Professional Documents
Culture Documents
Human Parvovirus B19 in The Bone Marrow
Human Parvovirus B19 in The Bone Marrow
Uploaded by
Kata TölgyesiOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Human Parvovirus B19 in The Bone Marrow
Human Parvovirus B19 in The Bone Marrow
Uploaded by
Kata TölgyesiCopyright:
Available Formats
rounds [cytology | microbiology and virology | immunology | hematology | histology]
Human Parvovirus B19 in the Bone Marrow
With Negative Viral Serologic Results
Deepali Gupta, MD, Sang L. Wu, MD, and Andy N.D. Nguyen, MD
From the Department of Pathology and Laboratory Medicine, University of Texas–Houston Medical School, Houston, TX
왘 Human parvovirus B19
왘 Tests to diagnose parvovirus B19 infection
왘 Persistent parvovirus B19 infection and suppressed serologic response
왘 Identification of inclusions in the erythroid precursors to diagnose active infection and confirmation with polymerase chain reaction for viral DNA
Case Presentation
Downloaded from https://academic.oup.com/labmed/article/32/8/429/2657187 by guest on 09 April 2022
A 28-year-old man with a
history of alcohol and
intravenous drug use presented
with delirium tremens, fever, and
progressive anemia. He had nu-
merous tattoos. Physical exami-
nation revealed marked pallor.
No organomegaly was identified.
CBC count revealed hemo-
globin, 10.3 g/dL (103 g/L);
WBC, 800/µL (0.8 × 109/L); and
platelet count, 14 × 103/µL (14 ×
109/L). The WBC differential
was as follows: 41% (0.41) neu-
trophils; 48% (0.48)
lymphocytes; 6% (0.06) mono-
cytes; 4% (0.04) eosinophils; and
1% (0.01) basophils. The reticu-
locyte count was less than 0.1%
(0.001). Blood cultures were neg-
ative, and radiologic studies re-
vealed no significant
abnormality. No HIV-1 or HIV-2
antibodies were detected, and
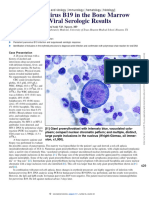
HIV-1 DNA by polymerase chain [I1] Giant proerythroblast with intensely blue, vacuolated cyto-
reaction (PCR) was negative. The
CD4 count was 1,300/µL (1.3 ×
plasm; compact nuclear chromatin pattern; and multiple, distinct,
9
10 /L). large purple inclusions in the nucleus (Wright-Giemsa, oil immer-
A bone marrow aspirate and sion, x1,000).
biopsy was performed to evaluate
the possible causes of pancytopenia. The marrow was hypocellular for the patient’s age and showed a red cell aplasia
(myeloid/erythroid ratio of 10.8:1). Numerous giant proerythroblasts were present in the aspirate, with a high nuclear/cytoplasmic
ratio; a narrow rim of intensely blue, vacuolated cytoplasm; compact uncondensed nuclear chromatin pattern; and multiple, dis-
tinct, large purple inclusions in the nucleus [I1]. The large size of the proerythroblasts could be appreciated by comparison with
adjacent neutrophils. Some of the early giant proerythroblasts showed multiple small cytoplasmic projections called “pseudopo-
dia” or “dog ears” [I2]. Intermediate giant proerythroblasts having frayed cytoplasm with decreased basophilia and coarse nuclear
chromatin with a large inclusion were also identified [I3]. This cell was larger than the early giant proerythroblast shown in [I1]
and [I2]. 429
In combination with pure red cell aplasia, these changes in erythroid precursors are highly suggestive of infection with
human parvovirus B19. DNA analysis using PCR for human parvovirus B19 DNA was performed, and the results were positive.
However, the IgG and IgM results for human parvovirus B19 were negative. The patient received intravenous immunoglobulins
for parvovirus B19. He left against medical advice 3 days later.
