects of Electric Current (Grade 8 Cbse)mithlikesfun2No ratings yet
- 8 Chemical Effects of Electric CurrentDocument5 pages8 Chemical Effects of Electric Currentandrew andrewNo ratings yet
- Importante Questions For CBSE Class 8 Science Chapter 14Document6 pagesImportante Questions For CBSE Class 8 Science Chapter 14Shubham KumarNo ratings yet
- Chemical Effects of Electric Current: AnswerDocument7 pagesChemical Effects of Electric Current: AnswerTvisha SolankiNo ratings yet
- CLASS VIII QUESTION BANK - 14. Chemical Effects of Electric CurrentDocument6 pagesCLASS VIII QUESTION BANK - 14. Chemical Effects of Electric CurrentSurbhi NayarNo ratings yet
- 8th Chemical Effects of Electric Current Solved QuestionsDocument3 pages8th Chemical Effects of Electric Current Solved QuestionsGururaj KulkarniNo ratings yet
- Notes-CHEMICAL EFFECTS OF ELECTRIC CURRENTDocument4 pagesNotes-CHEMICAL EFFECTS OF ELECTRIC CURRENTishaan14810No ratings yet
- Chemical Effects of Electric Current (Grade 8 CBSE)Document2 pagesChemical Effects of Electric Current (Grade 8 CBSE)mithlikesfun2No ratings yet
- 8th Chemical Effect of Electric Current Living Science SolutionDocument3 pages8th Chemical Effect of Electric Current Living Science SolutionAnshika BhagatNo ratings yet
- 8th Chemical Effects of Electric Current Solved QuestionsDocument3 pages8th Chemical Effects of Electric Current Solved QuestionsKavitha KNo ratings yet
- Chemical Effects of CurrentDocument7 pagesChemical Effects of CurrentRonak ShahNo ratings yet
- Rajkumar Chemistry 2-1Document13 pagesRajkumar Chemistry 2-1Gopala krishnanNo ratings yet
- Electrical Conductivity of Liquids Q & ADocument12 pagesElectrical Conductivity of Liquids Q & Ajayasree012754No ratings yet
- Electric CurrentDocument12 pagesElectric Currentstory manNo ratings yet
- (8th) Chemical Effects of Electric Current Solved AssignmentsDocument3 pages(8th) Chemical Effects of Electric Current Solved AssignmentssushantNo ratings yet
- 185 638283988626517543Document3 pages185 638283988626517543pqvrc6q25mNo ratings yet
- Elctrolysis XDocument6 pagesElctrolysis XManash SinghaNo ratings yet
- Assignment 2 ElectrolysisDocument4 pagesAssignment 2 ElectrolysisJayadevi ShanmugamNo ratings yet
- Electrolysis: Lesson 10 - Electrolysis (This Lesson Comes in The Science Part II)Document6 pagesElectrolysis: Lesson 10 - Electrolysis (This Lesson Comes in The Science Part II)SwarnapaliliyanageNo ratings yet
- How to Install Electric Bells, Annunciators, and AlarmsFrom EverandHow to Install Electric Bells, Annunciators, and AlarmsNo ratings yet
- Class 6 Electricity and Circuit Exercise Question and Answer Plus Inner (Extra) Question and AnswerDocument7 pagesClass 6 Electricity and Circuit Exercise Question and Answer Plus Inner (Extra) Question and Answerdebasishnath9593913213No ratings yet
- Chemical Effect of Current - 8thDocument14 pagesChemical Effect of Current - 8thVijay KumarNo ratings yet
- FinalDocument12 pagesFinalsameeryad72No ratings yet
- Electrolysis NotesDocument11 pagesElectrolysis NotesMichaela PowellNo ratings yet
- Grade 8 Ch14Document3 pagesGrade 8 Ch14parjercreation1No ratings yet
- Chemical Effectsof Electric Current-Class NotesDocument10 pagesChemical Effectsof Electric Current-Class NotesGaurav AggarwalNo ratings yet
- Long Ans Type QueDocument2 pagesLong Ans Type QueFor JunkNo ratings yet
- Project Report 12 BDocument13 pagesProject Report 12 BParth SaxenaNo ratings yet
- Finding of Emf of Electrochemical CellDocument20 pagesFinding of Emf of Electrochemical CellVaibhav Shankar100% (1)