© laboratorymedicine> august 2001> number 8> volume 32
Clinical Background
Human parvovirus B19 is a
small, nonenveloped, single-
stranded DNA virus, classified in
the family Parvoviridae, the sub-
family Parvovirinae, and genus
Erythrovirus. Parvoviruses are
icosahedral particles between 20
and 25 nm in diameter containing
60 copies of the structural pro-
teins. Virion DNA contains 5,596
nucleotides and has identical in-
verted terminal repeat sequences
at each end that permit self-prim-
ing. Nine mRNA species have
Downloaded from https://academic.oup.com/labmed/article/32/8/429/2657187 by guest on 09 April 2022
been identified in B19-infected
cells.1
Human parvovirus B19 is
known to cause erythema infec-
tiosum (fifth disease), polyarthri-
tis, aplastic crisis in various
hemolytic anemias, prolonged
erythroid suppression in immun-
odeficient patients, and fetal
[I2] Early giant proerythroblasts showing multiple small cytoplas- death in many cases of vertical
mic projections called "pseudopodia" or "dog ears" (Wright- transmission.2,3 It is cytotoxic to
Giemsa, oil immersion, x1,000). erythroid progenitor cells in vivo
and in vitro. It is known to enter
the red cell precursors through
the blood group P antigen.4
The presence of pure red cell
aplasia and full spectrum of char-
acteristic giant proerythroblasts
in the bone marrow are pathog-
nomonic of parvovirus B19 in-
fection.5 The pathogenesis of the
giant proerythroblasts and mech-
anism of erythroid hypoplasia in
parvovirus B19 infection are not
fully understood. Induction of
apoptosis has been suggested due
to the presence of cytoplasmic
blebs; a direct cytopathogenic
lytic effect of the virus on ery-
throid precursors has also been
suggested.5
In immunocompetent
patients, the infection typically
resolves in 2 to 3 weeks with the
production of IgG that neutral-
izes the virus infectivity for ery-
430 throid host cells.6 Persistent or
recurrent parvovirus B19 infec-
tion can be associated with sup-
[I3] Intermediate giant proerythroblasts with frayed cytoplasm with pressed serologic response. By
decreased basophilia and coarse nuclear chromatin with a large the age of 30 years, 30% to 60%
inclusion (Wright-Giemsa, oil immersion, x1,000). of the normal adult population is
estimated to have IgG antibodies
against parvovirus B19.7
laboratorymedicine> august 2001> number 8> volume 32 ©
Immunocompetent hosts rarely remain ill due to this viral infection,8 but in immunodeficient patients, the virus usually persists
for prolonged periods2 and can lead to chronic anemia.9
In persistently infected patients, an aberrant immune response can result in failure to produce IgG and IgM antibodies.2 Iden-
tification of characteristic inclusions in the erythroid precursors is diagnostic of active infection. Recognition of these changes
and confirmation with PCR for viral DNA will hasten appropriate therapy. This case represents the characteristic and unique mor-
phologic features of erythroid precursors in parvovirus B19 infection with nonreactive serology in an immunocompetent host.
Role of the Laboratory
IgG and IgM antibodies specific for parvovirus B19 can be measured by indirect immunofluorescence antibody (IFA) tech-
nique or enzyme immunoassay (EIA). For IFA, the patient’s serum is incubated with parvovirus B19 recombinant VP1 antigen in
insect cells stabilized on a glass slide. In the IgM test, specimens are pretreated to prevent interference with rheumatoid factors
and to reduce IgG competition. If anti–parvovirus B19 antibodies are present in the specimen, a stable complex is formed with
the antigen. Bound antibody then reacts with a fluorescein-labeled antihuman IgG or IgM antibody, and this 3-part complex is
visualized with the aid of a fluorescence microscope.10,11 The presence of IgM-class antibodies indicates recent infection. The
presence of IgG antibodies is consistent with previous exposure. A negative test result for both IgG and IgM antibodies indicates
Downloaded from https://academic.oup.com/labmed/article/32/8/429/2657187 by guest on 09 April 2022
the absence of antibodies to parvovirus.
Our patient had a negative test result for IgG and IgM antibodies, but the presence of red cell aplasia combined with typical
changes in erythroid precursors prompted PCR testing for parvovirus B19 DNA. Positive results confirmed infection with the
virus. The PCR was performed in a reference laboratory with the method described by Rogers et al.12 Confirmation of parvovirus
B19 infection can also be performed by immunohistochemistry.
Conclusion
Bone marrow changes, which include pure red cell aplasia and presence of giant proerythroblasts, are key to the diagnosis of
human parvovirus infection. Immunocompetent hosts can also have negative IgG and IgM antibodies. In such cases, infection can
be confirmed by PCR for viral DNA.
1. Anderson LJ. Human parvovirus B19. In: Richman DD, Whitley RJ, Hayden FG, eds. Clinical Virology. New York, NY: Churchill Livingstone; 1997:613-631.
2. Lundqvist A, Tolfvenstam T, Bostic J, et al. Clinical and laboratory findings in immunocompetent patients with persistent parvovirus B19 DNA in bone marrow. Scand J
Infect Dis. 1999;31:11-16.
3. Crook TW, Rogers BB, McFarland RD, et al. Unusual bone marrow manifestations of parvovirus B19 infection in immunocompromised patients. Hum Pathol. 2000;31:161-
168.
4. Brown KE, Anderson SM, Young NS. Erythrocyte P antigen: cellular receptor of B19 parvovirus. Science. 1993:262:114-117.
5. Koduri PR. Novel cytomorphology of the giant proerythroblasts of parvovirus B19 infection. Am J Hematol. 1998;58:95-99.
6. Anderson MJ, Higgins, Davis LR. Experimental parvovirus B19 infection in humans. J Infect Dis. 1985;152:257-265.
7. Woolf AD, Campion GV, Chishick A, et al. Clinical manifestations of human parvovirus B19 in adults. Arch Intern Med. 1989;149:1153-1156.
8. Faden H, Gary J GW, Anderson LJ. Chronic parvovirus infection in a presumably immunologically healthy woman. Clin Infect Dis. 1992;15:595-597.
9. Frickhofen N, Abkowitz JL, Safford M, et al. Persistent B19 parvovirus infection in patients infected with human immunodeficiency virus type 1 (HIV-1): a treatable cause
of anemia in AIDS. Ann Intern Med. 1990;113:926-933.
10. Cotmore SF, McKie VC, Anderson LJ, et al. Identification of the major structural and nonstructural proteins encoded by human parvovirus B19 and mapping of their genes
by prokaryotic expression of isolated genomic fragments. J Virol. 1986;60:548-557.
11. West NC, Meigh RC, Mackie M, et al. Parvovirus infection associated with aplastic crisis in a patient with HEMPAS. J Clin Pathol. 1986;39:1019-1020.
12. Rogers BB, Mak SK, Dailey JV, et al. Detection of parvovirus B19 DNA in amniotic fluid by PCR DNA amplification. Biotechniques. 1993;15: 406-410.
431
© laboratorymedicine> august 2001> number 8> volume 32
You might also like
- Fundamentals of Molecular Virology (2nd Ed.)Document11 pagesFundamentals of Molecular Virology (2nd Ed.)luz dey pastranaNo ratings yet
- Corona VirusesDocument42 pagesCorona VirusesstudymedicNo ratings yet
- Microbiology: Parvovirus, Papillomavirus, and PolyomavirusDocument3 pagesMicrobiology: Parvovirus, Papillomavirus, and PolyomavirusJustin TayabanNo ratings yet
- Zoo 1Document3 pagesZoo 1nurul rezki fitrianiazisNo ratings yet
- Journal of Virology-2018-Zou-e01881-17.fullDocument16 pagesJournal of Virology-2018-Zou-e01881-17.fullJavier Vallejo MontesinosNo ratings yet
- The Human Bone Marrow Is Host To The Dnas of Several VirusesDocument7 pagesThe Human Bone Marrow Is Host To The Dnas of Several VirusesAdote DrmNo ratings yet
- Diagnostic Laboratory Tests Epidemiology and Ecology: A. Specimens, Microscopic Examination, and CultureDocument3 pagesDiagnostic Laboratory Tests Epidemiology and Ecology: A. Specimens, Microscopic Examination, and CultureAnggaNo ratings yet
- Pathogenic VirusDocument283 pagesPathogenic VirusTamara ElyasNo ratings yet
- Smallest Viruses (The Only Dna Virus To Have Ssdna) .: Parvovirus B19Document8 pagesSmallest Viruses (The Only Dna Virus To Have Ssdna) .: Parvovirus B19AfreenNo ratings yet
- Ann Rev of Virology IPNVDocument30 pagesAnn Rev of Virology IPNVRodolfo VelazcoNo ratings yet
- Trofe Clark2016Document14 pagesTrofe Clark2016WEE LENG GANNo ratings yet
- Virus Structure ClassificationDocument9 pagesVirus Structure Classification007ginniNo ratings yet
- MLT503 - L2 - General Properties of VirusesDocument51 pagesMLT503 - L2 - General Properties of VirusesMuhammad FirdausNo ratings yet
- Virology Lec 1Document3 pagesVirology Lec 1Cristina CorpuzNo ratings yet