- DSR Elektronika BartsaDocument6 pagesDSR Elektronika BartsaBarNo ratings yet
- Danish ChemistryDocument13 pagesDanish ChemistryNaveen Kumar.RNo ratings yet
- Electricity and CircuitDocument6 pagesElectricity and CircuitVarun kumarNo ratings yet
- Chem Project.Document17 pagesChem Project.Shresth TiwariNo ratings yet
- Electric and WaterDocument11 pagesElectric and WaterCartoonn AroonnumchokNo ratings yet
- Water Level Indicator Circuit Using Bipolar Junction TransistorFrom EverandWater Level Indicator Circuit Using Bipolar Junction TransistorRating: 4.5 out of 5 stars4.5/5 (7)
- Fungal Pathogens BPTDocument2 pagesFungal Pathogens BPTIsha KanojiyaNo ratings yet
- ProjectDocument3 pagesProjectIsha KanojiyaNo ratings yet
- Bacteria and Virus General StructureDocument2 pagesBacteria and Virus General StructureIsha KanojiyaNo ratings yet
- Complement SystemDocument2 pagesComplement SystemIsha KanojiyaNo ratings yet
- Goniometry 220312161003Document39 pagesGoniometry 220312161003Isha KanojiyaNo ratings yet
- Screenshot - 2023 12 12 19 47 52 78Document17 pagesScreenshot - 2023 12 12 19 47 52 78Isha KanojiyaNo ratings yet
- Fungal Pathogens Bpt-MergedDocument51 pagesFungal Pathogens Bpt-MergedIsha KanojiyaNo ratings yet
- Unit 1first Aid Question Bank Template - DBUUDocument2 pagesUnit 1first Aid Question Bank Template - DBUUIsha KanojiyaNo ratings yet
- Lab Manual 1Document3 pagesLab Manual 1Isha KanojiyaNo ratings yet
- Unit 1Document19 pagesUnit 1Isha KanojiyaNo ratings yet
- Discovery of MicroorganismDocument2 pagesDiscovery of MicroorganismIsha KanojiyaNo ratings yet
- Shoulder JointDocument40 pagesShoulder JointIsha KanojiyaNo ratings yet
- PracResearch2 Grade-12 Q4 Mod5 Data-Collection-Presentation-and-Analysis Version4Document59 pagesPracResearch2 Grade-12 Q4 Mod5 Data-Collection-Presentation-and-Analysis Version4MichiOdevilasNo ratings yet
- HRM Notes A2Document114 pagesHRM Notes A2Hari Prasad AnupojuNo ratings yet
- CompTIA Security+ (Student Edition) PDFDocument615 pagesCompTIA Security+ (Student Edition) PDFAd Hi Ie100% (1)
- Migrate DHCP Scope(s) To Windows Server 2022 - PeteNetLiveDocument6 pagesMigrate DHCP Scope(s) To Windows Server 2022 - PeteNetLiveYudy KurniawanNo ratings yet
- Advertising Ethics 1927Document6 pagesAdvertising Ethics 1927amanfan00No ratings yet
- Accounting ProblemsDocument31 pagesAccounting ProblemsJanna Gunio100% (1)
- DigiIvy Products US 052814Document1 pageDigiIvy Products US 052814akiridino0% (1)
- Case Digest in Re Meling (Legal and Judicial Ethics)Document1 pageCase Digest in Re Meling (Legal and Judicial Ethics)Ivy Eunice EstorbaNo ratings yet
- MACT PetitionDocument6 pagesMACT PetitionAbhishekSinghNo ratings yet
- City of Manila Vs LaguioDocument6 pagesCity of Manila Vs LaguioAngelGempNo ratings yet
- Personal Project Handbook 2023-2024Document32 pagesPersonal Project Handbook 2023-2024Gayatri AroraNo ratings yet
- Globe Valves: Standard SpecificationDocument26 pagesGlobe Valves: Standard SpecificationHammad AshrafNo ratings yet
- Provisional Seniority Lists - LDC UDC and Sr. Clerk-01012024Document21 pagesProvisional Seniority Lists - LDC UDC and Sr. Clerk-01012024shubham patilNo ratings yet
- Fawaz HadiDocument1 pageFawaz HadiAnonymous LuvhmxPNo ratings yet
- AMIGA - Abandoned Places A Time For Heroes ManualDocument7 pagesAMIGA - Abandoned Places A Time For Heroes Manualjajagabor100% (1)
- Hurst Signals - Introducing The FLD Trading StrategyDocument7 pagesHurst Signals - Introducing The FLD Trading StrategyvewejNo ratings yet
- Capital MArket MCQDocument11 pagesCapital MArket MCQSoumit DasNo ratings yet
- Sample Plans PDFDocument40 pagesSample Plans PDFMichal SlavíčekNo ratings yet
- WB 36Document21 pagesWB 36Nitin BajpaiNo ratings yet
- Random-Access Memory - WikipediaDocument14 pagesRandom-Access Memory - WikipediaHeera SinghNo ratings yet
- 89-95 Parts ListDocument16 pages89-95 Parts ListChrystal SwartNo ratings yet
- Passive: (With Present Simple)Document2 pagesPassive: (With Present Simple)Ale AlejandraNo ratings yet
- Task 3 - PPT TemplateDocument2 pagesTask 3 - PPT TemplateSaloni Jain 1820343No ratings yet
- S111 EN 12 Aluminium Standard Inclinometric CasingDocument4 pagesS111 EN 12 Aluminium Standard Inclinometric CasingIrwan DarmawanNo ratings yet
- LOCKv101 - Mode 8Document18 pagesLOCKv101 - Mode 8Nurul KanGen DyaNo ratings yet
- CIO VP Director IT in Detroit MI Resume Remi DiesbourgDocument2 pagesCIO VP Director IT in Detroit MI Resume Remi DiesbourgRemi DiesbourgNo ratings yet
- Customer Engagement - A Literature Review: October 2016Document7 pagesCustomer Engagement - A Literature Review: October 2016Nlke NzkeNo ratings yet
Chemicql Effects of Electric Current (Excercise)
Chemicql Effects of Electric Current (Excercise)
Uploaded by
Isha Kanojiya0 ratings0% found this document useful (0 votes)
14 views14 pagesThis document contains 12 exercise questions related to electricity and conductivity. The questions cover topics like conductors and non-conductors, factors that affect conductivity, electroplating, and safety precautions around electricity during rain. Multiple choice and open-ended questions are included and answered in detail explaining concepts like how saltwater is a better conductor than pure water and why it is dangerous to perform electrical repairs outdoors during heavy rain.
Original Description:
Original Title
Chemicql Effects of Electric Current(Excercise)
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document contains 12 exercise questions related to electricity and conductivity. The questions cover topics like conductors and non-conductors, factors that affect conductivity, electroplating, and safety precautions around electricity during rain. Multiple choice and open-ended questions are included and answered in detail explaining concepts like how saltwater is a better conductor than pure water and why it is dangerous to perform electrical repairs outdoors during heavy rain.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
14 views14 pagesChemicql Effects of Electric Current (Excercise)
Chemicql Effects of Electric Current (Excercise)
Uploaded by
Isha KanojiyaThis document contains 12 exercise questions related to electricity and conductivity. The questions cover topics like conductors and non-conductors, factors that affect conductivity, electroplating, and safety precautions around electricity during rain. Multiple choice and open-ended questions are included and answered in detail explaining concepts like how saltwater is a better conductor than pure water and why it is dangerous to perform electrical repairs outdoors during heavy rain.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 14
Exercise Questions
1. Fillin the blanks.
(a) Most liquids that conduct
electricity are solutions of
and
(b) The passage of an electric current
throughasolution causes
effects.
(c) Ifyou pass current through copper
sulphate solution, copper gets
deposited on the plate connected to
the terminal of the
battery.
(a) The process of depositing a layer of
any desired metalon another material
by means of electricity is called
Soln:
(a) Most liquids that conduct electricity
are solutions of acids, bases and salts.