- Treacy Et Al 2010 First Report of Sudden Death Due To Myocarditis Caused by Adenovirus Serotype 3Document4 pagesTreacy Et Al 2010 First Report of Sudden Death Due To Myocarditis Caused by Adenovirus Serotype 3insyiisyaNo ratings yet
- DNA Ant TechnologyDocument19 pagesDNA Ant TechnologyJed Esperidion Solivio JumillaNo ratings yet
- General Concepts: Clinical ManifestationsDocument6 pagesGeneral Concepts: Clinical ManifestationsSyafda Heroni FirdausNo ratings yet
- Microbial Pathogenesis: SciencedirectDocument11 pagesMicrobial Pathogenesis: SciencedirectFerdinand Prayogo Cahyo SantosoNo ratings yet
- 4 Virology and VaccinesDocument25 pages4 Virology and VaccinesPaula CárdenasNo ratings yet
- 10.1371 Journal - PmedDocument3 pages10.1371 Journal - Pmedapi-3843865No ratings yet
- MIC 211 Papillomavirus Polyomavirus and Parvovirus IBD MergedDocument14 pagesMIC 211 Papillomavirus Polyomavirus and Parvovirus IBD MergedJack Ortega PuruggananNo ratings yet
- Viruses Morphology and Ultrastructure. Viruses Cultivation in Chicken Embryo and Laboratory Animals Organism!Document21 pagesViruses Morphology and Ultrastructure. Viruses Cultivation in Chicken Embryo and Laboratory Animals Organism!Amirs AmjadNo ratings yet
- Budgerigar Fledgling Disease (Papovavirus) in Pet BirdsDocument4 pagesBudgerigar Fledgling Disease (Papovavirus) in Pet BirdsFiroz RezaNo ratings yet
- CCHF 1Document17 pagesCCHF 1farzinhadiniaNo ratings yet
- NatureArticle TheLorenzAttractorExistsDocument2 pagesNatureArticle TheLorenzAttractorExistsMax LorenNo ratings yet
- Introduction To VirologyDocument39 pagesIntroduction To VirologybmackarelNo ratings yet
- BABESIOSISDocument34 pagesBABESIOSISRoderick83% (6)
- Virology Introductionby ShyamalDocument38 pagesVirology Introductionby ShyamalLyra Dennise LlidoNo ratings yet
- Diagnosis of Malaria Parasites Plasmodium Spp. in EndemicAreas: Current Strategies For An Ancient Disease Brian Gitta and Nicole KilianDocument12 pagesDiagnosis of Malaria Parasites Plasmodium Spp. in EndemicAreas: Current Strategies For An Ancient Disease Brian Gitta and Nicole KilianGitta BrianNo ratings yet
- Virus-PrionDocument28 pagesVirus-PrionHaikal FahreziNo ratings yet
- Anemia Caused by VirusDocument5 pagesAnemia Caused by VirusStephanie WirjomartaniNo ratings yet
- Birnaviridae J Gen VirologyDocument2 pagesBirnaviridae J Gen VirologyRodolfo VelazcoNo ratings yet
- KMTC Microbiology Notes March 2022 ClsaaDocument31 pagesKMTC Microbiology Notes March 2022 ClsaaFrancis ChegeNo ratings yet
- Van Der Hoek Et Al. (2004)Document6 pagesVan Der Hoek Et Al. (2004)SammerNo ratings yet
- Asssiment Crona Virus 2Document18 pagesAsssiment Crona Virus 2mariaNo ratings yet
- Viral OncogenesisDocument36 pagesViral OncogenesisyuanlupeNo ratings yet
- Case Study: A Case of An AIDS Patient With Cryptococcus Neoformans InfectionDocument5 pagesCase Study: A Case of An AIDS Patient With Cryptococcus Neoformans InfectionAdib HaikalNo ratings yet
- Virology Lectures: Dr. Mozahim AL-AttarDocument18 pagesVirology Lectures: Dr. Mozahim AL-AttarjasnaldNo ratings yet
- Neet Marathon RevisionDocument61 pagesNeet Marathon Revisionafaan18m3742No ratings yet
- Virology Lecture 1Document20 pagesVirology Lecture 1ao868598No ratings yet
- JadelDocument3 pagesJadelMariano, Adrian ImmanuelNo ratings yet
- Clinical Studies: Arthropod-Borne Virus Disease in FloridaDocument9 pagesClinical Studies: Arthropod-Borne Virus Disease in FloridaLúcia ReisNo ratings yet
- Adenoviruses: S Jane FlintDocument14 pagesAdenoviruses: S Jane FlintFrancisco BecerraNo ratings yet