(b) The passage of an electric current
through a solution causes chemical
effects.
(c) If you pass current through copper
sulphate solution, copper gets deposited
on the plate connected to the negative
terminalof the battery.
(d) The process of depositing a layer of
any desired metal on another material
by means of electricity is called
electroplating.
2. When the free ends of a tester are
dipped into a solution, the magnetic
needle shows deflection. Can you
explain the reason?
Soln:
The compass needle shows adeflection,
which concludes that current is flowing
through the wire. The circuit becomes
complete as the free ends of the tester
are immersed inside the solution. So, the
solution is conducting solution; hence,
deflection is obtained in the compass
needle.
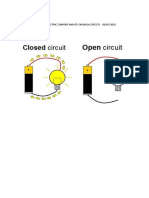
3. Name three liquids, which when
tested in the manner shown in Fig.14.9,
may cause the magnetic needle to
deflect.
Fig. 14.9
Soln:
I. Saltwater
I. Lemon juice
I. Vegetable oil
These liquids can be taken in a beaker to
showthe passage of electricity, as they
will showadeflection in the magnetic
needle.
4. The bulb does not glow in the setup
shown in Fig.14.10. List the possible
reasons. Explain your answer.
Fig. 14.10
Soln:
The possibility of the bulb not glowing
may be because of the following
reasons:
a. The liquid may be non-conducting. In
this case, the circuit is incomplete, and
the current does not pass through the
liquid.
b.Electric current may be weak for the
circuit is made up of a material which is
not a good conductor of electricity, or
there is insufficiernt energy in the battery
togenerate electricity.
5. A tester is used to check the
conduction of electricity through two
liquids, labelled A and B. It is found that
the bulb of the tester glows brightly for
liquid A, while it glows very dimly for
liquid B. You would conclude that
) liquid A is a better conductor than
liquid B.
(ii) liquid Bis a better conductor than
liquid A.
(ii) both liquids are equally
conducting.
(iv) conducting properties of liquid
cannot be compared in this manner.
Soln:
Liquid A is a better conductor than liquid
B.
The conductivity of the solution
determnines the amount of current
flowing through the solution. The greater
the conductivity, the grecater will be the
quantity of current passing through the
solution and the lesser the conductivity,
the quantity of current passing through
willbe correspondingly less. So, the
conductivity of liquid Ais more than the
conductivity of liquid B.
6. Does pure water conduct electricity?
If not, what can we do to make it
conduct?
Soln:
Pure water does not conduct electricity,
as it does not contain any type of salt.
Adding a smallamount of Common salt
(Sodiumn Chloride, i.e., Nacl) will turn the
water into aconducting medium.
7. In case of a fire, before the firemen
use the water hoses, they shut off the
main electricalsupply for the area.
Explain why they do this.
Soln:
In case of a fire, before the firemen use
the water hoses, they shut off the main
electrical supply for the area because
water sprayed from the hose might
conduct electricity which may come in
contact with the electrical appliances,
which increases the chance of electricity
passing through the wire. This may hurt
the firemen.
8. Achild staying in a coastal region
tests the drinking water and also
seawater with his tester. He finds that
the compass needle deflects more in
the case of seawater. Can you explain
the reason?
Soln:
The amount of dissolved salts present in
theseawater is more than that of the
drinking water. So, seawater will bea
better conductor than drinking water.
That is the reason behind the increased
deflection of the needle in the seawater
when compared to the drinking water.
9. Is it safe for the electrician to carry
out electrical repairs outdoors during
heavy downpours? Explain.
Soln:
No. It is not safe to repair electrical
appliances outdoors during heavy
downpours. Rainwater is composed of a
certain percentage of dissolved salts
making it conductive. This may cause
electric shocks and harm the electrician
while working outdoors during heavy
downpours.
O233
10. Pahelihad heard that rainwater is as
good as distilled water. So she
collected some rainwater in a clean
glass tumbler and tested it using a
tester. To her surprise, she found that
the compass needle showed deflection.
What could be the reasons?
Soln:
Rainwater is composed of acertain
percentage of dissolved salts making it
conductive. This results in the deflection
of the compass.
11. Prepare a list of objects around you
that are electroplated.
Soln:
Chromium plating: This is done on
exterior parts of automobiles in order to
obtain a shiny appearance.
Gold Plating: Silver ornaments are coated
with a thin layer of gold, and the product
is called Gold-plated Ornaments.
Zinc Plating: Iron used for Construction is
cOated with a Zinc layer in order to
protect them from corrosion and rusting.
12. The process that yousaw in Activity
14.7 is used for the purification of
copper. Athin plate of pure copper and
a thick rod of impure copper are used
as electrodes. Copper from the impure
rod is sought to be transferred to the
thin copper plate. Which electrode
should be attached to the positive
terminal of the battery and why?
Soln:
The thick rod of the impure copper plate
is to be attached to the positive terminal
of the battery because when the electric
current is passed through the copper
sulphate solution, it gets dissociated into
copper and sulphate. The free copper,
being positively charged, gets drawn to
the negative terminal of the battery and
gets deposited on it. On the other hand,
the loss of copper from the solution is
regained from the impure copper rod,
which is attached to the positive terminal
of the battery.
You might also like
- DuxebaxemaraviziwizubuzanDocument3 pagesDuxebaxemaraviziwizubuzanIsha KanojiyaNo ratings yet
- Class 12th Chemistry Project On Electrochemical CellsDocument11 pagesClass 12th Chemistry Project On Electrochemical CellsUdit Kanotra57% (37)
- APQP Checklist Report - Version 1.1.Dtd. 13.11.08Document51 pagesAPQP Checklist Report - Version 1.1.Dtd. 13.11.08robbie86290% (10)
- CPR Study Guide 2021: Online CPR Cheat SheetDocument14 pagesCPR Study Guide 2021: Online CPR Cheat SheetHenz Freeman0% (2)
- Family ResourcesDocument33 pagesFamily ResourcesJackie S LaymanNo ratings yet
- Chemical Effects of Electric CurrentDocument6 pagesChemical Effects of Electric CurrentImran ShahidNo ratings yet
- Chemical Effects of Electric CurrentDocument3 pagesChemical Effects of Electric Currentbharat patelNo ratings yet
- Grade 8 Science SyllabusDocument3 pagesGrade 8 Science SyllabusCRYSTAL GODNo ratings yet
- Viii Chapter 14Document6 pagesViii Chapter 14Shubh tiwari TiwariNo ratings yet
- Chemical Effects of Electric Current - Class 8 - NCERT Exercise Questions - PANTOMATHDocument3 pagesChemical Effects of Electric Current - Class 8 - NCERT Exercise Questions - PANTOMATHsourav9823No ratings yet
- NCERT Solutions For Class 8 Science Chapter 14Document4 pagesNCERT Solutions For Class 8 Science Chapter 14Padmalaya paloNo ratings yet
- 3543 93 Textbooksolution PDFDocument4 pages3543 93 Textbooksolution PDFAnushka RaoNo ratings yet
- CBSE NCERT Solutions For Class 8 Science Chapter 14: Back of Chapter QuestionsDocument4 pagesCBSE NCERT Solutions For Class 8 Science Chapter 14: Back of Chapter Questionsprem singhNo ratings yet
- Class 8 - Science - Chemical Effects of Electric CurrentDocument7 pagesClass 8 - Science - Chemical Effects of Electric CurrentSougrakpam SNo ratings yet
- Chemical Effects of ElectricDocument4 pagesChemical Effects of Electricali binNo ratings yet
- Delhi Public School Bangalore - East Chemistry Chemical Effects of Electric Current (Notes) NAME: - Class: Viii SecDocument3 pagesDelhi Public School Bangalore - East Chemistry Chemical Effects of Electric Current (Notes) NAME: - Class: Viii SecSkanda EnterprisesNo ratings yet