- VirologyDocument183 pagesVirologyPeachy Pie100% (1)
- Virology UG Class IV SemesterDocument109 pagesVirology UG Class IV Semestervallabhaneni rajesh100% (1)
- Parvovirus B19Document25 pagesParvovirus B19FreezingSoul FreezingSoulNo ratings yet
- Chapter 11Document14 pagesChapter 11Alec SavieNo ratings yet
- 9311 FullDocument6 pages9311 FullDaniela Alejandra EstupiñanNo ratings yet
- Oncogenic Viruses: Christopher B. Buck, Lee Ratner, and Giovanna TosatoDocument46 pagesOncogenic Viruses: Christopher B. Buck, Lee Ratner, and Giovanna TosatoJohan Rinto Even NapitupuluNo ratings yet
- Masters and Perlman 2013 in Fields Virology 1Document34 pagesMasters and Perlman 2013 in Fields Virology 1s_navroopNo ratings yet
- In Vitro Human Cells by ViralDocument10 pagesIn Vitro Human Cells by Viraljhonjairo5038No ratings yet
- TickDocument10 pagesTickmarioaNo ratings yet
- Agents of Quarantine Diseases 02Document15 pagesAgents of Quarantine Diseases 02AkasorachiNo ratings yet
- Sunarjati Sudigdoadi Usep Abdullah Husin Dept. of MicrobiologyDocument29 pagesSunarjati Sudigdoadi Usep Abdullah Husin Dept. of MicrobiologyFaishalNo ratings yet
- Virus-Klasifikasi, Sifat, GenetikaDocument24 pagesVirus-Klasifikasi, Sifat, GenetikaRamano Untoro PutroNo ratings yet
- Virus - "Poison" in LatinDocument5 pagesVirus - "Poison" in LatinTrisha Dela CruzNo ratings yet
- Acellular Microbes PPT Feb. 2024Document61 pagesAcellular Microbes PPT Feb. 202421-54405No ratings yet
- Herpes Simplex Viruses: State-Of-The-Art Clinical ArticleDocument15 pagesHerpes Simplex Viruses: State-Of-The-Art Clinical ArticleCozmina CirdeiNo ratings yet
- 2023 in Vitro Characterization of chIFITMs of Aseel and KadaknathDocument14 pages2023 in Vitro Characterization of chIFITMs of Aseel and KadaknathDr. SIVAKUMAR KARUPPUSAMYNo ratings yet
- Odontogenic TumorsDocument229 pagesOdontogenic TumorsKata TölgyesiNo ratings yet
- Papu Los Qu AmousDocument8 pagesPapu Los Qu AmousKata TölgyesiNo ratings yet
- Rotavirus Antigens in Infected CellsDocument11 pagesRotavirus Antigens in Infected CellsKata TölgyesiNo ratings yet
- Alterartion in WBCDocument26 pagesAlterartion in WBCKata TölgyesiNo ratings yet
- Erythroblastic SynartesisDocument11 pagesErythroblastic SynartesisKata TölgyesiNo ratings yet
- Anaphylactic and Anaphylactoid ReactionsDocument6 pagesAnaphylactic and Anaphylactoid ReactionsKata TölgyesiNo ratings yet
- Late Presentation of Pylephlebitis After Orthotopic Liver TransplantationDocument2 pagesLate Presentation of Pylephlebitis After Orthotopic Liver TransplantationKata TölgyesiNo ratings yet
- Fibrosis in Autoimmune and Cholestatic Liver DiseaseDocument14 pagesFibrosis in Autoimmune and Cholestatic Liver DiseaseKata TölgyesiNo ratings yet
- Enteric Disease Rotavirus Brachyspira Hyodysenteriae Moeser AASV2012Document2 pagesEnteric Disease Rotavirus Brachyspira Hyodysenteriae Moeser AASV2012Kata TölgyesiNo ratings yet
- Livedoid Vasculopathy A Review of Pathogenesis and Principles of ManagementDocument11 pagesLivedoid Vasculopathy A Review of Pathogenesis and Principles of ManagementKata TölgyesiNo ratings yet
- Congenital and Acquired Anomalies of The Portal Venous SystemDocument19 pagesCongenital and Acquired Anomalies of The Portal Venous SystemKata TölgyesiNo ratings yet
- Livedoid VasculopathyDocument15 pagesLivedoid VasculopathyKata TölgyesiNo ratings yet
- Fluorescent MicrosDocument55 pagesFluorescent Microstummalapalli venkateswara rao100% (2)