- PhysicsDocument9 pagesPhysicsGanesh VNo ratings yet
- Delhi Public School, Bangalore East Chemical Effects of Electric CurrentDocument3 pagesDelhi Public School, Bangalore East Chemical Effects of Electric CurrentSaket TNo ratings yet
- 8 ScienceDocument4 pages8 SciencePratibha GuptaNo ratings yet
- Chemical Effects of Electric CurrentDocument4 pagesChemical Effects of Electric Currentaadithya.v.5502.sssmscNo ratings yet
- Chemical Effect of Electric Current-5 (2021-22)Document24 pagesChemical Effect of Electric Current-5 (2021-22)Avyam SharmaNo ratings yet
- Chemical Effects of Electric CurrentDocument10 pagesChemical Effects of Electric Currentpragunjain2010No ratings yet
- PHYSICS NOTES - CHEMICAL EFFECTS OF ELECTRIC CURRENT - CLASS VIII - FINAL - CombinedDocument20 pagesPHYSICS NOTES - CHEMICAL EFFECTS OF ELECTRIC CURRENT - CLASS VIII - FINAL - CombinedB. Guru PrasadNo ratings yet
- Exemplar - Chemical Effects of CurrentDocument14 pagesExemplar - Chemical Effects of CurrentnitikaNo ratings yet
- Chemical Effects of Electric Current - NotesDocument2 pagesChemical Effects of Electric Current - Notesgmrhfamily2024No ratings yet
- 8 Science NCERT Solutions Chapter 14Document4 pages8 Science NCERT Solutions Chapter 14Raj AnandNo ratings yet
- Chemical Effects of Electric Current C W and H W 08 11 2023 20231108182020326Document3 pagesChemical Effects of Electric Current C W and H W 08 11 2023 20231108182020326dxvybzhcj8No ratings yet
- Chemical Effects NotesDocument3 pagesChemical Effects NotessabreenaapNo ratings yet
- C E O C 14: Hemical Ffects F UrrentDocument7 pagesC E O C 14: Hemical Ffects F UrrentPravat TiadiNo ratings yet
- Answer Key of Previous Worksheet On Chemical Effects of Electric Current (Grade 8 Cbse)Document3 pagesAnswer Key of Previous Worksheet On Chemical Effects of Electric Current (Grade 8 Cbse)mithlikesfun2No ratings yet
- 8 Chemical Effects of Electric CurrentDocument5 pages8 Chemical Effects of Electric Currentandrew andrewNo ratings yet
- Importante Questions For CBSE Class 8 Science Chapter 14Document6 pagesImportante Questions For CBSE Class 8 Science Chapter 14Shubham KumarNo ratings yet
- Chemical Effects of Electric Current: AnswerDocument7 pagesChemical Effects of Electric Current: AnswerTvisha SolankiNo ratings yet
- CLASS VIII QUESTION BANK - 14. Chemical Effects of Electric CurrentDocument6 pagesCLASS VIII QUESTION BANK - 14. Chemical Effects of Electric CurrentSurbhi NayarNo ratings yet
- 8th Chemical Effects of Electric Current Solved QuestionsDocument3 pages8th Chemical Effects of Electric Current Solved QuestionsGururaj KulkarniNo ratings yet
- Notes-CHEMICAL EFFECTS OF ELECTRIC CURRENTDocument4 pagesNotes-CHEMICAL EFFECTS OF ELECTRIC CURRENTishaan14810No ratings yet
- Chemical Effects of Electric Current (Grade 8 CBSE)Document2 pagesChemical Effects of Electric Current (Grade 8 CBSE)mithlikesfun2No ratings yet
- 8th Chemical Effect of Electric Current Living Science SolutionDocument3 pages8th Chemical Effect of Electric Current Living Science SolutionAnshika BhagatNo ratings yet
- 8th Chemical Effects of Electric Current Solved QuestionsDocument3 pages8th Chemical Effects of Electric Current Solved QuestionsKavitha KNo ratings yet
- Chemical Effects of CurrentDocument7 pagesChemical Effects of CurrentRonak ShahNo ratings yet
- Rajkumar Chemistry 2-1Document13 pagesRajkumar Chemistry 2-1Gopala krishnanNo ratings yet
- Electrical Conductivity of Liquids Q & ADocument12 pagesElectrical Conductivity of Liquids Q & Ajayasree012754No ratings yet
- Electric CurrentDocument12 pagesElectric Currentstory manNo ratings yet
- (8th) Chemical Effects of Electric Current Solved AssignmentsDocument3 pages(8th) Chemical Effects of Electric Current Solved AssignmentssushantNo ratings yet
- 185 638283988626517543Document3 pages185 638283988626517543pqvrc6q25mNo ratings yet
- Elctrolysis XDocument6 pagesElctrolysis XManash SinghaNo ratings yet
- Assignment 2 ElectrolysisDocument4 pagesAssignment 2 ElectrolysisJayadevi ShanmugamNo ratings yet
- Electrolysis: Lesson 10 - Electrolysis (This Lesson Comes in The Science Part II)Document6 pagesElectrolysis: Lesson 10 - Electrolysis (This Lesson Comes in The Science Part II)SwarnapaliliyanageNo ratings yet
- How to Install Electric Bells, Annunciators, and AlarmsFrom EverandHow to Install Electric Bells, Annunciators, and AlarmsNo ratings yet
- Class 6 Electricity and Circuit Exercise Question and Answer Plus Inner (Extra) Question and AnswerDocument7 pagesClass 6 Electricity and Circuit Exercise Question and Answer Plus Inner (Extra) Question and Answerdebasishnath9593913213No ratings yet
- Chemical Effect of Current - 8thDocument14 pagesChemical Effect of Current - 8thVijay KumarNo ratings yet
- FinalDocument12 pagesFinalsameeryad72No ratings yet
- Electrolysis NotesDocument11 pagesElectrolysis NotesMichaela PowellNo ratings yet
- Grade 8 Ch14Document3 pagesGrade 8 Ch14parjercreation1No ratings yet
- Chemical Effectsof Electric Current-Class NotesDocument10 pagesChemical Effectsof Electric Current-Class NotesGaurav AggarwalNo ratings yet
- Long Ans Type QueDocument2 pagesLong Ans Type QueFor JunkNo ratings yet
- Project Report 12 BDocument13 pagesProject Report 12 BParth SaxenaNo ratings yet
- Finding of Emf of Electrochemical CellDocument20 pagesFinding of Emf of Electrochemical CellVaibhav Shankar100% (1)
- DSR Elektronika BartsaDocument6 pagesDSR Elektronika BartsaBarNo ratings yet
- Danish ChemistryDocument13 pagesDanish ChemistryNaveen Kumar.RNo ratings yet
- Electricity and CircuitDocument6 pagesElectricity and CircuitVarun kumarNo ratings yet
- Chem Project.Document17 pagesChem Project.Shresth TiwariNo ratings yet
- Electric and WaterDocument11 pagesElectric and WaterCartoonn AroonnumchokNo ratings yet
- Water Level Indicator Circuit Using Bipolar Junction TransistorFrom EverandWater Level Indicator Circuit Using Bipolar Junction TransistorRating: 4.5 out of 5 stars4.5/5 (7)
- Fungal Pathogens BPTDocument2 pagesFungal Pathogens BPTIsha KanojiyaNo ratings yet
- ProjectDocument3 pagesProjectIsha KanojiyaNo ratings yet
- Bacteria and Virus General StructureDocument2 pagesBacteria and Virus General StructureIsha KanojiyaNo ratings yet
- Complement SystemDocument2 pagesComplement SystemIsha KanojiyaNo ratings yet
- Goniometry 220312161003Document39 pagesGoniometry 220312161003Isha KanojiyaNo ratings yet
- Screenshot - 2023 12 12 19 47 52 78Document17 pagesScreenshot - 2023 12 12 19 47 52 78Isha KanojiyaNo ratings yet
- Fungal Pathogens Bpt-MergedDocument51 pagesFungal Pathogens Bpt-MergedIsha KanojiyaNo ratings yet