- Cell and Molecular Biology Module 1 and 2Document8 pagesCell and Molecular Biology Module 1 and 2clarisseNo ratings yet
- Chapter 6 FixationDocument165 pagesChapter 6 FixationKelly Avila GripoNo ratings yet
- Euroimmun InfecciosasDocument300 pagesEuroimmun InfecciosasSusana Méndez GómezNo ratings yet
- IARC Sci Pub 163 - Chapter 10Document14 pagesIARC Sci Pub 163 - Chapter 10ashin411No ratings yet
- Interactions Between Antigen and AntibodyDocument25 pagesInteractions Between Antigen and AntibodyvmshanesNo ratings yet
- Human Parvovirus B19 in The Bone MarrowDocument3 pagesHuman Parvovirus B19 in The Bone MarrowKata TölgyesiNo ratings yet
- Shapiro 1983 CytometryDocument17 pagesShapiro 1983 CytometryMunir AliNo ratings yet
- Zincogen NBFDocument7 pagesZincogen NBFcodesolutionroNo ratings yet
- Rapid Antigen-Specific T Cell Enrichment (Rapid ARTE) - All Protocols - Applications - Miltenyi Biotec - DeutschlandDocument11 pagesRapid Antigen-Specific T Cell Enrichment (Rapid ARTE) - All Protocols - Applications - Miltenyi Biotec - Deutschlandzixu wangNo ratings yet
- Fluorescent Immunoassay (Autosaved)Document13 pagesFluorescent Immunoassay (Autosaved)Matt McAndrew GarciaNo ratings yet
- ELISADocument18 pagesELISAmukulpjrNo ratings yet
- Nadira Duwi 3191028 Naufal HafidDocument61 pagesNadira Duwi 3191028 Naufal HafidNadira RaraNo ratings yet
- Lec-9 ImmunofluorescenceDocument25 pagesLec-9 ImmunofluorescenceTMT100% (1)
- Staining Techniques: Connective TissueDocument6 pagesStaining Techniques: Connective TissuePamela Dawn PornesoNo ratings yet
- Immuno FluorescenceDocument21 pagesImmuno FluorescencePAVITHRA VNo ratings yet
- Modulation ContrastDocument30 pagesModulation ContrastNishalini NishaliniNo ratings yet
- Characteristics of Normal Cells Vs Transformed Cells: Ayman Saeed 2020-MPHIL-1243Document3 pagesCharacteristics of Normal Cells Vs Transformed Cells: Ayman Saeed 2020-MPHIL-1243Ujala AsadNo ratings yet
- Histopathology Laboratory OverviewDocument31 pagesHistopathology Laboratory OverviewaliNo ratings yet
- Molecular Cell Biology 7Th Edition Lodish Test Bank Full Chapter PDFDocument29 pagesMolecular Cell Biology 7Th Edition Lodish Test Bank Full Chapter PDFrorybridgetewe100% (14)
- Virology Assignment Re WriteDocument21 pagesVirology Assignment Re WriteDires AdmasNo ratings yet
- Techniques For Immune Function Analysis HandbookDocument248 pagesTechniques For Immune Function Analysis HandbookDante AvilésNo ratings yet
- UprgenyDocument9 pagesUprgenyJose Rafael Villafan BernalNo ratings yet
- 18 Test BankDocument16 pages18 Test BankNaNo ratings yet
- The Wd40 Protein Bamb Mediates Coupling of Bam Complexes Into Assembly Precincts in The Bacterial Outer MembraneDocument14 pagesThe Wd40 Protein Bamb Mediates Coupling of Bam Complexes Into Assembly Precincts in The Bacterial Outer MembraneAMMAR RAZANo ratings yet
- Serology PDFDocument201 pagesSerology PDFGEBEYAW ADDISU100% (1)
- Folleto Del Equipo HeliosDocument6 pagesFolleto Del Equipo HeliosJose Luis Albornoz HermosillaNo ratings yet
- Introduction To Cell & Molecular Biology Techniques: ImmunofluorescenceDocument38 pagesIntroduction To Cell & Molecular Biology Techniques: ImmunofluorescenceMarianne GonzalesNo ratings yet
- 15.antigen - Antibody Reactions Part 2Document34 pages15.antigen - Antibody Reactions Part 2Manas DixitNo ratings yet
- WORKSHEET 3 Lymphocyte ActivationDocument5 pagesWORKSHEET 3 Lymphocyte ActivationNeha ChoudharyNo ratings yet