- Unit 1first Aid Question Bank Template - DBUUDocument2 pagesUnit 1first Aid Question Bank Template - DBUUIsha KanojiyaNo ratings yet
- Lab Manual 1Document3 pagesLab Manual 1Isha KanojiyaNo ratings yet
- Unit 1Document19 pagesUnit 1Isha KanojiyaNo ratings yet
- Discovery of MicroorganismDocument2 pagesDiscovery of MicroorganismIsha KanojiyaNo ratings yet
- Shoulder JointDocument40 pagesShoulder JointIsha KanojiyaNo ratings yet
- PracResearch2 Grade-12 Q4 Mod5 Data-Collection-Presentation-and-Analysis Version4Document59 pagesPracResearch2 Grade-12 Q4 Mod5 Data-Collection-Presentation-and-Analysis Version4MichiOdevilasNo ratings yet
- HRM Notes A2Document114 pagesHRM Notes A2Hari Prasad AnupojuNo ratings yet
- CompTIA Security+ (Student Edition) PDFDocument615 pagesCompTIA Security+ (Student Edition) PDFAd Hi Ie100% (1)
- Migrate DHCP Scope(s) To Windows Server 2022 - PeteNetLiveDocument6 pagesMigrate DHCP Scope(s) To Windows Server 2022 - PeteNetLiveYudy KurniawanNo ratings yet
- Advertising Ethics 1927Document6 pagesAdvertising Ethics 1927amanfan00No ratings yet
- Accounting ProblemsDocument31 pagesAccounting ProblemsJanna Gunio100% (1)
- DigiIvy Products US 052814Document1 pageDigiIvy Products US 052814akiridino0% (1)
- Case Digest in Re Meling (Legal and Judicial Ethics)Document1 pageCase Digest in Re Meling (Legal and Judicial Ethics)Ivy Eunice EstorbaNo ratings yet
- MACT PetitionDocument6 pagesMACT PetitionAbhishekSinghNo ratings yet
- City of Manila Vs LaguioDocument6 pagesCity of Manila Vs LaguioAngelGempNo ratings yet
- Personal Project Handbook 2023-2024Document32 pagesPersonal Project Handbook 2023-2024Gayatri AroraNo ratings yet
- Globe Valves: Standard SpecificationDocument26 pagesGlobe Valves: Standard SpecificationHammad AshrafNo ratings yet
- Provisional Seniority Lists - LDC UDC and Sr. Clerk-01012024Document21 pagesProvisional Seniority Lists - LDC UDC and Sr. Clerk-01012024shubham patilNo ratings yet
- Fawaz HadiDocument1 pageFawaz HadiAnonymous LuvhmxPNo ratings yet
- AMIGA - Abandoned Places A Time For Heroes ManualDocument7 pagesAMIGA - Abandoned Places A Time For Heroes Manualjajagabor100% (1)
- Hurst Signals - Introducing The FLD Trading StrategyDocument7 pagesHurst Signals - Introducing The FLD Trading StrategyvewejNo ratings yet
- Capital MArket MCQDocument11 pagesCapital MArket MCQSoumit DasNo ratings yet
- Sample Plans PDFDocument40 pagesSample Plans PDFMichal SlavíčekNo ratings yet
- WB 36Document21 pagesWB 36Nitin BajpaiNo ratings yet
- Random-Access Memory - WikipediaDocument14 pagesRandom-Access Memory - WikipediaHeera SinghNo ratings yet
- 89-95 Parts ListDocument16 pages89-95 Parts ListChrystal SwartNo ratings yet
- Passive: (With Present Simple)Document2 pagesPassive: (With Present Simple)Ale AlejandraNo ratings yet
- Task 3 - PPT TemplateDocument2 pagesTask 3 - PPT TemplateSaloni Jain 1820343No ratings yet
- S111 EN 12 Aluminium Standard Inclinometric CasingDocument4 pagesS111 EN 12 Aluminium Standard Inclinometric CasingIrwan DarmawanNo ratings yet
- LOCKv101 - Mode 8Document18 pagesLOCKv101 - Mode 8Nurul KanGen DyaNo ratings yet
- CIO VP Director IT in Detroit MI Resume Remi DiesbourgDocument2 pagesCIO VP Director IT in Detroit MI Resume Remi DiesbourgRemi DiesbourgNo ratings yet
- Customer Engagement - A Literature Review: October 2016Document7 pagesCustomer Engagement - A Literature Review: October 2016Nlke NzkeNo ratings yet